The Role of Vitamin D in Skeletal Muscle Repair and Regeneration in Animal Models and Humans: A Systematic Review
Abstract
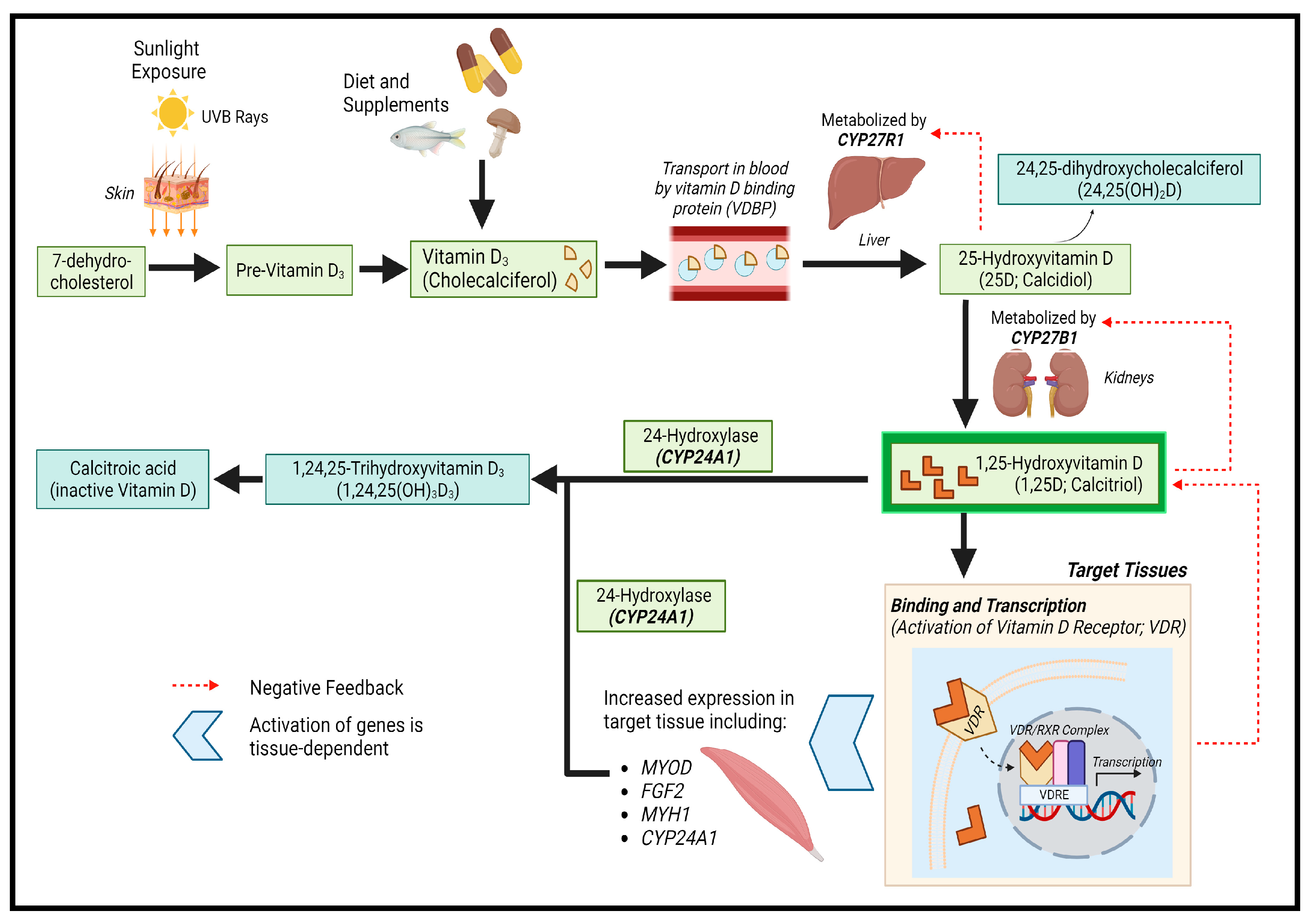
:1. Introduction
1.1. Whole-Body VDR Knockout Mice
1.2. Myocyte-VDR Knockout Mice
2. Materials and Methods
2.1. Search Strategies
- PubMed (searched in April, May, and September 2023);
- Web of Science (searched in September 2023);
- Cochrane Library (searched in September 2023);
- Scopus (searched in September 2023).
2.2. Selection of Studies
2.3. Data Extraction
2.4. Quality Assessment of Included Studies
3. Results
3.1. In Vitro Studies (Table 1)
3.1.1. Introduction to In Vitro Experiments
3.1.2. Main Findings and Their Implications
| Reference | Cell Model | Treatments | Notes and Effects |
|---|---|---|---|
| [42] Srikuea (2012) | C2C12 | 25D (2 µM) or 1,25D (20 nM) + CYP27B1 siRNA | ↑ Vdr mRNA with 25D and 1,25D treatment. Cyp27B1 knockdown caused and ↑ cell numbers with 25D treatment, suggesting inhibition by 25D |
| [50] Srikuea (2020) | C57BL/6 mouse SCs (male) | 100 nM 1,25D | ↑ VDR and CYP24A1 proteins in developmental, mature, and aged cells. ↓ responsiveness of SMSCs and ↓ VDR expression with aging |
| [51] Saito | C2C12 | Eldecalcitol 1, 10 or 100 nM | ↑ Vdr, MyoD and Igf1 mRNAs, ↑ expression of MHC Iia, Iib and Iix at 10–100 nM, ↑ fast myosin head chain proteins at 1 and 10 nM |
| [52] Okuno | C2C12 | 1,25D at 1, 10 or 100 nM | ↓ myoblast proliferation in differentiating phase ↑ fast myosin head chains in differentiated phase |
| [8] Girgis | C2C12 | 100 nM 25D or 100 nM 1,25D | 25D and 1,25D caused ↑ Vdr, Cyp27b1 and Cyp24a1 mRNAs, ↑ genes involved in G0/G1 arrest, ↓and in G1/S transition genes. ↓ myotube formation and myogenic regulatory factors, ↓ myostatin and ↑ myotube cross-sectional size with 1,25D |
| [27] Camperi | C2C12 myoblasts | 1,25D (10 or 100 nM) | ↓ myoblast proliferation and myogenic differentiation. ↑ VDR and myogenin protein. Lentiviral αVDR shRNA restored myoblast differentiation |
| [38] Hosoyama | Immortalis-ed mouse cells | 1 µM 1,25D | ↓ expression of myogenic regulatory factors, Myf5 and myogenin in proliferating myoblasts. ↓ myoblast-to-myoblast and myoblast-to-myotube fusion ↑ hypertrophy and protein anabolism |
| [13] Braga | C57BL/6 mouse SCs | 100 nM 1,25D | ↑ myogenic effect on satellite cells, ↑ myotube formation ↓ expression of MSTN |
| [12] Bass | C2C12 | Lentiviral anti-VDR shRNA | ↓ total protein content, lower myofiber area, ↓ myogenesis ↓ mitochondrial respiration related proteins and genes Activation of autophagic processes, no effect on muscle protein synthesis or anabolic signalling |
| [53] Irazoqui | C2C12 | Lentiviral anti-VDR shRNA | ↑ VDR and p38-dependent S-phase peaks, ↑ p21 and p27 cyclin-dependent kinase, ↑ myogenin expression. Induced cell arrest at G0/G1 phase and ↑ myogenic differentiation. |
| [39] Garcia (2013) | C2C12 | 100 nM 1,25D | ↑ Fgf1 and Vegfa expression, ↓ Fgf2 and Timp3 expression Vitamin D regulated angiogenic factors in skeletal muscle cells |
| [54] Garcia (2011) | C2C12 | 100 nM 1,25D | ↓ PCNA expression, ↑ MyoD, Desmin, Myogenin and Igf-II, ↓ Mstn, ↑Fst Vitamin D enhanced myoblast differentiation |
| [40] Mizutani | C2C12 myoblasts and myotubes | 100 nM and 1 µM 1,25D | ↑ expression of genes involved in promoting angiogenesis, myoblast differentiation, muscle hypertrophy and lipid metabolism |
| [55] Salles | C2C12 | 100 mM insulin + 5 mM leucine and 1,25D (0, 1 or 10 nM) | 1,25D enhanced the protein anabolic effects of insulin and leucine in myotubes in a dose-dependent manner |
| [56] Buitrago | C2C12 myoblasts | 1 nM 1,25D | ↑ Akt phosphorylation during both proliferation and early differentiation, ↑ expression of myosin head chains and myogenin, ↑ myoblast proliferation |
| [58] Ashcroft | C2C12 myoblasts | VDR-KD lentiviral plasmids | ↓ mitochondrial respiration, ↓ mitochondrial ATP production No changes in mitochondrial protein content, and markers for mitochondrial fission |
| [57] van der Meijden | C2C12 myotubes and myoblasts | 1 mM 25D or 100 nM 1,25D | ↑ Cyp24 and Vdr levels, ↓ Cyp27b1 and Myogenin, ↑ Myhc-1 -Iia, Iib and Iix, ↑ Increased myotube diameter with 25D. No changes in p-Akt, p-6 and total P6 levels, ↑ Total Akt with 25D and 1,25D, ↑ Cyp24a1 mRNA with 25D in myotubes and myoblasts. |
| [61] Olsson | Human myoblasts | 25D (100 nM) or 1,25D (1 nM or 100 nM) | ↑ VDR and CYP24A1 mRNA and proteins, ↓ cell proliferation, ↓ myotube formation, ↓ expression of myogenic regulatory factors ↓ cell cycle regulators, ↑ Cdk inhibitors p27 and p21, FOXO3, ↑ Notch signalling pathway components with 100 nM 1,25D. |
| [62] Ryan | Human skeletal muscle cells | 10 nM 1,25D | ↑ Increased oxygen consumption, ↑ mitochondrial volume and branching, ↑ expression of OPA1 and Mfn1, ↓ expression of mitochondrial fission proteins, ↓ phosphorylated pyruvate dehydrogenase and PDK4 |
| [41] Schnell | C2C12 myotubes | 100 nM Calcitriol ± PLIN2 siRNA | ↑ VDR and PLIN2 expression with calcitriol treatment, ↑ SDH activity with calcitriol treatment, ↑ intramyocellular lipid accumulation with calcitriol treatment, ↓ lipolysis and mitochondrial respiration with vitamin D + PLIN2 siRNA treatment |
| [63] Romeu Montenegro | Human skeletal muscle cells | 100 nM 1,25D | ↓ myocyte proliferation, ↑ myoblast differentiation, ↑ Akt and mTOR signalling cascade activity, ↑ mitochondrial oxygen consumption |
| [64] Hayakawa | Human myotubes | 10 nM 1,25D | ↓ expression of MAFbx and MuRF1, ↑ expression of IL-6 ↓ protein phosphatase 2A (PP2A) in human myotubes, ↑ AKT-1 |
| [45] Owens | Human myoblasts | 1, 25D (10 or 100 nM) | Improvements in myotube fusion and differentiation with 10 and 100 nM 1,25D. ↑ expression of MRF4, MYOG and VDR, ↑ myotube diameter, area, and number of myotubes per field. |
3.1.3. Conclusions: In Vitro Studies
3.2. Animal Studies (Table 3)
3.2.1. Introduction to Animal Models
3.2.2. Muscle Injury
3.2.3. Disease-Related Animal Models
| Reference | Model/Genetic Modification | Notes and Effects |
|---|---|---|
| [12] Bass | VDR knockout via lentiviral shRNA electro-transfer | ↑ skeletal muscle atrophy, ↑ autophagic processes ↓ mitochondrial metabolism. No changes to anabolic signalling or muscle protein synthesis |
| [24] Nakamura | VDR knockout | ↓ grip strength, ↓ muscle cross-sectional area, ↓muscle volume, ↑ Atrogin-1, ↑ MuRF1, ↑ Tnfa, ↑ Il-6 and ↑ Il-1b |
| mVDR knockout | ↓ muscle fiber cross-sectional area. Muscle atrophy upon immobilisation. | |
| neural crest- specific VDR knockout | ↑ muscle weight compared to mVDR mice, ↓ muscle weight upon immobilisation, ↓ muscle fiber cross-sectional area | |
| [28] Yu | CYP27B1 knockout | ↓ grip strength, ↓ muscle fiber size, ↓ MyoD, MyHC, Myf5 expression, ↓ BrdU positive cells |
| [66] Panda | CYP27B1 knockout | ↓ Body weight, ↓ serum Ca2+ and PO4 ↑ serum 25D, PTH, ↑ serum alkaline phosphate, ↑ urinary PO42− s |
| [3] Girgis (2015) | VDR knockout and vitamin D deficient mice | ↓ grip strength, ↓ muscle fiber size, ↓ myogenic regulatory factors, ↑ myostatin levels, ↓ calcium handling genes, ↓ sarco-endoplasmic reticulum calcium transport ATPase channels, ↑ atrophy-related gene MuRF1 |
| [2] Girgis (2019) | myocyte-specific vitamin D receptor knockout mice | ↓ voluntary running speed, distance, and grip strength, ↑ muscle fiber diameter, ↓ cell-cycle genes, ↓ cyclin-dependent kinases, ↓ calcium handling genes, calbindin, ↓ myostatin. No differences in muscle metabolism and fiber type observed. No increase in fibrosis. |
| [25] Cheung (2020a) | Cystinosis knockout | Normalized muscle function, muscle fiber size, collagen content, energy expenditure, muscle ATP content with vitamin D supplementation. Normalized expression of Igf1, Pax7, MyoD, Murf1 and myostatin. |
| [26] Cheung (2020b) | 5/6 nephrectomy, rats | Normalized muscle fiber size, ↑ muscle function ↓ expression of profibrotic genes, ↑ expression of anti-fibrotic genes, ↓ muscle fat infiltration, ↑ skeletal muscle mass regulation. Normalized muscle ATP content |
| [27] Camperi | Tumor-transplant, rats | ↓ circulating vitamin D levels, ↑ Vdr mRNA in tumor-bearing rats. ↑ Vdr mRNA expression after vitamin D administration. No significant change in body weight, gastrocnemius, or tibialis anterior size. |
| [42] Srikuea | BaCl2 induced injury, mice | ↑ Vdr and Cyp27b1 expression following injury |
| [31] Stratos | Crush injury, rats | ↑ cell proliferation, ↓ apoptosis with high dose vitamin D. No changes in twitch strength, ↑ tetanic strength, no changes in satellite cell number and myocyte size with high dose vitamin D. |
| [29] Srikuea and Hirunsai | BaCl2 induced injury, mice | ↑ Vdr expression, regulated Cyp24a1 and Cyp27b1 levels. ↓ cross-sectional area of regenerating skeletal myocytes, ↓ satellite cell differentiation, ↓ regenerative fiber formation No effect on regenerating muscle weight and fiber typing |
| [30] Dominguez-Faria | Old Wistar Rats | ↓ plasma 25D and tibialis anterior weights in vitamin D deplete diet fed rats, ↓ cell proliferation and notch signalling pathway genes, ↓ Bmp4, ↓ Fgf2, ↓ PCNA protein expression, ↓ Hes1 |
| [67] Bhat | Male rats Vitamin D deficient diet | ↓ muscle weight, ↓ lean body mass, ↓ Type II muscle fiber area, ↑ muscle protein degradation, ↓ muscle protein synthesis, ↑ Atrogin-1 and MuRF1, ↑ Psc2 and Psc8, ↓ MyoD, MyoG and Myf5 |
| [44] Bang | Rats | ↓ VDR expression in VDD group muscles at both 16 and 32-week timepoints, ↓ muscle fiber cross-sectional areas and volumes. |
| [43] Bass | In vivo electro-transfer of lentiviral VDR | ↑ muscle protein synthesis, ↑ expression anabolic signalling intermediates, ↑ hypertrophy, ↑ extracellular remodelling, ↑ satellite cell content, ↑ myofiber cross-sectional area |
3.2.4. Injury Studies
3.2.5. Vitamin D Deficiency
3.2.6. VDR Overexpression
3.2.7. Conclusions: Animal Studies
3.3. Human Studies (Table 4)
3.3.1. Introduction to Human Trials
3.3.2. Exercise-Induced Muscle Damage
3.3.3. Post-Surgery Studies
3.3.4. Other Relevant Studies
3.3.5. Conclusions: Human Studies
| Reference | Number of Subjects | Study Cohort Characteristics | Treatments/Surgeries Performed | Notes and Effects |
|---|---|---|---|---|
| [45], Owens | n = 20 | 18–30 years old, healthy male volunteers | 4000 IU/day oral 1,25D supplementation following eccentric exercise + MVC | ↑ MVC at 48 h and 7-days after muscle damage with 1,25D supplementation |
| [33], Brenna-Speranza | n = 19 | >50 years old, males and females with knee osteoarthritis | Knee replacement surgery | No significant differences in 25D levels between OA and asymptomatic patients, ↑ VDR, ↑ DBP, ↑ albumin levels in OA patients |
| [43], Bass | n = 37 | 18–35 years old, healthy males and females | 20 weeks resistance exercise training | ↑ muscle hypertrophy following long-term resistance training exercise |
| [73], Conzade | n = 975 | 63–93 years old | No treatments or surgeries performed | ↓ grip strength, ↓ gait speed, ↑ time during TUG test, ↑ sarcopenia in subjects with low serum 25D levels |
| [70], Ryu | n = 91 | 50–60 years old | Arthroscopic rotator cuff repair | Low serum 25D levels in patients were not correlated to the severity of rotator cuff tears |
| [32], Rhee | n = 36 | 40–80 years old | Arthroscopic rotator cuff repair | No correlation between tissue and serum vitamin D levels with fatty degeneration in muscle and healing failure post-surgery. |
| [44], Bang | n = 91 | 60–69 years old, females | No treatments or surgeries performed | ↓ lumbar muscularity, ↑ percentage of fatty infiltration in Vitamin D deficient subjects |
| [74], Dzik (2018) | n = 38 | average 48.2 years old, males and females with lower back pain | Vitamin D supplementation (3200 IU/day) for 5 weeks | ↓ markers of free radical damage of lipids and proteins, ↓ oxidative stress in paraspinal muscle with vitamin D supplementation |
| [75], Dzik (2019) | n = 14 | average 45.2–51.2-year-old patients with lower back pain | Vitamin D supplementation (3200 IU/day) for 5 weeks | ↑ muscle atrophy, ↑ oxidative stress, ↓ mitochondrial function with vitamin D deficiency |
| [76], Scott | n = 686 | 50–59 years old | No treatments or surgeries performed | ↓ leg strength, ↓ muscle quality, ↓ physical activity in patients with low 25D levels |
| [77], Gerdhem | n = 986 | 75 years old, females | No treatments or surgeries | ↓ muscle strength and physical activity |
| [72], Gordon | n = 79 | 49 years old | 1,25D treatment with calcitriol (0.5 mcg-9 mcg) or paricalcitol (0.5–42 mcg) | ↑ thigh muscle cross-sectional area, ↑ muscle strengths with active vitamin D (calcitriol or paricalcitol) treatment |
| [68], Barker (2013a) | n = 14 | 32 years old, males and females | Intense-stretch shortening contraction | ↓ peak isometric forces after intense exercise at all time-points (except day 7) ↓ muscle strength in subjects with low pre-exercise 25D levels |
| [69], Barker (2013b) | n = 28 | 30 years old | 4000 IU Cholecalciferol (Vitamin D) for 35 days | ↑ muscle function and strength following intense exercise with Vitamin D supplementation |
| [71], Ekinci | n = 75 | ≥65 years old, females | 3 g CaHMB + 1000 IU Vitamin D + 36 g Protein supplementation following orthopaedic surgery | ↓ wound healing time, ↑ mobilisation, ↑muscle strength with combined treatment post orthopaedic surgery |
| [34], Shim | n = 18 | >50 years old, females | Distal Radial Fracture repair + 1000 IU Vitamin D supplementation | ↑ medical VDR and muscle CSA after vitamin D supplantation Normalized serum 25D levels in vitamin D deficient patients post-surgery |
| [35], Cancienne | n = 982 | >50 years old | Arthroscopic rotator cuff repair | ↑ rate of revision rotator cuff surgery in patients with vitamin D deficiency |
| [36], Kim | n = 24 | 41–75 years old | Arthroscopic rotator cuff repair | ↑ IL1β and IL6 in deltoid and supraspinatus muscles in vitamin D deficient subjects. No functional outcomes were assessed. |
| [37], Gronborg | n= 136 | 18–50 years old | Vitamin D fortified diet | ↑ grip strength and knee extension in active treatment groups |
| [78], Ceglia | n = 21 | ≥65 years old, females | 4000 IU/day oral Vitamin D3 supplementation | ↑ serum 25D levels, ↑ intramyonuclear VDR, ↑ muscle fiber CSA at 4 months post-supplementation |
4. Interaction between Vitamin D and Physical Exercise
5. Discussion
Comparisons between Animal and Human Studies
6. Strengths and Limitations
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1,25D | 1,25(OH)2vitamin D |
| 25D | 25(OH)vitamin D |
| CaHMB | calcium β-hydroxy-β-methylbutyrate |
| CKD | chronic kidney disease |
| CSA | cross-sectional area |
| DBP | vitamin D binding protein |
| FC | floxed controls |
| IL6 | interleukin 6 |
| MHC | myosin-heavy chain |
| MVC | maximum voluntary contraction |
| mVDR | myocyte-specific Vitamin D Receptor knockout mice |
| PCNA | proliferating cell nuclear antigen |
| PI3K | phosphoinositide 3-kinases |
| PLIN2 | perilipin-2 |
| PP2A | protein phosphatase 2A |
| PTH | parathyroid hormone |
| RCR | rotator cuff repair |
| SERCA | sarco-endoplasmic reticulum transport ATPase |
| SMSC | skeletal muscle stem cells |
| TA | tibialis anterior |
| TUG | Time Up and Go |
| VDD | Vitamin D deficient |
| VDDR | Vitamin D deficient replacement |
| VDR | Vitamin D Receptor |
| VDRKO | VDR knockout mice |
References
- Fernández-Lázaro, D.; Garrosa, E.; Seco-Calvo, J.; Garrosa, M. Potential Satellite Cell-Linked Biomarkers in Aging Skeletal Muscle Tissue: Proteomics and Proteogenomics to Monitor Sarcopenia. Proteomes 2022, 10, 29. [Google Scholar] [CrossRef]
- Girgis, C.M.; Cha, K.M.; So, B.; Tsang, M.; Chen, J.; Houweling, P.J.; Schindeler, A.; Stokes, R.; Swarbrick, M.M.; Evesson, F.J.; et al. Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function. J. Cachexia Sarcopenia Muscle 2019, 10, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Girgis, C.M.; Cha, K.M.; Houweling, P.J.; Rao, R.; Mokbel, N.; Lin, M.; Clifton-Bligh, R.J.; Gunton, J.E. Vitamin D Receptor Ablation and Vitamin D Deficiency Result in Reduced Grip Strength, Altered Muscle Fibers, and Increased Myostatin in Mice. Calcif. Tissue Int. 2015, 97, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: Production, Metabolism and Mechanisms of Action. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.J.; Mokbel, N.; Cheng, K.; Gunton, J.E. Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology 2014, 155, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Endo, I.; Inoue, D.; Mitsui, T.; Umaki, Y.; Akaike, M.; Yoshizawa, T.; Kato, S.; Matsumoto, T. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 2003, 144, 5138–5144. [Google Scholar] [CrossRef] [PubMed]
- Burne, T.H.; McGrath, J.J.; Eyles, D.W.; Mackay-Sim, A. Behavioural characterization of vitamin D receptor knockout mice. Behav. Brain Res. 2005, 157, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Hosoyama, T.; Tomida, M.; Yamamoto, Y.; Nakamichi, Y.; Kato, S.; Kawai-Takaishi, M.; Ishizuka, S.; Nishita, Y.; Tange, C.; et al. Influence of vitamin D on sarcopenia pathophysiology: A longitudinal study in humans and basic research in knockout mice. J. Cachexia Sarcopenia Muscle 2022, 13, 2961–2973. [Google Scholar] [CrossRef]
- Bass, J.J.; Kazi, A.A.; Deane, C.S.; Nakhuda, A.; Ashcroft, S.P.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; et al. The mechanisms of skeletal muscle atrophy in response to transient knockdown of the vitamin D receptor in vivo. J. Physiol. 2021, 599, 963–979. [Google Scholar] [CrossRef]
- Braga, M.; Simmons, Z.; Norris, K.C.; Ferrini, M.G.; Artaza, J.N. Vitamin D induces myogenic differentiation in skeletal muscle derived stem cells. Endocr. Connect. 2017, 6, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Agostini, D.; Gervasi, M.; Ferrini, F.; Bartolacci, A.; Stranieri, A.; Piccoli, G.; Barbieri, E.; Sestili, P.; Patti, A.; Stocchi, V.; et al. An Integrated Approach to Skeletal Muscle Health in Aging. Nutrients 2023, 15, 1802. [Google Scholar] [CrossRef]
- Domingues-Faria, C.; Vasson, M.-P.; Goncalves-Mendes, N.; Boirie, Y.; Walrand, S. Skeletal muscle regeneration and impact of aging and nutrition. Ageing Res. Rev. 2016, 26, 22–36. [Google Scholar] [CrossRef]
- Forcina, L.; Cosentino, M.; Musarò, A. Mechanisms Regulating Muscle Regeneration: Insights into the Interrelated and Time-Dependent Phases of Tissue Healing. Cells 2020, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef]
- Collins, C.A.; Olsen, I.; Zammit, P.S.; Heslop, L.; Petrie, A.; Partridge, T.A.; Morgan, J.E. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005, 122, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Lepper, C.; Partridge, T.A.; Fan, C.-M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 2011, 138, 3639–3646. [Google Scholar] [CrossRef]
- Seale, P.; Sabourin, L.A.; Girgis-Gabardo, A.; Mansouri, A.; Gruss, P.; Rudnicki, M.A. Pax7 is required for the specification of myogenic satellite cells. Cell 2000, 102, 777–786. [Google Scholar] [CrossRef]
- Pang, K.T.; Loo, L.S.W.; Chia, S.; Ong, F.Y.T.; Yu, H.; Walsh, I. Insight into muscle stem cell regeneration and mechanobiology. Stem Cell Res. Ther. 2023, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Nakamura, S.; Sato, Y.; Kobayashi, T.; Kaneko, Y.; Ito, E.; Soma, T.; Okada, H.; Miyamoto, K.; Oya, A.; Matsumoto, M.; et al. Vitamin D protects against immobilization-induced muscle atrophy via neural crest-derived cells in mice. Sci. Rep. 2020, 10, 12242. [Google Scholar] [CrossRef]
- Cheung, W.W.; Hao, S.; Wang, Z.; Ding, W.; Zheng, R.; Gonzalez, A.; Zhan, J.-Y.; Zhou, P.; Li, S.; Esparza, M.C.; et al. Vitamin D repletion ameliorates adipose tissue browning and muscle wasting in infantile nephropathic cystinosis-associated cachexia. J. Cachexia Sarcopenia Muscle 2020, 11, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.W.; Ding, W.; Hoffman, H.M.; Wang, Z.; Hao, S.; Zheng, R.; Gonzalez, A.; Zhan, J.-Y.; Zhou, P.; Li, S.; et al. Vitamin D ameliorates adipose browning in chronic kidney disease cachexia. Sci. Rep. 2020, 10, 14175. [Google Scholar] [CrossRef] [PubMed]
- Camperi, A.; Pin, F.; Costamagna, D.; Penna, F.; Menduina, M.L.; Aversa, Z.; Zimmers, T.; Verzaro, R.; Fittipaldi, R.; Caretti, G.; et al. Vitamin D and VDR in cancer cachexia and muscle regeneration. Oncotarget 2017, 8, 21778–21793. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ren, B.; Chen, H.; Goltzman, D.; Yan, J.; Miao, D. 1,25-Dihydroxyvitamin D deficiency induces sarcopenia by inducing skeletal muscle cell senescence. Am. J. Transl. Res. 2021, 13, 12638–12649. [Google Scholar]
- Srikuea, R.; Hirunsai, M. Effects of intramuscular administration of 1α,25(OH)2D3 during skeletal muscle regeneration on regenerative capacity, muscular fibrosis, and angiogenesis. J. Appl. Physiol. 2016, 120, 1381–1393. [Google Scholar] [CrossRef]
- Domingues-Faria, C.; Chanet, A.; Salles, J.; Berry, A.; Giraudet, C.; Patrac, V.; Denis, P.; Bouton, K.; Goncalves-Mendes, N.; Vasson, M.-P.; et al. Vitamin D deficiency down-regulates Notch pathway contributing to skeletal muscle atrophy in old wistar rats. Nutr. Metab. 2014, 11, 47. [Google Scholar] [CrossRef]
- Stratos, I.; Li, Z.; Herlyn, P.; Rotter, R.; Behrendt, A.-K.; Mittlmeier, T.; Vollmar, B. Vitamin D increases cellular turnover and functionally restores the skeletal muscle after crush injury in rats. Am. J. Pathol. 2013, 182, 895–904. [Google Scholar] [CrossRef]
- Rhee, S.-M.; Park, J.H.; Jeong, H.J.; Kim, Y.K.; Lee, K.; Oh, J.H. Serum Vitamin D Level Correlations With Tissue Vitamin D Level and Muscle Performance Before and After Rotator Cuff Repair. Am. J. Sports Med. 2023, 51, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Brennan-Speranza, T.C.; Mor, D.; Mason, R.S.; Bartlett, J.R.; Duque, G.; Levinger, I.; Levinger, P. Skeletal muscle vitamin D in patients with end stage osteoarthritis of the knee. J. Steroid Biochem. Mol. Biol. 2017, 173, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Shim, B.J.; Lee, M.H.; Lim, J.Y.; Gong, H.S. A longitudinal histologic evaluation of vitamin D receptor expression in the skeletal muscles of patients with a distal radius fracture. Osteoporos. Int. 2021, 32, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Cancienne, J.M.; Brockmeier, S.F.; Kew, M.E.; Werner, B.C. Perioperative Serum 25-Hydroxyvitamin D Levels Affect Revision Surgery Rates After Arthroscopic Rotator Cuff Repair. Arthroscopy 2019, 35, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.T.; Lee, S.H.; Lee, J.K.; Chung, S.W. Influence of Vitamin D Deficiency on the Expression of Genes and Proteins in Patients With Medium Rotator Cuff Tears. Am. J. Sports Med. 2023, 51, 2650–2658. [Google Scholar] [CrossRef]
- Grønborg, I.M.; Tetens, I.; Andersen, E.W.; Kristensen, M.; Larsen, R.E.K.; Tran, T.L.L.; Andersen, R. Effect of vitamin D fortified foods on bone markers and muscle strength in women of Pakistani and Danish origin living in Denmark: A randomised controlled trial. Nutr. J. 2019, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Hosoyama, T.; Iida, H.; Kawai-Takaishi, M.; Watanabe, K. Vitamin D Inhibits Myogenic Cell Fusion and Expression of Fusogenic Genes. Nutrients 2020, 12, 2192. [Google Scholar] [CrossRef]
- Garcia, L.A.; Ferrini, M.G.; Norris, K.C.; Artaza, J.N. 1,25(OH)2vitamin D3 enhances myogenic differentiation by modulating the expression of key angiogenic growth factors and angiogenic inhibitors in C2C12 skeletal muscle cells. J. Steroid Biochem. Mol. Biol. 2013, 133, 1–11. [Google Scholar] [CrossRef]
- Mizutani, S.; Oyabu, M.; Yamamoto, A.; Uchitomi, R.; Sugimoto, T.; Kamei, Y. Vitamin D Activates Various Gene Expressions, Including Lipid Metabolism, in C2C12 Cells. J. Nutr. Sci. Vitaminol. 2022, 68, 65–72. [Google Scholar] [CrossRef]
- Schnell, D.M.; Walton, R.G.; Vekaria, H.J.; Sullivan, P.G.; Bollinger, L.M.; Peterson, C.A.; Thomas, D.T. Vitamin D produces a perilipin 2-dependent increase in mitochondrial function in C2C12 myotubes. J. Nutr. Biochem. 2019, 65, 83–92. [Google Scholar] [CrossRef]
- Srikuea, R.; Zhang, X.; Park-Sarge, O.K.; Esser, K.A. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: Potential role in suppression of myoblast proliferation. Am. J. Physiol. Cell Physiol. 2012, 303, C396–C405. [Google Scholar] [CrossRef]
- Bass, J.J.; Nakhuda, A.; Deane, C.S.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; Kadi, F.; Andersen, D.; et al. Overexpression of the vitamin D receptor (VDR) induces skeletal muscle hypertrophy. Mol. Metab. 2020, 42, 101059. [Google Scholar] [CrossRef]
- Bang, W.-S.; Lee, D.-H.; Kim, K.-T.; Cho, D.-C.; Sung, J.-K.; Han, I.-B.; Kim, D.-H.; Kwon, B.K.; Kim, C.H.; Park, K.-S.; et al. Relationships between vitamin D and paraspinal muscle: Human data and experimental rat model analysis. Spine J. 2018, 18, 1053–1061. [Google Scholar] [CrossRef]
- Owens, D.J.; Sharples, A.P.; Polydorou, I.; Alwan, N.; Donovan, T.; Tang, J.; Fraser, W.D.; Cooper, R.G.; Morton, J.P.; Stewart, C.; et al. A systems-based investigation into vitamin D and skeletal muscle repair, regeneration, and hypertrophy. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E1019–E1031. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; DeLuca, H.F. Is the Vitamin D Receptor Found in Muscle? Endocrinology 2011, 152, 354–363. [Google Scholar] [CrossRef]
- Girgis, C.M.; Mokbel, N.; Cha, K.M.; Houweling, P.J.; Abboud, M.; Fraser, D.R.; Mason, R.S.; Clifton-Bligh, R.J.; Gunton, J.E. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 2014, 155, 3227–3237. [Google Scholar] [CrossRef]
- Pojednic, R.M.; Ceglia, L.; Olsson, K.; Gustafsson, T.; Lichtenstein, A.H.; Dawson-Hughes, B.; Fielding, R.A. Effects of 1,25-dihydroxyvitamin D3 and vitamin D3 on the expression of the vitamin d receptor in human skeletal muscle cells. Calcif. Tissue Int. 2015, 96, 256–263. [Google Scholar] [CrossRef]
- Capiati, D.; Benassati, S.; Boland, R.L. 1,25(OH)2-vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. J. Cell. Biochem. 2002, 86, 128–135. [Google Scholar] [CrossRef]
- Srikuea, R.; Hirunsai, M.; Charoenphandhu, N. Regulation of vitamin D system in skeletal muscle and resident myogenic stem cell during development, maturation, and ageing. Sci. Rep. 2020, 10, 8239. [Google Scholar] [CrossRef]
- Saito, H.; Kishimoto, K.N.; Mori, Y.; Okuno, H.; Tanaka, M.; Itoi, E. A vitamin D analogue, eldecalcitol, enhances expression of fast myosin heavy chain subtypes in differentiated C2C12 myoblasts. J. Orthop. Sci. 2017, 22, 345–350. [Google Scholar] [CrossRef]
- Okuno, H.; Kishimoto, K.N.; Hatori, M.; Itoi, E. 1α,25-dihydroxyvitamin D₃ enhances fast-myosin heavy chain expression in differentiated C2C12 myoblasts. Cell Biol. Int. 2012, 36, 441–447. [Google Scholar] [CrossRef]
- Irazoqui, A.P.; Boland, R.L.; Buitrago, C.G. Actions of 1,25(OH)2-vitamin D3 on the cellular cycle depend on VDR and p38 MAPK in skeletal muscle cells. J. Mol. Endocrinol. 2014, 53, 331–343. [Google Scholar] [CrossRef]
- Garcia, L.A.; King, K.K.; Ferrini, M.G.; Norris, K.C.; Artaza, J.N. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 2011, 152, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- Salles, J.; Chanet, A.; Giraudet, C.; Patrac, V.; Pierre, P.; Jourdan, M.; Luiking, Y.C.; Verlaan, S.; Migné, C.; Boirie, Y.; et al. 1,25(OH)2-vitamin D3 enhances the stimulating effect of leucine and insulin on protein synthesis rate through Akt/PKB and mTOR mediated pathways in murine C2C12 skeletal myotubes. Mol. Nutr. Food Res. 2013, 57, 2137–2146. [Google Scholar] [CrossRef]
- Buitrago, C.G.; Arango, N.S.; Boland, R.L. 1α,25(OH)2D3-dependent modulation of Akt in proliferating and differentiating C2C12 skeletal muscle cells. J. Cell. Biochem. 2012, 113, 1170–1181. [Google Scholar] [CrossRef]
- van der Meijden, K.; Bravenboer, N.; Dirks, N.F.; Heijboer, A.C.; den Heijer, M.; de Wit, G.M.J.; Offringa, C.; Lips, P.; Jaspers, R.T. Effects of 1,25(OH)2 D3 and 25(OH)D3 on C2C12 Myoblast Proliferation, Differentiation, and Myotube Hypertrophy. J. Cell. Physiol. 2016, 231, 2517–2528. [Google Scholar] [CrossRef]
- Ashcroft, S.P.; Bass, J.J.; Kazi, A.A.; Atherton, P.J.; Philp, A. The vitamin D receptor regulates mitochondrial function in C2C12 myoblasts. Am. J. Physiol. Cell Physiol. 2020, 318, C536–C541. [Google Scholar] [CrossRef]
- Shepherd, S.O.; Cocks, M.; Tipton, K.D.; Ranasinghe, A.M.; Barker, T.A.; Burniston, J.G.; Wagenmakers, A.J.M.; Shaw, C.S. Preferential utilization of perilipin 2-associated intramuscular triglycerides during 1 h of moderate-intensity endurance-type exercise. Exp. Physiol. 2012, 97, 970–980. [Google Scholar] [CrossRef]
- Bosma, M.; Hesselink, M.K.; Sparks, L.M.; Timmers, S.; Ferraz, M.J.; Mattijssen, F.; van Beurden, D.; Schaart, G.; de Baets, M.H.; Verheyen, F.K.; et al. Perilipin 2 improves insulin sensitivity in skeletal muscle despite elevated intramuscular lipid levels. Diabetes 2012, 61, 2679–2690. [Google Scholar] [CrossRef]
- Olsson, K.; Saini, A.; Strömberg, A.; Alam, S.; Lilja, M.; Rullman, E.; Gustafsson, T. Evidence for Vitamin D Receptor Expression and Direct Effects of 1α,25(OH)2D3 in Human Skeletal Muscle Precursor Cells. Endocrinology 2016, 157, 98–111. [Google Scholar] [CrossRef]
- Ryan, Z.C.; Craig, T.A.; Folmes, C.D.; Wang, X.; Lanza, I.R.; Schaible, N.S.; Salisbury, J.L.; Nair, K.S.; Terzic, A.; Sieck, G.C.; et al. 1α,25-Dihydroxyvitamin D3 Regulates Mitochondrial Oxygen Consumption and Dynamics in Human Skeletal Muscle Cells. J. Biol. Chem. 2016, 291, 1514–1528. [Google Scholar] [CrossRef]
- Romeu Montenegro, K.; Carlessi, R.; Cruzat, V.; Newsholme, P. Effects of vitamin D on primary human skeletal muscle cell proliferation, differentiation, protein synthesis and bioenergetics. J. Steroid Biochem. Mol. Biol. 2019, 193, 105423. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, N.; Fukumura, J.; Yasuno, H.; Fujimoto-Ouchi, K.; Kitamura, H. 1α,25(OH)2D3 downregulates gene expression levels of muscle ubiquitin ligases MAFbx and MuRF1 in human myotubes. Biomed. Res. 2015, 36, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Panda, D.K.; Miao, D.; Tremblay, M.L.; Sirois, J.; Farookhi, R.; Hendy, G.N.; Goltzman, D. Targeted ablation of the 25-hydroxyvitamin D 1α-hydroxylase enzyme: Evidence for skeletal, reproductive, and immune dysfunction. Proc. Natl. Acad. Sci. USA 2001, 98, 7498–7503. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Kalam, R.; Qadri, S.S.; Madabushi, S.; Ismail, A. Vitamin D deficiency-induced muscle wasting occurs through the ubiquitin proteasome pathway and is partially corrected by calcium in male rats. Endocrinology 2013, 154, 4018–4029. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.; Henriksen, V.T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Schneider, E.D.; Dixon, B.M.; Weaver, L.K. Higher serum 25-hydroxyvitamin D concentrations associate with a faster recovery of skeletal muscle strength after muscular injury. Nutrients 2013, 5, 1253–1275. [Google Scholar] [CrossRef]
- Barker, T.; Schneider, E.D.; Dixon, B.M.; Henriksen, V.T.; Weaver, L.K. Supplemental vitamin D enhances the recovery in peak isometric force shortly after intense exercise. Nutr. Metab. 2013, 10, 69. [Google Scholar] [CrossRef]
- Ryu, K.J.; Kim, B.H.; Lee, Y.; Dan, J.; Kim, J.H. Low Serum Vitamin D Is Not Correlated With the Severity of a Rotator Cuff Tear or Retear After Arthroscopic Repair. Am. J. Sports Med. 2015, 43, 1743–1750. [Google Scholar] [CrossRef]
- Ekinci, O.; Yanık, S.; Terzioğlu Bebitoğlu, B.; Yılmaz Akyüz, E.; Dokuyucu, A.; Erdem, Ş. Effect of Calcium β-Hydroxy-β-Methylbutyrate (CaHMB), Vitamin D, and Protein Supplementation on Postoperative Immobilization in Malnourished Older Adult Patients With Hip Fracture: A Randomized Controlled Study. Nutr. Clin. Pract. 2016, 31, 829–835. [Google Scholar] [CrossRef]
- Gordon, P.L.; Sakkas, G.K.; Doyle, J.W.; Shubert, T.; Johansen, K.L. Relationship between vitamin D and muscle size and strength in patients on hemodialysis. J. Ren. Nutr. 2007, 17, 397–407. [Google Scholar] [CrossRef]
- Conzade, R.; Grill, E.; Bischoff-Ferrari, H.A.; Ferrari, U.; Horsch, A.; Koenig, W.; Peters, A.; Thorand, B. Vitamin D in Relation to Incident Sarcopenia and Changes in Muscle Parameters Among Older Adults: The KORA-Age Study. Calcif. Tissue Int. 2019, 105, 173–182. [Google Scholar] [CrossRef]
- Dzik, K.; Skrobot, W.; Flis, D.J.; Karnia, M.; Libionka, W.; Kloc, W.; Kaczor, J.J. Vitamin D supplementation attenuates oxidative stress in paraspinal skeletal muscles in patients with low back pain. Eur. J. Appl. Physiol. 2018, 118, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Dzik, K.P.; Skrobot, W.; Kaczor, K.B.; Flis, D.J.; Karnia, M.J.; Libionka, W.; Antosiewicz, J.; Kloc, W.; Kaczor, J.J. Vitamin D Deficiency Is Associated with Muscle Atrophy and Reduced Mitochondrial Function in Patients with Chronic Low Back Pain. Oxid. Med. Cell. Longev. 2019, 2019, 6835341. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Blizzard, L.; Fell, J.; Ding, C.; Winzenberg, T.; Jones, G. A prospective study of the associations between 25-hydroxy-vitamin D, sarcopenia progression and physical activity in older adults. Clin. Endocrinol. 2010, 73, 581–587. [Google Scholar] [CrossRef]
- Gerdhem, P.; Ringsberg, K.A.M.; Obrant, K.J.; Akesson, K. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos. Int. 2005, 16, 1425–1431. [Google Scholar] [CrossRef]
- Ceglia, L.; Niramitmahapanya, S.; da Silva Morais, M.; Rivas, D.A.; Harris, S.S.; Bischoff-Ferrari, H.; Fielding, R.A.; Dawson-Hughes, B. A randomized study on the effect of vitamin D₃ supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J. Clin. Endocrinol. Metab. 2013, 98, E1927–E1935. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, M.M.; Gołaś, A.; Maszczyk, A.; Kaczka, P.; Zając, A. Influence of Sunlight and Oral D3 Supplementation on Serum 25(OH)D Concentration and Exercise Performance in Elite Soccer Players. Nutrients 2020, 12, 1311. [Google Scholar] [CrossRef]
- Bunout, D.; Barrera, G.; Leiva, L.; Gattas, V.; de la Maza, M.P.; Avendaño, M.; Hirsch, S. Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Exp. Gerontol. 2006, 41, 746–752. [Google Scholar] [CrossRef]
- Jastrzębska, M.; Kaczmarczyk, M.; Jastrzębski, Z. Effect of Vitamin D Supplementation on Training Adaptation in Well-Trained Soccer Players. J. Strength Cond. Res. 2016, 30, 2648–2655. [Google Scholar] [CrossRef]
- Bezuglov, E.; Tikhonova, A.; Zueva, A.; Khaitin, V.; Waśkiewicz, Z.; Gerasimuk, D.; Żebrowska, A.; Rosemann, T.; Nikolaidis, P.; Knechtle, B. Prevalence and Treatment of Vitamin D Deficiency in Young Male Russian Soccer Players in Winter. Nutrients 2019, 11, 2405. [Google Scholar] [CrossRef]
- Houston, D.K.; Marsh, A.P.; Neiberg, R.H.; Demons, J.L.; Campos, C.L.; Kritchevsky, S.B.; Delbono, O.; Tooze, J.A. Vitamin D Supplementation and Muscle Power, Strength and Physical Performance in Older Adults: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2023, 117, 1086–1095. [Google Scholar] [CrossRef]
- Dzik, K.P.; Grzywacz, T.; Łuszczyk, M.; Kujach, S.; Flis, D.J.; Kaczor, J.J. Single bout of exercise triggers the increase of vitamin D blood concentration in adolescent trained boys: A pilot study. Sci. Rep. 2022, 12, 1825. [Google Scholar] [CrossRef] [PubMed]
- Alfaqih, M.S.; Tarawan, V.M.; Sylviana, N.; Goenawan, H.; Lesmana, R.; Susianti, S. Effects of Vitamin D on Satellite Cells: A Systematic Review of In Vivo Studies. Nutrients 2022, 14, 4558. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Declan Fleming, R.Y.; Wolfe, R.R. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J. Clin. Investig. 1995, 95, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Haegens, A.; Schols, A.M.; van Essen, A.L.; van Loon, L.J.; Langen, R.C. Leucine induces myofibrillar protein accretion in cultured skeletal muscle through mTOR dependent and -independent control of myosin heavy chain mRNA levels. Mol. Nutr. Food Res. 2012, 56, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Latham, C.M.; Brightwell, C.R.; Keeble, A.R.; Munson, B.D.; Thomas, N.T.; Zagzoog, A.M.; Fry, C.S.; Fry, J.L. Vitamin D Promotes Skeletal Muscle Regeneration and Mitochondrial Health. Front. Physiol. 2021, 12, 660498. [Google Scholar] [CrossRef]
- Murphy, M.M.; Lawson, J.A.; Mathew, S.J.; Hutcheson, D.A.; Kardon, G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 2011, 138, 3625–3637. [Google Scholar] [CrossRef] [PubMed]
- Haß, U.; Kochlik, B.; Herpich, C.; Rudloff, S.; Norman, K. Effects of an Omega-3 Supplemented, High-Protein Diet in Combination with Vibration and Resistance Exercise on Muscle Power and Inflammation in Old Adults: A Pilot Randomized Controlled Trial. Nutrients 2022, 14, 4274. [Google Scholar] [CrossRef] [PubMed]
- Jannas-Vela, S.; Espinosa, A.; Candia, A.A.; Flores-Opazo, M.; Peñailillo, L.; Valenzuela, R. The Role of Omega-3 Polyunsaturated Fatty Acids and Their Lipid Mediators on Skeletal Muscle Regeneration: A Narrative Review. Nutrients 2023, 15, 871. [Google Scholar] [CrossRef]
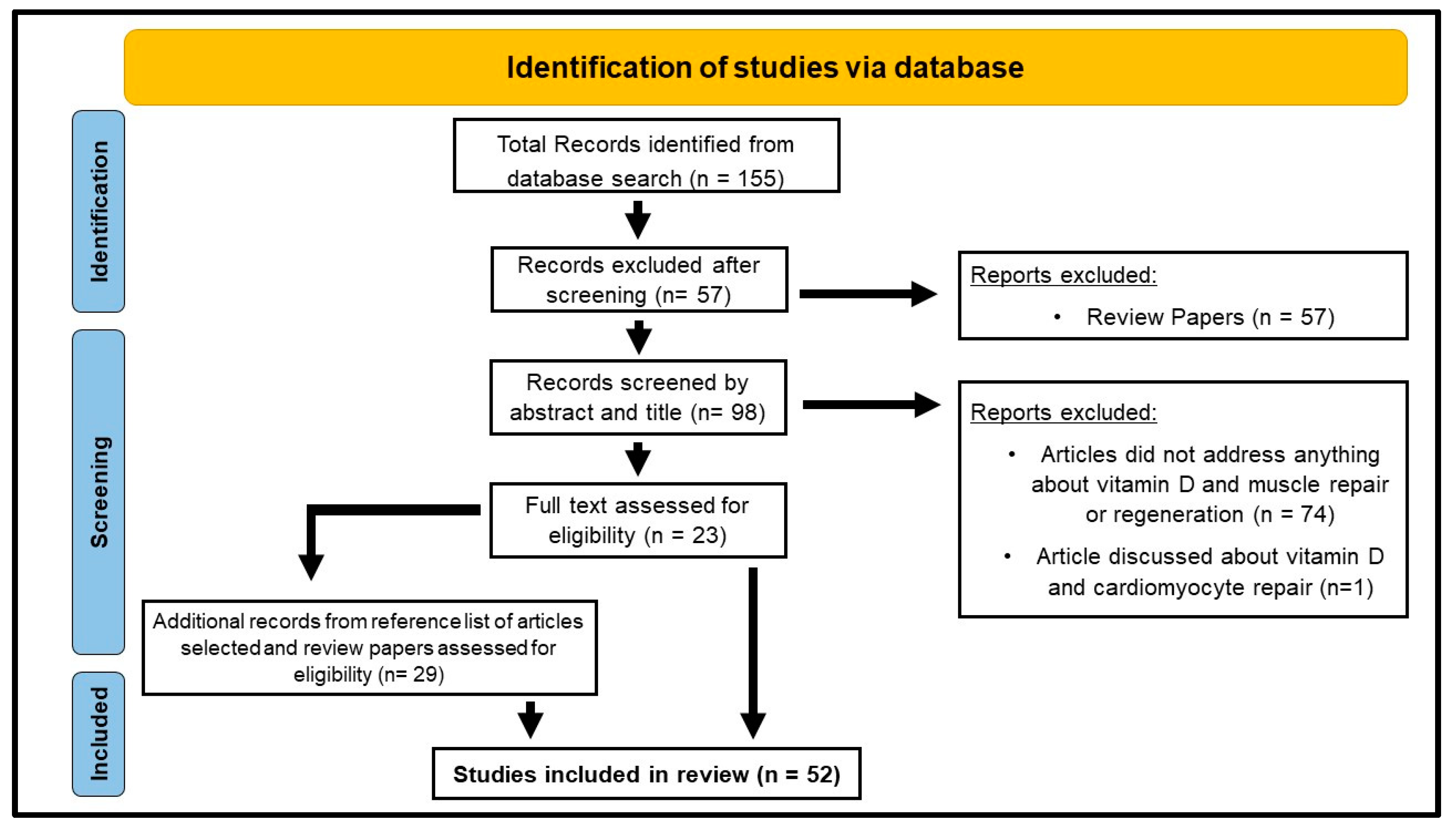
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agoncillo, M.; Yu, J.; Gunton, J.E. The Role of Vitamin D in Skeletal Muscle Repair and Regeneration in Animal Models and Humans: A Systematic Review. Nutrients 2023, 15, 4377. https://doi.org/10.3390/nu15204377
Agoncillo M, Yu J, Gunton JE. The Role of Vitamin D in Skeletal Muscle Repair and Regeneration in Animal Models and Humans: A Systematic Review. Nutrients. 2023; 15(20):4377. https://doi.org/10.3390/nu15204377
Chicago/Turabian StyleAgoncillo, Miguel, Josephine Yu, and Jenny E. Gunton. 2023. "The Role of Vitamin D in Skeletal Muscle Repair and Regeneration in Animal Models and Humans: A Systematic Review" Nutrients 15, no. 20: 4377. https://doi.org/10.3390/nu15204377
APA StyleAgoncillo, M., Yu, J., & Gunton, J. E. (2023). The Role of Vitamin D in Skeletal Muscle Repair and Regeneration in Animal Models and Humans: A Systematic Review. Nutrients, 15(20), 4377. https://doi.org/10.3390/nu15204377







