Practical Application and Methodological Considerations on the Basics of Sports Nutrition in Basketball: A Comprehensive Systematic Review of Observational and Interventional Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Eligibility
2.2. Search Strategy
2.3. Data Extraction and Synthesis
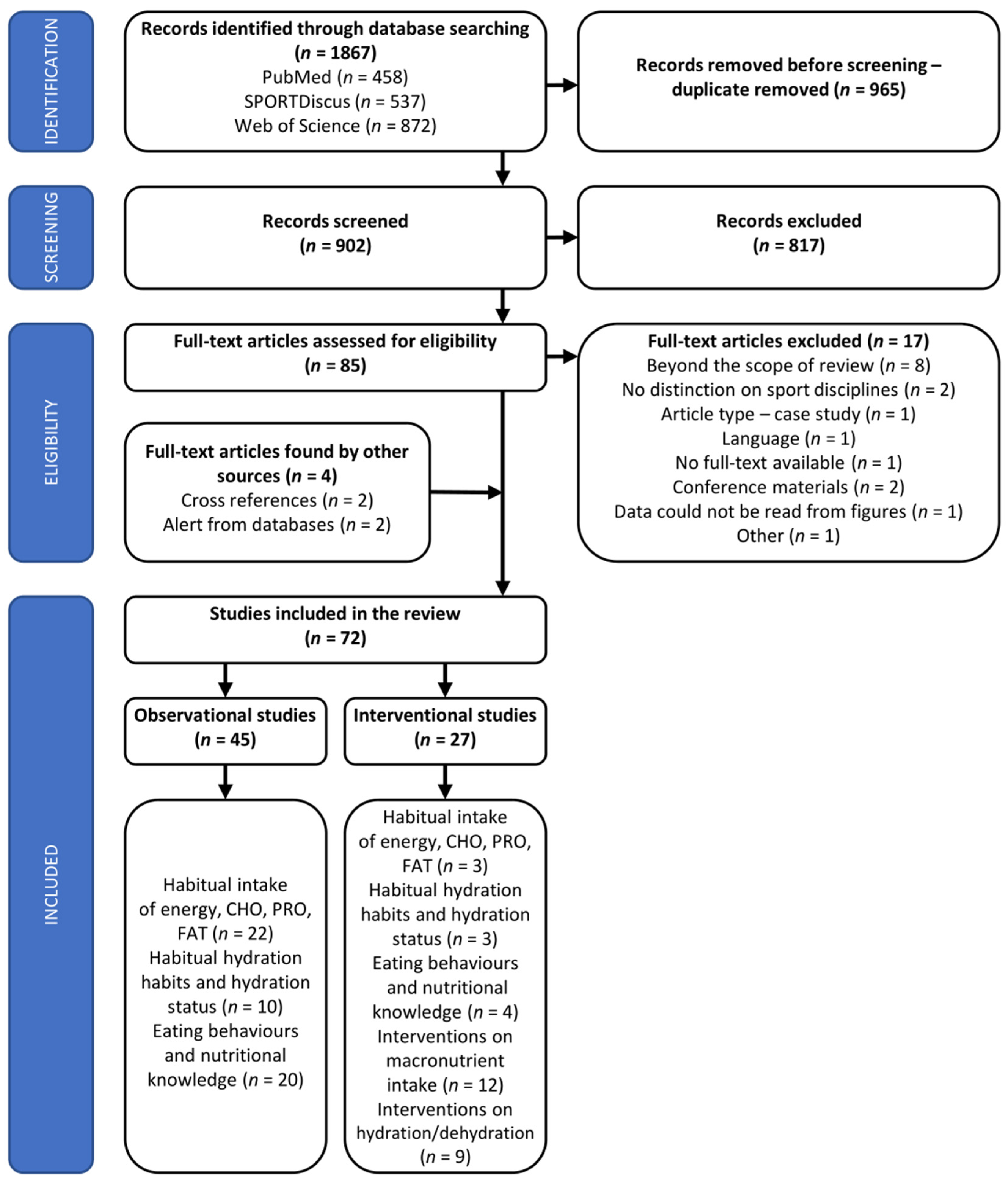
2.4. Assessment of Studies’ Quality
3. Results
3.1. Study Quality and Risk of Bias
3.2. Energy and Macronutrients Intake
3.3. Hydration Practices and Hydration Status
3.4. Dietary Interventions on Dehydration and Hydration Strategies
3.5. Dietary Interventions on Macronutrients’ Manipulations
3.6. Eating Behaviours and Nutritional Knowledge
3.6.1. Frequency and Timing of Meals Consumption, and Breakfast Consumption Habits
3.6.2. Food Groups’ Contribution to Daily Food Rations and Composition of Pre- and Post-Exercise Meals
3.6.3. Alcohol Use and Smoking Habits
3.6.4. Disordered Eating Behaviours
3.6.5. Nutritional Knowledge and Dietary Counseling Interventions
4. Discussion
4.1. Energy Intake and Energy Balance
4.2. Habitual Macronutrients Intake
4.3. Hydration—Habits, Practices, and Implications with Basketball Performance
4.4. Macronutrients’ Alternations and Manipulations
4.5. Studies on Para-Athlete Basketball Players
4.6. Strengths and Limitations
5. Conclusions
- (1)
- Each basketball player should periodically undergo professional evaluation of exercise- and total energy expenditures and physical activity levels during different periods of the athletic season, with the use of standard, valid, and accurate methods and equipment and performed by trained and experienced personnel.
- (2)
- Special emphasis should be paid to the proper periodization of energy and macronutrients’ intake according to training macro- and microcycles, training/non-training (match/non-match) days, as well as timing of meal consumption according to pre- and post-exercise schedule. Energy and macronutrients must be adjusted to actual and individual athletic requirements. Proper provision of CHO is of particular importance. However, the results of the current systematic review do not allow for framing basketball-specific recommendations on CHO intake; thus, the athletes should follow the most up-to-date recommendations for the general athletic population.
- (3)
- Basketball players at each age, level of training experience, or degree of full-body abilities must be provided with nutritional education courses. Taking into account numerous adverse lifestyle and nutritional behaviours in junior basketball players, these activities need to be undertaken from the very beginning of their sports career to prevent transferring adverse nutritional practices to later years of life and sports practice, as well as for developing proper diet, health, and lifestyle behaviours that ensure optimal growth and physiological and physical development.
- (4)
- Based on the included studies, modifying and improving eating habits in basketball players seem to be a longer term process. Thus, nutritional education courses should be planned as longer-lasting programs and should comprise group and individual meetings. Finally, periodical monitoring of their effectiveness should also be introduced.
- (5)
- The scope of nutritional education should be individually tailored to specific and pre-identified needs of particular groups of basketball players. Nevertheless, based on the results of current systematic review, special attention should be paid to (a) nutritional characteristics of particular food groups and proper frequency of their consumption and distribution between meals, including pre- and post-exercises eating occasions; (b) making athletes aware of the importance and necessity of proper periodization (at a micro and macro scale) and timing in energy and macronutrients’ intake; (c) providing them with basic abilities to estimate energy and macronutrients’ intake with particular foods; (d) proper hydration practices, including self-evaluation of an adequate pre-exercise hydration status and exercise-induced fluid losses based on simple indices e.g., urine colour or body mass change, respectively; fluid replenishment strategies during trainings/competitions; and post-exercise rehydration protocols; (e) the importance of proper nutritional and hydration practices on the days of heavy trainings/competition, especially due to the fact that they are commonly neglected by the athletes particularly at such occasions.
- (1)
- Sport-specific tools for the evaluation of diet, eating habits, or nutritional knowledge must be developed, validated, and widely introduced in the research practice.
- (2)
- Any dietary intervention or supplementation protocol in basketball players needs to originate from a well-thought-out and clearly pre-specified research hypotheses, and the hypotheses must be supported by the underlying presumable—but probable—physiological mechanisms. No random protocols can be implemented.
- (3)
- To frame any basketball-specific dietary recommendations, there is apparently the necessity for conducting interventional studies on alternations/supplementation with carbohydrate, protein, and fat, while considering acute, short-, and/or long-term protocols, as well as aspects related to diet periodization and consumption timing.
- (4)
- With respect to hydration strategies, special emphasis must be paid to post-exercise rehydration protocols, while none of the up-to-date studies in basketball players have investigated this aspect. Concurrently, the concern related to the most effective fluids in replenishing fluids losses during exercise in basketball players is still to be resolved. The factors determining the applicability of carbohydrate-electrolyte solutions concerning actual discipline-specific performance need to be disclosed and described in various groups of basketball players.
- (5)
- Protocols of interventional studies, including a plan of statistical analysis, must be prospectively registered in the relevant databases. The presentation of study results need to cover all pre-specified outcomes and all studied subgroups/treatments.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ziv, G.; Lidor, R. Physical Attributes, Physiological Characteristics, On-Court Performances and Nutritional Strategies of Female and Male Basketball Players. Sports Med. 2009, 39, 547–568. [Google Scholar] [CrossRef]
- Stojanović, E.; Stojiljković, N.; Scanlan, A.T.; Dalbo, V.J.; Berkelmans, D.M.; Milanović, Z. The Activity Demands and Physiological Responses Encountered during Basketball Match-Play: A Systematic Review. Sports Med. 2018, 48, 111–135. [Google Scholar] [CrossRef]
- Calleja-González, J.; Terrados, N.; Mielgo-Ayuso, J.; Delextrat, A.; Jukic, I.; Vaquera, A.; Torres, L.; Schelling, X.; Stojanovic, M.; Ostojic, S.M. Evidence-Based Post-Exercise Recovery Strategies in Basketball. Phys. Sportsmed. 2016, 44, 74–78. [Google Scholar] [CrossRef]
- Aksović, N.; Milanović, F.; Bjelica, B.; Nikolić, D.; Jovanović, N.; D’Onofrio, R. Analysis and Overview Wheelchair Basketball. Ital. J. Sports Rehabil. Posturology 2021, 10, 2335–2348. [Google Scholar]
- McInnes, S.E.; Carlson, J.S.; Jones, C.J.; McKenna, M.J. The Physiological Load Imposed on Basketball Players during Competition. J. Sports Sci. 1995, 13, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Maughan, R.J.; Gleeson, M.; Bilsborough, J.; Jeukendrup, A.; Morton, J.P.; Phillips, S.M.; Armstrong, L.; Burke, L.M.; Close, G.L.; et al. UEFA Expert Group Statement on Nutrition in Elite Football. Current Evidence to Inform Practical Recommendations and Guide Future Research. Br. J. Sports Med. 2021, 55, 416. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Ferreira, D.; Caetano, C.; Granja, D.; Pinto, R.; Mendes, B.; Sousa, M. Nutrition and Supplementation in Soccer. Sports 2017, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Danielik, K.; Książek, A.; Zagrodna, A.; Słowińska-Lisowska, M. How do Male Football Players Meet Dietary Recommendations? A Systematic Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 9561. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, H.; Karczemna, A.; Włodarek, D. Nutrition for Female Soccer Players—Recommendations. Medicina 2020, 56, 28. [Google Scholar] [CrossRef]
- Carruthers, J. Nutritional Practices, Interventions and Recommendations for Junior Rugby League Players. Sports Nutr. Ther. 2016, 1, 110. [Google Scholar] [CrossRef]
- Davis, J.K.; Oikawa, S.Y.; Halson, S.; Stephens, J.; O’Riordan, S.; Luhrs, K.; Sopena, B.; Baker, L.B. In-Season Nutrition Strategies and Recovery Modalities to Enhance Recovery for Basketball Players: A Narrative Review. Sports Med. 2022, 52, 971–993. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Ott, I.; Calleja-González, J.; Mielgo-Ayuso, J. Ergo-Nutritional Intervention in Basketball: A Systematic Review. Nutrients 2022, 14, 638. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.; Jordan, Z.; McArthur, A. Developing the Review Question and Inclusion Criteria. AJN 2014, 114, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Baranauskas, M.; Tubelis, L.; Stukas, R.; Švedas, E.; Samsonienė, L.; Karanauskienė, D. Some Aspects of Nutrition and Moderate Body Weight Reduction in Lithuanian Olympic Sport Centre Female Basketball Players. Balt. J. Sport Health Sci. 2011, 2, 3–10. [Google Scholar] [CrossRef]
- Baranauskas, M.; Tubelis, L.; Stukas, R.; Švedas, E.; Samsonienė, L.; Karanauskienė, D. Lithuanian Olympic Basketball Players’ Nutrition during the Training Mezzo-Cycles Designed for Strength Training. Balt. J. Sport Health Sci. 2013, 3, 3–10. [Google Scholar] [CrossRef]
- Baranauskas, M.; Jablonskienė, V.; Abaravičius, J.A.; Stukas, R. Cardiorespiratory Fitness and Diet Quality Profile of the Lithuanian Team of Deaf Women’s Basketball Players. Int. J. Environ. Res. Public Health 2020, 17, 6749. [Google Scholar] [CrossRef]
- Dzimbova, T. Nutritional Paterns of Children Involved in Four Different Sports: Ski, Gymnastics, Football, and Basketball. PESH 2020, 9, 189–194. [Google Scholar] [CrossRef]
- Eskici, G.; Ersoy, G. An Evaluation of Wheelchair Basketball Players’ Nutritional Status and Nutritional Knowledge Levels. J. Sports Med. Phys. Fitness 2016, 56, 259–268. [Google Scholar]
- Ferro, A.; Garrido, G.; Villacieros, J.; Pérez, J.; Grams, L. Nutritional Habits and Performance in Male Elite Wheelchair Basketball Players During a Precompetitive Period. Adapt. Phys. Activ. Q. 2017, 34, 295–310. [Google Scholar] [CrossRef]
- Gacek, M. Sense of Self-Efficacy and the Content of Energy and Nutrients in the Diet of Elite Polish Basketball Players. Rocz. Panstw. Zakł. Hig. 2022, 73, 183–189. [Google Scholar]
- Grams, L.; Garrido, G.; Villacieros, J.; Ferro, A. Marginal Micronutrient Intake in High-Performance Male Wheelchair Basketball Players: A Dietary Evaluation and the Effects of Nutritional Advice. PLoS ONE 2016, 11, e0157931. [Google Scholar] [CrossRef]
- Hickson, J.F.; Schrader, J.; Trischler, L.C. Dietary Intakes of Female Basketball and Gymnastics Athletes. J. Am. Diet. Assoc. 1986, 86, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Hickson, J.F.; Coleman, A.E.; Wolinsky, I.; Buck, B. Preseason Nutritional Profile of High School Basketball Athletes. J. App. Sport Sci. Res. 1990, 4, 131–134. [Google Scholar]
- Kostopoulos, N.; Apostoldis, N.; Mexis, D.; Mikellidi, A.; Nomikos, T. Dietary Intake and the Markers of Muscle Damage in Elite Basketball Players after a Basketball Match. J. Phys. Educ. Sport 2017, 17, 394–401. [Google Scholar]
- Leinus, K.; Ööpik, V. Habitual Nutrient Intake and Energy Expenditure of Students Participating in Recreational Sports. Nutr. Res. 1998, 18, 683–691. [Google Scholar] [CrossRef]
- Nepocatych, S.; Balilionis, G. Analysis of Dietary Intake and Body Composition of Female Athletes over a Competitive Season. Monten. J. Sports Sci. Med. 2017, 6, 57–65. [Google Scholar] [CrossRef]
- Nikić, M.; Pedišić, Ž.; Šatalić, Z.; Jakovljević, S.; Venus, D. Adequacy of Nutrient Intakes in Elite Junior Basketball Players. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 516–523. [Google Scholar] [CrossRef]
- Nowak, R.K.; Knudsen, K.S.; Schulz, L.O. Body Composition and Nutrient Intakes of College Men and Women Basketball Players. J. Am. Diet. Assoc. 1988, 88, 575–578. [Google Scholar] [CrossRef]
- Papandreou, D.; Eystathiadis, P.; Bouzoukiu, V.; Hassapidou, M.; Tsitskaris, G.; Garefis, A. Dietary Assessment, Anthropometric Measurements and Nutritional Status of Greek Professional Athletes. Nutr. Food Sci. 2007, 37, 338–344. [Google Scholar] [CrossRef]
- Quintas, M.E.; Ortega, R.M.; López-Sobaler, A.M.; Garrido, G.; Requejo, A.M. Influence of Dietetic and Anthropometric Factors and of the Type of Sport Practised on Bone Density in Different Groups of Women. Eur. J. Clin. Nutr. 2003, 57, S58–S62. [Google Scholar] [CrossRef]
- Schröder, H.; Navarro, E.; Mora, J.; Seco, J.; Torregrosa, J.M.; Tramullas, A. Dietary Habits and Fluid Intake of a Group of Elite Spanish Basketball Players: A Need for Professional Advice? Eur. J. Sport Sci. 2004, 4, 1–15. [Google Scholar] [CrossRef]
- Shimizu, Y.; Mutsuzaki, H.; Tachibana, K.; Hotta, K.; Wadano, Y. Investigation of the Female Athlete Triad in Japanese Elite Wheelchair Basketball Players. Medicina 2019, 56, 10. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Santos, D.A.; Matias, C.N.; Rocha, P.M.; Petroski, E.L.; Minderico, C.S.; Sardinha, L.B. Changes in Regional Body Composition Explain Increases in Energy Expenditure in Elite Junior Basketball Players over the Season. Eur. J. Appl. Physiol. 2012, 112, 2727–2737. [Google Scholar] [CrossRef]
- Silva, A.M.; Santos, D.A.; Matias, C.N.; Minderico, C.S.; Schoeller, D.A.; Sardinha, L.B. Total Energy Expenditure Assessment in Elite Junior Basketball Players: A Validation Study Using Doubly Labeled Water. J. Strength Cond. Res. 2013, 27, 1920–1927. [Google Scholar] [CrossRef]
- Silva, A.M.; Matias, C.N.; Santos, D.A.; Thomas, D.; Bosy-Westphal, A.; MüLLER, M.J.; Heymsfield, S.B.; Sardinha, L.B. Compensatory Changes in Energy Balance Regulation over One Athletic Season. Med. Sci. Sports Exerc. 2017, 49, 1229–1235. [Google Scholar] [CrossRef]
- Toti, E.; Raguzzini, A.; Fedullo, A.L.; Cavedon, V.; Milanese, C.; Bernardi, M.; Mariani, B.M.; Massaro, L.; Mellara, F.; Sciarra, T.; et al. Longitudinal Effects of Dietary Advice on Wheelchair Basketball Athletes: Nutritional and Environmental Aspects. Sustainability 2021, 13, 5244. [Google Scholar] [CrossRef]
- Toti, E.; Cavedon, V.; Raguzzini, A.; Fedullo, A.L.; Milanese, C.; Bernardi, E.; Bellito, S.; Bernardi, M.; Sciarra, T.; Peluso, I. Dietary Intakes and Food Habits of Wheelchair Basketball Athletes Compared to Gym Attendees and Individuals Who Do Not Practice Sport Activity. Endocr. Metab. Immune Disord. Drug Targets 2021, 22, 38–48. [Google Scholar] [CrossRef]
- Zanders, B.R.; Currier, B.S.; Harty, P.S.; Zabriskie, H.A.; Smith, C.R.; Stecker, R.A.; Richmond, S.R.; Jagim, A.R.; Kerksick, C.M. Changes in Energy Expenditure, Dietary Intake, and Energy Availability Across an Entire Collegiate Women’s Basketball Season. J. Strength Cond. Res. 2021, 35, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, I.S.; Lopez, R.M.; Kuo, Y.-T.; Shapiro, B.S. Efficacy of an Educational Intervention for Improving the Hydration Status of Female Collegiate Indoor-Sport Athletes. J. Ath. Train. 2021, 56, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Arnaoutis, G.; Kavouras, S.A.; Angelopoulou, A.; Skoulariki, C.; Bismpikou, S.; Mourtakos, S.; Sidossis, L.S. Fluid Balance During Training in Elite Young Athletes of Different Sports. J. Strength Cond. Res. 2015, 29, 3447–3452. [Google Scholar] [CrossRef]
- Baker, L.B.; Dougherty, K.A.; Chow, M.; Kenney, W.L. Progressive Dehydration Causes a Progressive Decline in Basketball Skill Performance. Med. Sci. Sports Exerc. 2007, 39, 1114–1123. [Google Scholar] [CrossRef]
- Barnes, K.A.; Anderson, M.L.; Stofan, J.R.; Dalrymple, K.J.; Reimel, A.J.; Roberts, T.J.; Randell, R.K.; Ungaro, C.T.; Baker, L.B. Normative Data for Sweating Rate, Sweat Sodium Concentration, and Sweat Sodium Loss in Athletes: An Update and Analysis by Sport. J. Sports Sci. 2019, 37, 2356–2366. [Google Scholar] [CrossRef]
- Brandenburg, J.P.; Gaetz, M. Fluid Balance of Elite Female Basketball Players Before and During Game Play. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 347–352. [Google Scholar] [CrossRef]
- Broad, E.M.; Burke, L.M.; Cox, G.R.; Heeley, P.; Riley, M. Body Weight Changes and Voluntary Fluid Intakes during Training and Competition Sessions in Team Sports. Int. J. Sport Nutr. 1996, 6, 307–320. [Google Scholar] [CrossRef]
- Heishman, A.D.; Daub, B.D.; Miller, R.M.; Freitas, E.D.S.; Bemben, M.G. Longitudinal Hydration Assessment in Collegiate Basketball Players Over Various Training Phases. J. Strength Cond. Res. 2021, 35, 1089–1094. [Google Scholar] [CrossRef]
- Logan-Sprenger, H.M.; McNaughton, L.R. Characterizing Thermoregulatory Demands of Female Wheelchair Basketball Players during Competition. Res. Sports Med. 2020, 28, 256–267. [Google Scholar] [CrossRef]
- Osterberg, K.L.; Horswill, C.A.; Baker, L.B. Pregame Urine Specific Gravity and Fluid Intake by National Basketball Association Players During Competition. J. Athl. Train. 2009, 44, 53–57. [Google Scholar] [CrossRef]
- Taim, B.C.; Suppiah, H.T.; Wee, J.; Lee, M.; Lee, J.K.W.; Chia, M. Palatable Flavoured Fluids without Carbohydrates and Electrolytes Do Not Enhance Voluntary Fluid Consumption in Male Collegiate Basketball Players in the Heat. Nutrients 2021, 13, 4197. [Google Scholar] [CrossRef]
- Thigpen, L.K.; Green, J.M.; O’Neal, E.K. Hydration Profile and Sweat Loss Perception of Male and Female Division II Basketball Players During Practice. J. Strength Cond. Res. 2014, 28, 3425–3431. [Google Scholar] [CrossRef]
- Vukasinović-Vesić, M.; Andjelković, M.; Stojmenović, T.; Dikić, N.; Kostić, M.; Curcić, D. Sweat Rate and Fluid Intake in Young Elite Basketball Players on the FIBA Europe U20 Championship. Vojnosanit. Pregl. 2015, 72, 1063–1068. [Google Scholar] [CrossRef]
- Baker, L.B.; Conroy, D.E.; Kenney, W.L. Dehydration Impairs Vigilance-Related Attention in Male Basketball Players. Med. Sci. Sports Exerc. 2007, 39, 976–983. [Google Scholar] [CrossRef]
- Carvalho, P.; Oliveira, B.; Barros, R.; Padrão, P.; Moreira, P.; Teixeira, V.H. Impact of Fluid Restriction and Ad Libitum Water Intake or an 8% Carbohydrate-Electrolyte Beverage on Skill Performance of Elite Adolescent Basketball Players. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 214–221. [Google Scholar] [CrossRef]
- Dougherty, K.A.; Baker, L.B.; Chow, M.; Kenney, W.L. Two Percent Dehydration Impairs and Six Percent Carbohydrate Drink Improves Boys Basketball Skills. Med. Sci. Sports Exerc. 2006, 38, 1650–1658. [Google Scholar] [CrossRef]
- Hoffman, J.; Stavsky, H.; Folk, B. The Effect of Water Restriction on Anaerobic Power and Vertical Jumping Height in Basketball Players. Int. J. Sports Med. 1995, 16, 214–218. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Williams, D.R.; Emerson, N.S.; Hoffman, M.W.; Wells, A.J.; McVeigh, D.M.; McCormack, W.P.; Mangine, G.T.; Gonzalez, A.M.; Fragala, M.S. L-Alanyl-L-Glutamine Ingestion Maintains Performance during a Competitive Basketball Game. J. Int. Soc. Sports Nutr. 2012, 9, 4. [Google Scholar] [CrossRef]
- Louis, J.; Dinu, D.; Leguy, E.; Jacquet, M.; Slawinski, J.; Tiollier, E. Effect of Dehydration on Performance and Technique of Three-Point Shooting in Elite Basketball. J. Sports Med. Phys. Fitness 2018, 58, 1710–1711. [Google Scholar] [CrossRef]
- Minehan, M.R.; Riley, M.D.; Burke, L.M. Effect of Flavor and Awareness of Kilojoule Content of Drinks on Preference and Fluid Balance in Team Sports. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 81–92. [Google Scholar] [CrossRef]
- Afman, G.; Garside, R.M.; Dinan, N.; Gant, N.; Betts, J.A.; Williams, C. Effect of Carbohydrate or Sodium Bicarbonate Ingestion on Performance During a Validated Basketball Simulation Test. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 632–644. [Google Scholar] [CrossRef]
- Daniel, N.V.S.; Zimberg, I.Z.; Estadella, D.; Garcia, M.C.; Padovani, R.C.; Juzwiak, C.R. Effect of the Intake of High or Low Glycemic Index High Carbohydrate-Meals on Athletes’ Sleep Quality in Pre-Game Nights. An. Acad. Bras. Ciênc. 2019, 91, e20180107. [Google Scholar] [CrossRef]
- Gentle, H.L.; Love, T.D.; Howe, A.S.; Black, K.E. A Randomised Trial of Pre-Exercise Meal Composition on Performance and Muscle Damage in Well-Trained Basketball Players. J. Int. Soc. Sports Nutr. 2014, 11, 33. [Google Scholar] [CrossRef]
- Ghiasvand, R.; Djalali, M.; Djazayery, S.; Keshavarz, S.; Hosseini, M.; Askari, G.; Jani, N.; Fardad, N.; Fatehi, F. Effect of Eicosapentaenoic Acid (EPA) and Vitamin e on the Blood Levels of Inflammatory Markers, Antioxidant Enzymes, and Lipid Peroxidation in Iranian Basketball Players. Iran. J. Public Health 2010, 39, 15–21. [Google Scholar]
- Ho, C.-F.; Jiao, Y.; Wei, B.; Yang, Z.; Wang, H.-Y.; Wu, Y.-Y.; Yang, C.; Tseng, K.-W.; Huang, C.-Y.; Chen, C.-Y.; et al. Protein Supplementation Enhances Cerebral Oxygenation during Exercise in Elite Basketball Players. Nutrition 2018, 53, 34–37. [Google Scholar] [CrossRef]
- Marques, C.G.; Santos, V.C.; Levada-Pires, A.C.; Jacintho, T.M.; Gorjão, R.; Pithon-Curi, T.C.; Cury-Boaventura, M.F. Effects of DHA-Rich Fish Oil Supplementation on the Lipid Profile, Markers of Muscle Damage, and Neutrophil Function in Wheelchair Basketball Athletes before and after Acute Exercise. Appl. Physiol. Nutr. Metab. 2015, 40, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, M.; Zajac, A.; Mikolajec, K.; Zydek, G.; Langfort, J. No Modification in Blood Lipoprotein Concentration but Changes in Body Composition After 4 Weeks of Low Carbohydrate Diet (LCD) Followed by 7 Days of Carbohydrate Loading in Basketball Players. J. Hum. Kin. 2018, 65, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, M.; Chycki, J.; Zajac, A.; Maszczyk, A.; Zydek, G.; Langfort, J. Anaerobic Performance after a Low-Carbohydrate Diet (LCD) Followed by 7 Days of Carbohydrate Loading in Male Basketball Players. Nutrients 2019, 11, 778. [Google Scholar] [CrossRef] [PubMed]
- Ronghui, S. The Reasearch on the Anti-Fatigue Effect of Whey Protein Powder in Basketball Training. Open Biomed. Eng. J. 2015, 9, 330–334. [Google Scholar] [CrossRef]
- Shi, D. Oligosaccharide and Creatine Supplementation on Glucose and Urea Nitrogen in Blood and Serum Creatine Kinase in Basketball Athletes. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2005, 25, 587–589. [Google Scholar]
- Taylor, L.W.; Wilborn, C.; Roberts, M.D.; White, A.; Dugan, K. Eight Weeks of Pre- and Postexercise Whey Protein Supplementation Increases Lean Body Mass and Improves Performance in Division III Collegiate Female Basketball Players. Appl. Physiol. Nutr. Metab. 2016, 41, 249–254. [Google Scholar] [CrossRef]
- Wilborn, C.D.; Taylor, L.W.; Outlaw, J.; Williams, L.; Campbell, B.; Foster, C.A.; Smith-Ryan, A.; Urbina, S.; Hayward, S. The Effects of Pre- and Post-Exercise Whey vs. Casein Protein Consumption on Body Composition and Performance Measures in Collegiate Female Athletes. J. Sports Sci. Med. 2013, 12, 74–79. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.P.; Savović, J.; Page, M.J.; Sterne, J.A. Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2) 2019. Available online: https://www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2 (accessed on 20 December 2022).
- Higgins, J.P.; Li, T.; Sterne, J.A. Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB 2). Additional Considerations for Crossover Trials. 2021. Available online: https://www.riskofbias.info/welcome/rob-2-0-tool/rob-2-for-crossover-trials (accessed on 20 December 2022).
- National Heart, Lung and Blood Institute Quality Assessment Tool for Before-After (Pre-POST) Studies with No Control Group. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 20 December 2022).
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological Guidance for Systematic Reviews of Observational Epidemiological Studies Reporting Prevalence and Cumulative Incidence Data. Int. J. Evid. Based Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef]
- Ma, L.-L.; Wang, Y.-Y.; Yang, Z.-H.; Huang, D.; Weng, H.; Zeng, X.-T. Methodological Quality (Risk of Bias) Assessment Tools for Primary and Secondary Medical Studies: What Are They and Which Is Better? Military Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Schroder, H.; Navarro, E.; Mora, J.; Seco, J.; Torregrosa, J.M.; Tramullas, A. The Type, Amount, Frequency and Timing of Dietary Supplement Use by Elite Players in the First Spanish Basketball League. J. Sports Sci. 2002, 20, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Ott, I.; Mielgo-Ayuso, J.; Calleja-González, J. A Glimpse of the Sports Nutrition Awareness in Spanish Basketball Players. Nutrients 2021, 14, 27. [Google Scholar] [CrossRef]
- Sánchez-Díaz, S.; Yanci, J.; Raya-González, J.; Scanlan, A.T.; Castillo, D. A Comparison in Physical Fitness Attributes, Physical Activity Behaviors, Nutritional Habits, and Nutritional Knowledge Between Elite Male and Female Youth Basketball Players. Front. Psychol. 2021, 12, 685203. [Google Scholar] [CrossRef]
- Boumosleh, J.M.; el Hage, C.; Farhat, A. Sports Nutrition Knowledge and Perceptions among Professional Basketball Athletes and Coaches in Lebanon-a Cross-Sectional Study. BMC Sports Sci. Med. Rehabil. 2021, 13, 53. [Google Scholar] [CrossRef]
- del Mar Bibiloni, M.; Vidal-Garcia, E.; Carrasco, M.; Julibert, A.; Pons, A.; Tur, J.A. Hydration Habits before, during and after Training and Competition Days among Amateur Basketball Players. Nutr. Hosp. 2018, 35, 612–619. [Google Scholar]
- Gacek, M.; Wojtowicz, A. Personal Resources and Nutritional Behavior of Polish Basketball Players. J. Phy. Edu. Sport 2021, 21, 130–139. [Google Scholar]
- Gorrell, S.; Nagata, J.M.; Hill, K.B.; Carlson, J.L.; Shain, A.F.; Wilson, J.; Alix Timko, C.; Hardy, K.K.; Lock, J.; Peebles, R. Eating Behavior and Reasons for Exercise among Competitive Collegiate Male Athletes. Eat. Weight Disord. 2021, 26, 75–83. [Google Scholar] [CrossRef]
- Kampouri, D.; Kotopoulea-Nikolaidi, M.; Daskou, S.; Giannopoulou, I. Prevalence of Disordered Eating in Elite Female Athletes in Team Sports in Greece. Eur. J. Sport Sci. 2019, 19, 1267–1275. [Google Scholar] [CrossRef]
- Mavra, N.; Ivkovic, G.; Zderic, I. The Dietary Habits of Croatian Women Basketball Players and Menstrual Irregularities. In Proceedings of the 7th International Scientific Conference on Kinesiology, Opatija, Croatia, 22–25 May 2014; pp. 98–102. [Google Scholar]
- Michou, M.; Costarelli, V. Disordered Eating Attitudes in Relation to Anxiety Levels, Self-Esteem and Body Image in Female Basketball Players. J. Exerc. Sci. Fitness 2011, 9, 109–115. [Google Scholar] [CrossRef]
- Monthuy-Blanc, J.; Maïano, C.; Morin, A.J.S.; Stephan, Y. Physical Self-Concept and Disturbed Eating Attitudes and Behaviors in French Athlete and Non-Athlete Adolescent Girls: Direct and Indirect Relations. Body Image 2012, 9, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Musaiger, A.O.; Ragheb, M.A. Dietary Habits of Athletes in Bahrain. Nutr. Health 1994, 10, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, E.; Spałkowska, A. Zachowania Żywieniowe Sportowców Wyczynowao Uprawiających Siatkówkę i Koszykówkę. Rocz. Państw. Zakł. Hig. 2012, 63, 483–489. [Google Scholar]
- Wells, E.K.; Chin, A.D.; Tacke, J.A.; Bunn, J.A. Risk of Disordered Eating Among Division I Female College Athletes. Int. J. Exerc. Sci. 2015, 8, 256–264. [Google Scholar] [PubMed]
- Tsoufi, A.; Maraki, M.I.; Dimitrakopoulos, L.; Famisis, K.; Grammatikopoulou, M.G. The Effect of Professional Dietary Counseling: Elite Basketball Players Eat Healthier during Competition Days. J. Sports Med. Phys. Fitness 2017, 57, 1305–1310. [Google Scholar] [CrossRef]
- Čabarkapa, D.; Fry, A.C.; Deane, M.A.; Akers, J.D. The Relationship Between Breakfast Consumption and Basketball Shooting Performance. Facta Univ. Ser. Phys. Educ. Sport 2020, 18, 311–322. [Google Scholar] [CrossRef]
- Davis, J.-K.; Freese, E.C.; Wolfe, A.S.; Basham, S.A.; Stein, K.M.W. Evaluation of Omega-3 Status in Professional Basketball Players. J. Strength Cond. Res. 2021, 35, 1794–1799. [Google Scholar] [CrossRef]
- Sawka, M.N.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, S.J.; Stachenfeld, N.S. American College of Sports Medicine Position Stand. Exercise and Fluid Replacement. Med. Sci. Sports Exerc. 2007, 39, 377–390. [Google Scholar]
- Casa, D.J.; Armstrong, L.E.; Hillman, S.K.; Montain, S.J.; Reiff, R.V.; Rich, B.S.; Roberts, W.O.; Stone, J.A. National Athletic Trainers’ Association Position Statement: Fluid Replacement for Athletes. J. Athl. Train. 2000, 35, 212–224. [Google Scholar] [PubMed]
- Mountjoy, M.; Sundgot-Borgen, J.K.; Burke, L.M.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.K.; Meyer, N.L.; et al. IOC Consensus Statement on Relative Energy Deficiency in Sport (RED-S): 2018 Update. Br. J. Sports Med. 2018, 52, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Sansone, P.; Makivic, B.; Csapo, R.; Hume, P.; Martínez-Rodríguez, A.; Bauer, P. Body Fat of Basketball Players: A Systematic Review and Meta-Analysis. Sports Med. Open 2022, 8, 26. [Google Scholar] [CrossRef]
- Scanlan, A.T.; Dascombe, B.J.; Kidcaff, A.P.; Peucker, J.L.; Dalbo, V.J. Gender-Specific Activity Demands Experienced During Semiprofessional Basketball Game Play. Int. J. Sports Physiol. Perform. 2015, 10, 618–625. [Google Scholar] [CrossRef]
- Spiteri, T.; Newton, R.U.; Binetti, M.; Hart, N.H.; Sheppard, J.M.; Nimphius, S. Mechanical Determinants of Faster Change of Direction and Agility Performance in Female Basketball Athletes. J. Strength Cond. Res. 2015, 29, 2205–2214. [Google Scholar] [CrossRef]
- Ribeiro Gonçalves, B.; Mota, H.R.; Sampaio-Jorge, F.; Morales, A.P.; Leite, T.C. Correlation between Body Composition and the Performance of Vertical Jumps in Basketball Players. J. Exerc. Physiol. Online 2015, 18, 69–78. [Google Scholar]
- Moon, J.M.; Zabriskie, H.A.; Harty, P.S.; Currier, B.S.; Blumkaitis, J.C.; Stecker, R.A.; Jagim, A.; Kerksick, C.M. Comparison of Energy Expenditure Observed between Scheduled Activities in Collegiate Team-Sport Female Athletes. Sports 2021, 9, 50. [Google Scholar] [CrossRef]
- Ali Nabli, M.; Abdelkrim, N.B.; Castagna, C.; Jabri, I.; Batikh, T.; Chamari, K. Energy Demands and Metabolic Equivalents (METS) in U-19 Basketball Refereeing During Official Games. J. Sports Med. Dop. Stud. 2017, 7, 190. [Google Scholar] [CrossRef]
- Peklaj, E.; Reščič, N.; Koroušic´ Seljak, B.; Rotovnik Kozjek, N. Is RED-S in Athletes Just Another Face of Malnutrition? Clin. Nutr. ESPEN 2022, 48, 298–307. [Google Scholar] [CrossRef]
- Williams, C.; Rollo, I. Carbohydrate Nutrition and Team Sport Performance. Sports Med. 2015, 45, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN Exercise & Sports Nutrition Review Update: Research & Recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [PubMed]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and Exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Durkalec-Michalski, K.; Nowaczyk, P.M.; Siedzik, K. Effect of a Four-Week Ketogenic Diet on Exercise Metabolism in CrossFit-Trained Athletes. J. Int. Soc. Sports Nutr. 2019, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- McSwiney, F.T.; Wardrop, B.; Hyde, P.N.; Lafountain, R.A.; Volek, J.S.; Doyle, L. Keto-Adaptation Enhances Exercise Performance and Body Composition Responses to Training in Endurance Athletes. Metabolism 2018, 81, 25–34. [Google Scholar] [CrossRef]
- Volek, J.S.; Noakes, T.; Phinney, S.D. Rethinking Fat as a Fuel for Endurance Exercise. Eur. J. Sport Sci. 2015, 15, 13–20. [Google Scholar] [CrossRef]
- Bonfanti, N.; Ma, V. Are There Nutritional Recommendations for Wheelchair Basketball Available? Sports Nutr. Ther. 2017, 2, 1. [Google Scholar]
- Grossmann, F.; Perret, C.; Roelands, B.; Meeusen, R.; Flueck, J.L. Fluid Balance and Thermoregulatory Responses during Wheelchair Basketball Games in Hot vs. Temperate Conditions. Nutrients 2022, 14, 2930. [Google Scholar] [CrossRef]
- Capling, L.; Tam, R.; Beck, K.L.; Slater, G.J.; Flood, V.M.; O’Connor, H.T.; Gifford, J.A. Diet Quality of Elite Australian Athletes Evaluated Using the Athlete Diet Index. Nutrients 2020, 13, 126. [Google Scholar] [CrossRef]
- Capling, L.; Gifford, J.A.; Beck, K.L.; Flood, V.M.; Slater, G.J.; Denyer, G.S.; O’Connor, H.T. Development of an Athlete Diet Index for Rapid Dietary Assessment of Athletes. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 643–650. [Google Scholar] [CrossRef]
| Reference | Survey Method | Season/Training Macrocycle | Gender/n | Age (Years) | Body Mass (kg) | Energy | Carbohydrate | Protein | Fat | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (kcal∙day−1) | (kcal∙kgBM−1∙day−1) | (g∙day−1) | (g∙kgBM−1∙day−1) | (% EI) | (g∙day−1) | (g∙kgBM−1∙day−1) | (% EI) | (g∙day−1) | (g∙kgBM−1∙day−1) | (% EI) | ||||||
| Baranauskas et al. 2021 [15] § | 3 × 24 h FD | - | F/10 | 16.2 ± 0.4 | 70.6 ± 4.6 | 2781 ± 210 | 40.0 ± 3.8 | 353 ǂ | 5.0 ± 0.4 | 50.3 ± 1.9 | 106 ǂ | 1.5 ± 0.2 | 15.0 ± 0.5 | 113 ǂ | 1.6 ± 0.2 | 34.7 ± 1.5 |
| Baranauskas et al. 2013 [16] | 24 h DR | training mezzo-cycles designed for strength training | M/39 F/13 | 18.6 ± 1.8 16.1 ± 0.5 | 85.7 ± 9.9 69.5 ± 8.0 | 4521.2 ± 1341.7 2854.5 ± 428.1 | 52.9 ± 14.8 41.6 ± 8.4 | 514 ǂ 375 ǂ | 6.0 ± 1.9 5.4 ± 1.3 | 44.8 ± 5.4 52.0 ± 5.1 | 163 ǂ 104 ǂ | 1.9 ± 0.6 1.5 ± 0.3 | - | - | - | 40.7 ± 5.2 33.5 ± 4.2 |
| Baranauskas et al. 2020 [17] | 7-day DR | special preparatory period for the competition | F/14 | 26.4 ± 4.5 | 65.2 ± 7.8 | 2579 ± 590 | 39.6 ǂ | 326 ǂ | 5.0 ± 1.3 | - | 85 ǂ | 1.3 ± 0.3 | - | - | - | 38.1 ± 4.1 |
| Dzimbova 2020 [18] | FFQ | - | -/16 | 15.4 ± 1.2 | 64.4 ± 10.8 | 2204 ± 624 | 32.2 ǂ | 379.7 ± 123.4 | 5.9 ǂ | - | 90.8 ± 28.9 | 1.4 ǂ | - | 59.8 ± 17.3 | 0.9 ǂ | - |
| Eskici and Ersoy 2016 [19] | 24 h DR | athletes at the training camp | F/22 | 25.5 ± 7.2 | 57.4 ± 8.6 | 2867.8 ± 523.6 | 50.0 ǂ | 297.3 ± 74.9 | 5.2 ǂ | 42.7 ± 8.8 | 92.6 ± 16.7 | 1.6 ǂ | 13.2 ± 1.9 | 142.7 ± 37.7 | 2.5 ǂ | 44.05 ± 8.0 |
| Ferro et al. 2017 [20] | 3-day FD on CD (food weighing) | pre-competitive period: May June | M/11 | 30 ± 6 | 74.8 ± 14.9 75.1 ± 14.5 | 2492 ± 362 2470 ± 497 | 34.8 ± 9.8 34.7 ± 12.6 | 281 ǂ 318 ǂ | 3.76 ± 1.30 4.24 ± 1.92 | 45.3 ± 7.3 49.3 ± 8.2 | 126 ǂ 111 ǂ | 1.68 ± 0.64 1.48 ± 0.45 | 19.1 ± 4.8 17.0 ± 2.8 | 104 ǂ 92 ǂ | 1.39 ± 0.43 1.23 ± 0.41 | 35.5 ± 4.7 32.1 ± 5.3 |
| Gacek 2022 [21] | 3-day FD (2 TDs + 1 RD) | - | M/48 | 26.6 ± 4.5 | - | 1795.5 ± 547.9 | - | 258.2 ± 105.9 | - | 52.4 ± 9.2 | 79.3 ± 23.4 | - | 18.2 ± 3.0 | 58.5 ± 24.5 | - | 29.4 ± 9.4 |
| Grams et al. 2016 [22] § | 3-day FD on CD (food weighing) | training camps held in the pre-competitive season | M/8 | 29.9 ± 6.5 | 75.0 ± 16.2 | 2441 ± 341 | 32.5 ǂ | 233 ǂ | 3.1 | - | 120 ǂ | 1.6 ± 0.7 | - | - | - | 35.1 ± 4.7 |
| Hickson et al. 1986 [23] | 3 × 24 h DR on CD weekdays | competitive season | F/13 | 19.4 ± 0.3 | 68.3 ± 1.6 | 1995 ± 151 (SEM) | 30 ± 8 | - | - | - | - | - | - | - | - | - |
| Hickson et al. 1990 [24] | 3-day FD on CD weekdays (food weighing) | pre-season | M/12 | 16.4 ± 0.7 15–18 | 77.0 ± 8.9 | 3400 ± 702 | 45 ± 10 | - | - | 53 | - | - | 13 | - | - | 34 |
| Kostopoulos et al. 2017 [25] | 3 × 24 h DR on 2 non-CDs (1 weekday and 1 weekend day) + 1 match day | competitive season (playoff stage): average from 3 × 24 h DR intake at the match day | -/18 | 24 ± 4 | - | - | 24.7 ± 7.2 24.0 ± 8.7 | - | 2.6 ± 0.8 2.8 ± 0.8 | 45.3 ± 10.5 45.7 ± 11.9 | - | 1.5 ± 0.9 1.4 ± 1.0 | 23.0 ± 7.4 22.7 ± 9.7 | - | 0.95 ± 0.36 0.88 ± 0.33 | 33.9 ± 5.9 33.3 ± 6.2 |
| Leinus and Ööpik 1998 [26] | 4-day FD on 2 TDs + 2 RDs (food weighing) | RD TD RD+TD RD TD RD + TD | M/7 F/7 | 21.1 ± 2.6 20.6 ± 1.9 | 81.6 ± 9.3 63.1 ± 7.7 | 3545 ± 970 2531 ± 719 2986 ± 767 2185 ± 666 1752 ± 394 1968 ± 449 | 43.4 ǂ 31.0 ǂ 36.6 ǂ 34.6 ǂ 27.8 ǂ 31.2 ǂ | 384 ǂ 294 ǂ 335 ǂ 252 ǂ 221 ǂ 240 ǂ | 4.7 ± 1.3 3.6 ± 1.1 4.1 ± 0.9 4.0 ± 1.3 3.5 ± 1.1 3.8 ± 0.9 | 43.0 ± 9.1 46.7 ± 9.4 44.9 ± 8.1 47.0 ± 5.0 48.4 ± 5.4 47.7 ± 4.5 | 114 ǂ 81 ǂ 90 ǂ 57 ǂ 50 ǂ 50 ǂ | 1.4 ± 0.4 1.0 ± 0.3 1.1 ± 0.2 0.9 ± 0.2 0.8 ± 0.3 0.8 ± 0.2 | 13.0 ± 2.7 12.8 ± 2.4 12.9 ± 1.7 11.4 ± 1.5 11.5 ± 2.0 11.5 ± 1.3 | 180 ǂ 122 ǂ 147 ǂ 107 ǂ 76 ǂ 95 ǂ | 2.2 ± 0.7 1.5 ± 0.6 1.8 ± 0.6 1.7 ± 0.7 1.2 ± 0.3 1.5 ± 0.5 | 44.1 ± 8.5 40.5 ± 7.4 42.3 ± 7.4 41.7 ± 5.8 40.2 ± 6.1 41.0 ± 5.2 |
| Nepocatych and Balilionis 2017 [27] | 3-day FD (2 week- and 1 weekend day) | beginning of the competitive season end of the competitive season | F/10 | 18–22 | 78.7 ± 16.8 80.1 ± 18.6 | 2208 ± 373 2567 ± 834 | 29 ± 8 34 ± 15 | 254 ± 51 304 ±74 | 3.4 ± 1.0 4.1 ± 1.5 | 46 ± 6 50 ± 14 | 92 ± 29 97 ± 38 | 1.3 ± 0.6 1.4 ± 0.7 | 17 ± 5 15 ± 2 | 87 ± 19 111 ± 42 | 1.2 ± 0.3 1.6 ± 0.9 | 35 ± 5 39 ± 7 |
| Nikić et al. 2014 [28] | FFQ | - | M/57 | 15.6 ± 0.9 | 78.0 ± 10.7 | 3962 ± 1376 | 51.1 ± 16.5 | 487.8 ± 171.8 | 6.3 ± 2.1 | - | 140.0 ± 58.2 | 1.8 ± 0.7 | - | 165.6 ± 64.4 | 2.1 ǂ | - |
| Nowak et al. 1998 [29] | 3-day FD on CD (weekdays) | pre-competitive season | M/16 F/10 | 18.9 ± 1.29 19.4 ± 0.97 | 83.4 ± 9.09 71.7 ± 3.50 | 3558 ± 1078 1730 ± 573 | 42.7 ǂ 24.1 ǂ | 437 ± 158 229 ± 95 | 5.2 ǂ 3.2 ǂ | 48 52 | 159 ± 70 68 ± 28 | 1.9 ǂ 0.9 ǂ | 17 16 | 139 ± 48 63 ± 19 | 1.7 ǂ 0.9 ǂ | 34 32 |
| Papandreou et al. 2007 [30] | 5-day FD (week- and weekend days) | - | M/8 F/13 | 20 ± 4 25 ± 5 | 90 ± 9 62 ± 8 | 1901 ± 323 1487 ± 636 (n = 8) | 21 ± 4 25 ± 13 | 220 ± 42 170 ±71 | 1.9 ± 1.1 2.9 ± 1.1 | 46 ± 3 47 ± 11 | 80 ± 10 66 ± 24 | 1.1 ± 0.9 1.1 ± 0.9 | 17 ± 2 18 ± 5 | 83 ± 17 64 ± 36 | 1.1 ± 0.9 1.1 ± 0.9 | 39 ± 4 36 ± 9 |
| Quintas et al. 2003 [31] | 5-day FD (week- and weekend days) | - | F/26 | 17.2 ± 2.1 | 70.5 ± 11.02 | 2580 ± 698 ǂ (10,807 ± 2921 kJ∙day−1) | 36.6 ǂ (153 kJ∙kg−1∙day−1 ǂ) | - | - | - | 99 ǂ | 1.4 ± 0.41 | - | - | - | - |
| Schröder et al. 2004 [32] | 24 h DR | training & competition | M/50 | 25.1 ± 4.0 | 93.0 ± 11.0 | 4228 ± 215 ǂ (17.7 ± 0.9 MJ∙day−1) | 45.8 ǂ (191.8 ± 68.6 kJ∙kg−1∙day−1) | 424.2 ± 165.9 | 4.6 ± 1.7 | 40.3 ± 7.7 | 211.3 ± 99.5 | 2.3 ± 1.0 | 19.7 ± 4.9 | 185.3 ± 78.6 | 2.1 ± 0.92 | 39.0 ± 7.7 |
| Shimizu et al. 2019 [33] | FFQ | - | F/13 | 28.9 ± 8.1 | - | 1636.1 ± 439.5 | - | - | - | - | 57.5 ± 18.9 | - | - | - | - | - |
| Silva et al. 2012 [34] | 7-day FD | longitudinal approach over 34 weeks: beginning of the pre-season competitive period assessment beginning of the pre-season competitive period assessment | M/7 F/2 | 16.0 ± 0.5 16.8 ± 0.7 16.3 ± 0.5 16.8 ± 0.7 | 77.7 ± 6.6 79.9 ± 6.8 64.3 ± 7.1 65.7 ± 6.5 | 3003 ± 831 ǂ (12,570 ± 3478 kJ∙day−1) 3239 ± 422 ǂ (13,559 ± 1765 kJ∙day−1) 2392 ± 382 ǂ (10,015 ± 1600 kJ∙day−1) 1801 ± 49 ǂ (7537 ± 204 kJ∙day−1) | 38.7 ǂ (162 kJ∙kg−1∙day−1ǂ) 40.6 ǂ (170 kJ∙kg−1∙day−1) 37.3 ǂ (156 kJ∙kg−1∙day−1 ǂ) 27.5 ǂ (115 kJ∙kg−1∙day−1 ǂ) | 395 ± 125 427 ± 81 333 ± 71 227 ± 13 | 5.1 ǂ 5.3 ǂ 5.2 ǂ 3.5 ǂ | - | 143 ± 27 150 ± 19 104 ± 17 82 ± 14 | 1.8 ǂ 1.9 ǂ 1.6 ǂ 1.2 ǂ | - | 95 ± 28 104 ± 16 72 ± 19 63 ± 3 | 1.2 ǂ 1.3 ǂ 1.1 ǂ 0.96 ǂ | - |
| Silva et al. 2013 [35] | 7-day FD | competitive period | M/12 F/7 | 17.0 ± 0.7 16.9 ± 0.7 | 80.9 ± 7.7 64.0 ± 5.4 | 2895 ± 479 1807 ± 46 | 35.8 ǂ 28.2 ǂ | 365.5 ± 64.4 218.8 ± 1.8 | 4.5 ǂ 3.4 ǂ | 50.5 ± 3.8 48.4 ± 0.8 | 135.4 ± 23.5 82.0 ± 14.3 | 1.7 ǂ 1.3 ǂ | 18.7 ± 2.8 18.8 ± 2.7 | 93.5 ± 20.7 64.1 ± 1.2 | 1.2 ǂ 1.0 ǂ | 29.1 ± 2.4 31.4 ± 1.4 |
| Silva et al. 2017 [36] | DXA/DLW | competitive phase | -/24 | - | - | 4347 ± 756 | - | - | - | - | - | - | - | - | - | - |
| Toti et al. 2021 [37] § | 3-day FD on CD (2 working days + 1 weekend day/holiday) | - | M/16 M/12 F/9 | 27 (24–31) 19 (18–21) 26 (19–30) | 74.2 ± 12.3 57.2 ± 11.7 61.0 ± 10.6 | 2441 ǂ 1853 ǂ 1635 ǂ | 32.9 ± 10.4 32.4 ± 8.8 26.8 ± 5.1 | 267 ǂ 257 ǂ 226 ǂ | 3.6 ± 1.2 4.5 ± 1.5 3.7 ± 0.9 | 43.0 55.0 55.0 | 11 1 ǂ 74 ǂ 61 ǂ | 1.5 (1.1–2.0) 1.3 (1.1–1.4) 1.0 (0.8–1.0) | 19.0 17.0 17.0 | 96 ǂ 57 ǂ 55 ǂ | 1.3 ± 0.4 1.0 ± 0.3 0.9 ± 0.2 | 37.0 27.0 27.0 |
| Toti et al. 2021 [38] | 3-day FD on CD (2 working days + 1 weekend day/holiday) | Training camp before the 2019 European Championship | M/15 | 28.5 ± 1.5 | 74.8 ± 3.2 | 2438 ǂ | 32.6 ± 2.8 | 269 ǂ | 3.6 ± 0.3 | 43.9 ± 1.2 | 112 ǂ | 1.5 ± 0.1 | 18.4 ± 0.7 | - | - | 36.9 ± 0.6 |
| Zanders et al. 2021 [39] | 4 CD of recording of food intake via mobile app | Entire women’s collegiate basketball season: phase I (heavy practicing + non-conference games) phase II (heavy practicing + conference league play) phase III (postseason conference tournament) phase IV (off-season workout) phase V (off-season workout) | F/13 | 19.8 ± 1.3 | 74.6 ± 9.1 | 2506 ± 271 2354 ± 533 2326 ± 456 2517 ± 334 2422 ± 276 | 33.7 ± 3.1 31.9 ± 7.8 31.5 ± 7.3 33.8 ± 3.7 32.7 ± 4.9 | 282.4 ± 60.3 272.2 ± 73.2 244.8 ± 42.2 299.9 ± 36.4 263.2 ± 36.8 | 3.8 ± 0.7 3.7 ± 1.1 3.3 ± 0.7 4.0 ± 0.4 3.6 ± 0.7 | - | 97.9 ± 18.8 87.3 ± 13.9 87.5 ± 17.0 78.0 ± 13.9 84.7 ± 16.3 | 1.31 ± 0.22 1.18 ± 0.19 1.19 ± 0.28 1.05 ± 0.19 1.15 ± 0.26 | - | 113.0 ± 26.1 98.4 ± 27.1 112.7 ± 29.3 87.3 ± 18.8 93.3 ± 28.5 | 1.50 ± 0.34 1.37 ± 0.38 1.51 ± 0.42 1.23 ± 0.31 1.23 ± 0.35 | - |
| Reference | Gender/n Age (years) | Body Mass (kg) | Environmental Conditions: Temperature & Relative Humidity | Type of Practice & Duration | Fluid Intake | Indices of Hydration State | Additional Notes |
|---|---|---|---|---|---|---|---|
| Abbasi et al. 2021 [40] § | F/10 n/a | n/a | n/a n/a | n/a | 553.35 ± 122.91 mL (during practice) | PRE-PRACTICE Urine specific gravity (USG): 1.017 ± 0.006 Urine colour (UC): 4 ± 1 Incidence of dehydration (DEH)†: 40% POST-PRACTICE USG: 1.021 ± 0.005; UC: 5 ± 2 Incidence of DEH †: 60% Body mass (BM) loss ¥: −0.6 ± 0.3% Sweat rate: 0.6 ± 0.1 L∙h−1 Fluid replacement: 59.4 ± 27.3% Hydration Awareness Questionnaire: 121 ± 8 | - |
| Arnaoutis et al. 2015 [41] | M/12 15.5 ± 0.5 | 78.8 ± 8.9 | 28.8 °C n/a | A typical day of training 86.0 min | n/a | FIRST MORNING URINE SAMPLE USG: 1.026 ± 0.005; UC: 5.0 ± 1.0 PRE-TRANING USG: 1.024 ± 0.005; UC: 4.0 ± 1.0 Incidence of euhydration (EUH) ǂ: 16.7% POST-TRAINING USG: 1.026 ± 0.005; UC: 5.0 ± 1.0 BM loss ¥: −1.0 ± 0.01% or −0.79 ± 0.01 kg | - |
| Baker et al. 2007 [42] § | M/17 21.1 ± 2.4 (17–28) | 81.6 ± 12.1 (63.6–104.5) | n/a n/a | - | - | FIRST EVALUATION UC: 5 ± 1; USG: 1.024 ± 0.004; urine osmolality (UO): 820 ± 210 mOsm∙L−1 SECOND EVALUATION UC: 5 ± 1; USG: 1.022 ± 0.006; UO: 774 ± 201 mOsm∙L−1 THIRD EVALUATION UC: 5 ± 1; USG: 1.023 ± 0.005; UO: 795 ± 180 mOsm∙L−1 FOURTH EVALUATION UC: 5 ± 1; USG: 1.025 ± 0.006; UO: 826 ± 181 mOsm∙L−1 FIFTH EVALUATION UC: 5 ± 2; USG: 1.021 ± 0.006; UO: 771 ± 240 mOsm∙L−1 | Baseline measurements were taken on five different occasions before introducing different hydration/dehydration strategies |
| Barnes et al. 2019 [43] | M, F/196 23 ± 5 | 92.1 ± 18.0 | 22.4 ± 1.7 °C 51 ± 12% | n/a 2.1 ± 0.8 h | n/a | Whole body sweat loss: 0.95 ± 0.42 L∙h−1 Whole body sweat [Na+]: 35.4 ± 11.2 mmol∙L−1 Rate of sweat Na+ loss: 34.5 ± 21.2 mmol∙h−1 | - |
| Brandenburg and Gaetz 2012 [44] | F/17 24.2 ± 3 | 78.8 ± 8 | 22.5–23.5 °C 44–50% | GAME I (preceded by 40-min warm-up) 17.0 ± 4.4 min | GAME I Warm-up: 0.35 ± 0.2 L Game: 1.22 ± 0.5 L Fluid intake in relation to sweat loss: 77.8 ± 32% | GAME I Pre-game USG: 1.005 ± 0.002 (1.002–1.008) Sweat loss: −1.99 ± 0.75 L BM loss ¥: −0.7 ± 0.8 (−2.1–0.5)% BM loss ¥: −0.6 ± 0.5 (−1.5–0.4) kg | Two games played on consecutive days against the same opponent |
| GAME II (preceded by 40-min warm-up) 16.4 ± 4.7 min | GAME II Warm-up: 0.25 ± 0.1 L Game: 1.40 ± 0.6 L Fluid intake in relation to sweat loss: 78.0 ± 21% | GAME II Pre-game USG: 1.010 ± 0.005 (1.005–1.022) Sweat loss: −1.99 ± 0.60 L BM loss ¥: −0.6 ± 0.6 (−2.0–0.1)% BM loss ¥: −0.5 ± 0.5 (−1.6–0.1) kg | |||||
| Broad et al. 1996 [45] | M/19 16.0–18.0 | 92.65 ± 8.33 | WINTER | WINTER | WINTER | WINTER | Testing sessions represent a typical program of weight training, field training, and competition sessions over a 1-week period; data were collected during a minimum of two matches, four training sessions, and two weight training sessions |
| 20.1 ± 0.0 °C 37.0 ± 0.2% | Weight training session - | 113 ± 149 mL∙h−1 | Sweat rate: 337 ± 120 mL∙h−1 BM loss ¶: −0.4 ± 0.3% | ||||
| 19.9 ± 1.4 °C 24.1 ± 3.3% | Court/field training 123 ± 18 min | 489 ± 177 mL∙h−1 | Sweat rate: 1039 ± 169 mL∙h−1 BM loss ¶: −1.2 ± 0.4% | ||||
| 18.9 ± 0.9 °C 36.3 ± 5.8% | Competition 85 ± 24 min | 917 ± 460 mL∙h−1 | Sweat rate: 1587 ± 362 mL∙h−1 BM loss ¶: −1.0 ± 0.6% | ||||
| SUMMER | SUMMER | SUMMER | SUMMER | ||||
| 22.5 ± 0.0 °C 52.1 ± 6.4% | Weight training session - | 236 ± 292 mL∙h−1 | Sweat rate: 389 ± 121 mL∙h−1 BM loss ¶: −0.3 ± 0.4% | ||||
| 27.4 ± 2.5 °C 33.7 ± 6.3% | Court/field training 103 ± 38 min | 797 ± 234 mL∙h−1 | Sweat rate:1371 ± 235 mL∙h−1 BM loss ¶: −1.0 ± 0.5% | ||||
| 23.3 ± 2.6 °C 41.4 ± 10.6% | Competition 89 ± 21 min | 1079 ± 613 mL∙h−1 | Sweat rate: 1601 ± 371 mL∙h−1 BM loss¶: −0.9 ± 0.7% | ||||
| F/12 16–18 | 68.16 ± 5.42 | WINTER | WINTER | WINTER | WINTER | ||
| 20.9 °C 65.9% | Weight training session - | 23 ± 60 mL∙h−1 | Sweat rate: 246 ± 133 mL∙h−1 BM loss ¶: −0.4 ± 0.2% | ||||
| 17.2 ± 1.9 °C 56.2 ± 11.8% | Court/field training 114 ± 23 min | 330 ± 156 mL∙h−1 | Sweat rate: 687 ± 114 mL∙h−1 BM loss ¶: −1.0 ± 0.4% | ||||
| 17.0 ± 1.3 °C 58.1 ± 15.6% | Competition 81 ± 7 min | 601 ± 167 mL∙h−1 | Sweat rate: 976 ± 254 mL∙h−1 BM loss ¶: −0.7 ± 0.5% | ||||
| SUMMER | SUMMER | SUMMER | SUMMER | ||||
| 21.4 ± 0.4 °C 48.6 ± 3.5% | Weight training session - | 38 ± 62 mL∙h−1 | Sweat rate: 389 ± 121 mL∙h−1 BM loss ¶: −0.3 ± 0.4% | ||||
| 25.1 ± 0.9 °C 42.8 ± 6.8% | Court/field training 114 ± 7 min | 413 ± 162 mL∙h−1 | Sweat rate:1371 ± 235 mL∙h−1 BM loss ¶: −1.0 ± 0.5% | ||||
| 25.6 ± 1.5 °C 59.6 ± 7.5% | Competition 93 ± 2 min | 599 ± 170 mL∙h−1 | Sweat rate: 1601 ± 371 mL∙h−1 BM loss ¶: −0.9 ± 0.7% | ||||
| Heishman et al. 2021 [46] | M/15 20.4 ± 1.7 | 95.1 ± 7.4 | n/a n/a | n/a | n/a | YEAR 1 PRE-SEASON USG: 1.020 ± 0.009 Incidence of EUH/DEH/significant DEH #: 44.0/55.5/0.5% | Pre-season and competitive season; 2 consecutive years. |
| COMPETITIVE SEASON USG: 1.022 ± 0.009 Incidence of EUH/DEH/significant DEH #: 38.5/60.7/0.5% Playing time ≤ 15 min–USG: 1.021 ± 0.002 Playing time > 15 min—USG: 1.021 ± 0.006 | |||||||
| M/16 18.9 ± 4.9 | 94.7 ± 9.7 | n/a n/a | n/a | n/a | YEAR 2 PRE-SEASON USG: 1.019 ± 0.001 Incidence of EUH/DEH #: 42.9 / 57.1 / 0.0% | ||
| COMPETITIVE SEASON USG: 1.021 ± 0.004 Incidence of EUH/DEH/significant DEH #: 31.0/65.7/3.3% Playing time ≤ 15 min—USG: 1.022 ± 0.0012 Playing time > 15 min—USG: 1.022 ± 0.001 | |||||||
| Logan-Sprenger and McNaughton 2020 [47] | F/11 18–41 | 65.9 ± 16.1 | 22.1 ± 1.2 °C 55 ± 2% | 18.27 ± 11.08 min | n/a | WHOLE SAMPLE (n = 11) (mean ± SD calculated based on raw data from original paper) Pre-game USG: 1.014 ± 0.006 BM loss: −0.5 ± 0.4% Δ in core temperature (Tc): 1.0 ± 0.6 °C (n = 10) Highest Tc: 38.6 ± 0.6°C (n = 10) Δ in skin temperature (Tsk): 6.1 ± 1.5°C (n = 10) Incidence of DEH †: 9% | Testing during a four-game series over four consecutive nights with the same game start time; each player was tested twice in the four-day period |
| 55.9 ± 6.9 | 20.88 ± 10.52 min | n/a | SPINAL CORD INJURED GROUP (n = 7) (mean ± SD calculated based on raw data from the original paper) Pre-game USG: 1.016 ± 0.005 BM loss: −0.4 ± 0.5% Δ in Tc: 1.0 ± 0.5 °C (n = 6) Highest Tc: 38.6 ± 0.5 °C (n = 6) Δ in Tsk: 5.6 ± 1.3 °C (n = 6) | ||||
| 83.4 ± 11.4 | 13.68 ± 12.00 min | n/a | NON-SPINAL CORD INJURED GROUP (n = 4) (mean ± SD calculated based on raw data from the original paper) Pre-game USG: 1.011 ± 0.005 BM loss: −0.6 ± 0.3% Δ in Tc: 0.9 ± 0.8 °C Highest Tc: 38.5 ± 0.8 °C Δ in Tsk: 6.8 ± 1.7 °C | ||||
| Osterberg et al. 2009 [48] | M/29 n/a | 99 ± 18 (76–140) | 20–22 °C 18–22% | Game - Game - Game 21.0 ± 8.0 min | GAME I 1.1 ± 0.7 L GAME II 1.0 ± 0.5 L AVERAGE 1.0 ± 0.6 (0.1–2.9) L | GAME I Pre-game USG: 1.020 ± 0.006 Sweat loss: −1.9 ± 0.7 L BM loss ¥: −1.2 ± 0.5% GAME II Pre-game USG: 1.019 ± 0.008 Sweat loss: −2.4 ± 0.9 L BM loss ¥: −1.6 ± 0.7% AVERAGE Incidence of DEH (USG > 1.020): 52% Sweat loss: −2.2 ± 0.8 (1.0–4.6) L BM loss¥: −1.4 ± 0.6 (0.5–3.2)% Sweat Na concentration: 41.6 ± 11.5 (21.3–58.1) mEq∙L−1 Total Na loss: −82.2 ± 38.2 (33.2–161.4) mEq NaCl loss: −4.8 ± 2.3 (1.9–9.5) g Na replacement: 16.6 ± 14.6 (0–49.7)% Sweat K concentration: 4.9 ± 0.7 (3.1–5.8) mEq∙L−1 Total K loss: −9.7 ± 2.7 (5.7–14.3) mEq | Athletes competed in 5 to 7 games throughout 9 to 10 days; measurements were taken from each player on 2 occasions, from 2 to 4 days apart |
| Schröder et al. 2004 [32] | M/50 25.1 ± 4.0 | 93.0 ± 11.0 | n/a n/a | Training - Competition - | 646 ± 352 mL∙h−1 § 882 ± 486 mL∙h−1 § Total daily intake: 3126 ± 1226 mL | - | - |
| Taim et al. 2021 [49] § | M/18 23.1 ± 1.3 | 76.5 ± 12.1 | n/a | - | - | USG: 1.018 ± 0.008 Incidence of EUH: 44.4% (USG ≤ 1.020) or 77.8% (USG ≤ 1.025) Incidence of DEH: 55.6% (USG > 1.020) or 22.2% (USG > 1.025) | - |
| Thigpen et al. 2014 [50] | M/11 21 ± 1 | 85.4 ± 7.6 | 22.5 ± 0.1 °C n/a | Morning conditioning practices 45.0 min | 523 ± 250 mL | Sweat loss: −969 ± 250 mL Sweat rate: 1263 ± 326 mL∙h−1 BM loss: −1.1 ± 0.3% | - |
| 19.6 ± 2.5 °C n/a | Afternoon sport-specific practices 170.0 min | 1535 ± 571 mL | Sweat loss: −2471 ± 495 mL Sweat rate: 872 ± 175 mL∙h−1 BM loss: −2.9 ± 0.6% Pre-practice USG: 1.026 ± 0.004 Incidence of minimal/significant/serious DEH: 18/68/14% * | ||||
| F/11 19 ± 1 | 75.3 ± 10.1 | 23.9 ± 1.0 °C n/a | Morning conditioning practices 95.0 min | 744 ± 230 mL | Sweat loss: −1112 ± 271 mL Sweat rate: 702 ± 171 mL∙h−1 BM loss: −1.5 ± 0.3% | ||
| 23.7 ± 0.8 °C n/a | Afternoon sport-specific practices 170.0 min | 1101 ± 411 mL | Sweat loss: −1910 ± 441 mL Sweat rate: 674 ± 156 mL∙h−1 BM loss: −2.5 ± 0.4% Pre-practice USG: 1.022 ± 0.008 (n = 10) Incidence of minimal/significant/serious DEH: 25 / 55 / 20% * | ||||
| Vukasinović-Vesić et al. 2015 [51] | M/96 19.0 ± 0.79 (16–20) | 90.6 ± 12.4 (62–144) | 30 ± 2 °C (27.2–32.5 °C) 55 ± 4% (48–58%) | Game 18.8 ± 10.5 min (0.15–40 min) | Fluid intake 1.87 ± 0.82 L (0.38–3.98 L) Fluid intake rate 1.79 ± 0.8 L∙h−1 (0.4–19 L∙h−1) | PRE-GAME USG: 1024 ± 0.6; UC: 5.67 ± 1.12; UO: 883 ± 229 mOsm Incidence of DEH based on USG: 80% (> 1.020); UC (>4): 95%; UO: 75% (>700 mOsm) POST-GAME USG: 1026 ± 6; UC: 5.97 ± 1.37 UO: 852 ± 228 mOsm Incidence of DEH based on USG: 85%; UC: 95%; UO: 75% Sweat rate: 2.7 ± 0.9 (0.23–5.54) L∙h−1 BM loss: −0.9 ± 0.7 (−1.0–2.9) kg Level of DEH: 0.99 ± 0.7 (−1.25–2.95)% | Evaluation during the FIBA Europe U20 Championship |
| Reference | Study Design | Gender/n | Age (years) | Intervention | Experimental Procedures † | Outcomes |
|---|---|---|---|---|---|---|
| Baker et al. 2007 [52] | Six-arm randomized cross-over placebo-controlled trial (double-blind with respect to euhydration [EUH] trials) | M/11 | 21 ± 3 (17–28) | (1) EUH with lemon/lime-flavored carbohydrate–electrolyte solution (CES; 6% carbohydrate [CHO] and 18.0 mM NaCl)—EUH-CES (2) EUH with a placebo (PLA; lemon/lime-flavored water and 18.0 mM NaCl)—EUH-PLA (3) 1% dehydration (DEH) (4) 2% DEH (5) 3% DEH (6) 4% DEH Results presented as means of two implemented EUH conditions (EUH-CES and EUH-PLA) and four distinct DEH conditions (1% DEH—4% DEH) | (1) BASELINE evaluation (blood sampling, blood pressure [BP], heart rate [HR], core body temperature [Tc], Test of Variables of Attention [TOVA], ratings of fatigue) (2) Procedure of EUH/DEH obtaining via EXERCISE/HEAT exposure during interval-walking protocol (9 bouts × 15 min walking at 50% maximal oxygen uptake [VO2max] with 5-min rest between bouts, temperature: 40°C, relative humidity: 20%) (3) POST-EXERCISE/HEAT evaluation (4) Recovery (50 min + 20 min travel) (5) Basketball drill test (4 bouts × 15 min of drills with a 5-min break between quarters [QR] and a 10-min break at halftime) (6) Ratings of fatigue at the HALFTIME drill test (7) Post-drill test evaluation—END | PHYSIOLOGICAL VARIABLES Tc POST-EXERCISE/HEAT: ↑ DEH vs. EUH END: ↔ DEH vs. EUH Δ Plasma volume (PV) POST-EXERCISE/HEAT: ↓ DEH vs. EUH Serum glucose POST-EXERCISE/HEAT: ↓ DEH vs. EUH, ↑ EUH-CES vs. EUH-PLA, ↑ EUH-CES vs. DEH RATINGS OF FATIGUE Lightheadedness, hotness POST-EXERCISE/HEAT, HALFTIME, END: ↑ DEH vs. EUH Total body fatigue POST-EXERCISE/HEAT, HALFTIME: ↑ DEH vs. EUH END: ↔ DEH vs. EUH VIGILANCE—TOVA—TARGET-INFREQUENT CONDITION Sensitivity (Δ from BASELINE) POST-EXERCISE/HEAT: ↔ DEH vs. EUH END: ↓ DEH vs. EUH Response time, omission errors (Δ from BASELINE) POST-EXERCISE/HEAT: ↔ DEH vs. EUH END: ↑ DEH vs. EUH Commission errors (Δ from BASELINE) POST-EXERCISE/HEAT, END: ↔ DEH vs. EUH VIGILANCE—TOVA—TARGET-FREQUENT CONDITION Sensitivity (Δ from BASELINE) POST-EXERCISE/HEAT: ↔ DEH vs. EUH END: ↓ DEH vs. EUH Response time (Δ from BASELINE) POST-EXERCISE/HEAT: ↔ DEH vs. EUH END: ↑ DEH vs. EUH Omission and commission errors (Δ from BASELINE) POST-EXERCISE/HEAT: ↑ DEH vs. EUH END: ↔ DEH vs. EUH |
| Baker et al. 2007 [42] | Six-arm randomized cross-over placebo-controlled trial (double-blind concerning EUH trials) | M/17 | 21.1 ± 2.4 (17—28) | (1) EUH with lemon/lime-flavored CHO–electrolyte solution CES; 6% CHO and 18.0 mM NaCl)—EUH-CES (2) EUH with a PLA (lemon/lime-flavored water and 18.0 mM NaCl)—EUH-PLA (3) 1% DEH (4) 2% DEH (5) 3% DEH (6) 4% DEH Results presented for EUH-PLA and separately for four distinct DEH conditions EUH-CES excluded from the presentation due to a lack of differences between EUH conditions and for simplification | (1) BASELINE evaluation (blood sampling, BP, HR, Tc) (2) Procedure of EUH/DEH obtaining via EXERCISE/HEAT exposure during interval-walking protocol (9 bouts × 15 min walking at 50% VO2max with 5-min rest between bouts, temperature: 40 °C, relative humidity: 20%) (3) POST-EXERCISE/HEAT evaluation (same as at baseline and additionally ratings of fatigue, rate of perceived exertion [RPE]) (4) Recovery (70 min) (5) RECOVERY evaluation (same as at POST-EXERCISE/HEAT) (6) Basketball drill test (4 bouts × 15 min of drills with a 5-min break between QRs and a 10-min break at HALFTIME) (7) HALFTIME drill test (after 2nd QR) evaluation (same as at POST-EXERCISE/HEAT) (8) Post-drill test (after 4th QR) evaluation (same as at POST-EXERCISE/HEAT)—END | PHYSIOLOGICAL VARIABLES HR POST-EXERCISE/HEAT: ↑ 1–4% DEH vs. EUH RECOVERY: ↔ 1–3% DEH vs. EUH, ↑ 4% DEH vs. EUH HALFTIME: ↔ 1–4% DEH vs. EUH END: ↔ 1, 3, 4% DEH vs. EUH, ↑ 2% DEH vs. EUH Tc POST-EXERCISE/HEAT: ↔1% DEH vs. EUH, ↑ 2–4% DEH vs. EUH RECOVERY, HALFTIME: ↔ 1–3% DEH vs. EUH, ↑ 4% DEH vs. EUH END: ↔ 1–4% DEH vs. EUH Mean arterial pressure (MAP) POST-EXERCISE/HEAT: ↔ 1–2% DEH vs. EUH, ↓ 3–4% DEH vs. EUH RECOVERY: ↔1–4% DEH vs. EUH BLOOD VARIABLES (at the END of the whole protocol) Glucose: ↔ 1–4% DEH vs. EUH Sodium: ↔ 1% DEH vs. EUH; ↑ 2–4% DEH vs. EUH, Osmolality, Δ PV (change from BASELINE): ↓ 1–4% DEH vs.EUH Protein: ↔ 1–2% DEH vs. EUH, ↑ 3–4% DEH vs. EUH RATINGS OF FATIGUE Lightheadedness, leg fatigue POST-EXERCISE/HEAT, END: ↔ 1–2% DEH vs. EUH, ↑ 3–4% DEH vs. EUH Windedness, hotness, muscle cramping POST-EXERCISE/HEAT: ↔ 1–2% DEH vs. EUH, ↑ 3–4% DEH vs. EUH END: ↔ 1–4% DEH vs. EUH Upper and total body fatigue POST-EXERCISE/HEAT: ↔ 1–2% DEH vs. EUH, ↑ 3–4% DEH vs. EUH END: ↔ 1–3% DEH vs. EUH, ↑ 4% DEH vs. EUH Side stitch/ache POST-EXERCISE/HEAT, END: ↔ 1–4% DEH vs. EUH BASKETBALL PERFORMANCE Comparison between EUH conditions No advantage of EUH-CES condition over EUH-PLA concerning basketball performance Comparisons between EUH and levels of DEH Baseline jump shots: ↔ 1–3% DEH vs. EUH, ↓ 4% DEH vs. EUH Lay-up shots: ↔ 1–2% DEH vs. EUH, ↓ 3–4% DEH vs. EUH Foul line jump shots: ↔ 1–4% DEH vs. EUH Total shots on the move: ↔1% DEH vs. EUH, ↓ 2–4% DEH vs. EUH, 20 court widths sprint: ↔ 1–2% DEH vs. EUH, ↑ 3–4% DEH vs. EUH Ladder suicide sprint: ↑ 1–4% DEH vs. EUH Total sprint time, all timed drills: ↔ 1% DEH vs. EUH, ↑ 2–4% DEH vs. EUH All shots: ↔ 1% DEH vs. EUH, ↓ 2–4% DEH vs. EUH RPE POST-EXERCISE/HEAT: ↔ 1–2% DEH vs. EUH, ↑ 3–4% DEH vs. EUH HALFTIME, END: ↔ 1–4% DEH vs. EUH |
| Carvalho et al. 2011 [53] | Three-arm randomized cross-over trial | M/12 | 14.8 ± 0.45 (14—15) | Three training sessions under distinct hydration conditions: (1) No fluid (NF) ingestion (2) Ad libitum ingestion of water (W, 3.8 mg∙L−1 Na) (3) Ad libitum ingestion of CES (7.2% sugar, 0.8% maltodextrin, 510 mg∙L−1 Na) | (1) Baseline evaluation (BM) (2) 90-min training (3) 30-min evaluation of basketball performance drills (4) Post-exercise evaluation (urine sampling, RPE, beverage acceptability) | BM loss: ↑ NF (−2.46 ± 0.87%) vs. W (−1.08 ± 0.67%) vs. CES (−0.65 ± 0.62%), ↑ W vs. CES Sweat rate, urine colour (UC): ↔ between conditions Fluid intake, beverage acceptability: ↔ W vs. CES RPE (6—20): ↑ NF vs. W, ↑ NF vs. CSB Basketball performance (2- and 3-point shooting, free-throw shooting, suicide sprints, defensive zigzag): ↔ between conditions |
| Dougherty et al. 2006 [54] | Three-arm randomized placebo-controlled double-blind cross-over trial | M/15 | 13.5 ± 1.3 (12—15) | (1) 2% DEH—DEH (2) EUH with CES (6% CHO and 18.0 mmol∙L−1 Na)—EUH-CES (3) EUH with a flavored water PLA (0% CHO and 18.0 mmol∙L−1 Na)–EUH-PLA | (1) BASELINE evaluation (urine sampling, BM, BP, HR, Tc) (2) Procedure of EUH/DEH obtaining via EXERCISE/HEAT exposure during interval-exercise protocol (6 bouts of 15-min treadmill/cycle ergometer exercise at 50%VO2 max with 5-min rests; temperature: 35 °C, relative humidity: 20%) (3) POST-EXERCISE/HEAT evaluation (urine sampling, BM, BP, HR, Tc, RPE, fluid intake, ratings of fatigue) (4) 1 h recovery (5) RECOVERY evaluation (urine sampling, BM, BP, HR, Tc, fluid intake) (6) Basketball drill test (4QRs × 12 min of drills with 10-min break at HALFTIME) (7) HALFTIME drill test (after 2nd QR) evaluation (same as at POST-EXERCISE/HEAT) (8) Post-drill test (after 4th QR) evaluation (POST-EXERCISE/HEAT)—END | PHYSIOLOGICAL VARIABLES HR POST-EXERCISE/HEAT: ↑ DEH vs. EUH-PLA, ↑ DEH vs. EUH-CES RECOVERY, HALFTIME: ↔ DEH vs. EUH-PLA, ↔ DEH vs. EUH-CES END: ↑ DEH vs. EUH-PLA, ↔DEH vs. EUH-CES Tc POST-EXERCISE/HEAT: ↑ DEH vs. EUH-PLA, ↑ DEH vs. EUH-CES RECOVERY, HALFTIME: ↔ DEH vs. EUH-PLA, ↔ DEH vs. EUH-CES END: ↑ DEH vs. EUH-PLA, ↑ DEH vs. EUH-CES MAP POST-EXERCISE/HEAT, RECOVERY: ↔ DEH vs. EUH-PLA, ↔ DEH vs. EUH-CES RPE POST-EXERCISE/HEAT: ↑ DEH vs. EUH-PLA, ↑ DEH vs. EUH-CES HALFTIME, END: ↔ DEH vs. EUH-PLA, ↔ DEH vs. EUH-CES RATINGS OF FATIGUE Lightheadedness POST-EXERCISE/HEAT: ↑ DEH vs. EUH-PLA, ↑ DEH vs. EUH-CES HALFTIME, END: ↔ DEH vs. EUH-PLA, ↑ DEH vs. EUH-CES Windedness, hotness POST-EXERCISE/HEAT: ↔ DEH vs. EUH-PLA, ↑ DEH vs. EUH-CES HALFTIME, END: ↔ DEH vs. EUH-PLA; ↔ DEH vs. EUH-CES Upper-body fatigue POST-EXERCISE/HEAT: ↔ DEH vs. EUH-PLA, ↔ DEH vs. EUH-CES HALFTIME: ↑ DEH vs. EUH-PLA, ↑ DEH vs. EUH-CES END: ↔ DEH vs. EUH-PLA, ↑ DEH vs. EUH-CES Total body fatigue POST-EXERCISE/HEAT, HALFTIME: ↔ DEH vs. EUH-PLA, ↔ DEH vs. EUH-CES END: ↔ DEH vs. EUH-PLA, ↑ DEH vs. EUH-CES Side stitch/ache, muscle cramping, leg fatigue POST-EXERCISE/HEAT, HALFTIME, END: ↔ DEH vs. EUH-PLA, ↔ DEH vs. EUH-CES BASKETBALL PERFORMANCE Around the world shots, free throws: ↔ DEH vs. EUH-PLA; ↓ DEH vs. EUH-CES 3-point shots: ↓ DEH vs. EUH-PLA, ↓ DEH vs. EUH-CES Combined shooting: ↓ DEH vs. EUH-PLA, ↓ DEH vs. EUH-CES, ↑ EUH-CES vs. EUH PLA Short-range (layups) shooting: ↔ between conditions 10 widths sprinting: ↑ DEH vs. EUH-PLA, ↑ DEH vs. EUH-CES, ↔ EUH-CES vs. EUH-PLA Suicides sprinting, average and total sprints’ times: ↑ DEH vs. EUH-PLA, ↑ DEH vs. EUH-CES, ↓ EUH-CES vs. EUH-PLA Lateral movement drills—zigzags, lane slides, average and total lateral movements’ times: ↑ DEH vs. EUH-PLA, ↑ DEH vs. EUH-CES, ↔ EUH-CES vs. EUH-PLA Individual full-court combination times: ↔ between conditions Individual key combination times: ↑ DEH vs. EUH-PLA, ↑ DEH vs. EUH-CES Average and total defensive drills’ times: ↔ DEH vs. EUH-PLA, ↑ DEH vs. EUH-CES Time to complete 10 vertical jumps (VJ) and maximum VJ height: ↔ between conditions |
| Hoffman et al. 1995 [55] | Two-arm balanced cross-over design | M/10 | 17.3 ± 0.9 | Two stimulated ‘2 × 2 full-court’ basketball games under distinct hydration conditions: (1) drinking water permitted—Wa (2) restriction from any fluid consumption—NWa Ambient temperature: 20.8 ± 0.9 °C, relative humidity: 0.64 ± 0.05% | (1) 15-min standardized warm-up (2) PRE evaluation of dynamic strength of the lower limb and anaerobic power (3) Game—1st half (field goal attempts [FGA] and free throw attempts [FTA] evaluation, BM measurements [at 7, 14 and 20 min]) (4) HALF-game evaluation of dynamic strength of the lower limb and anaerobic power (5) 15-min break (6) Game—2nd half (FGA, FTA, BM at 7, 14, and 20 min)—POST | BM loss at NWa condition (compared to PRE evaluation) HALF: −1.1 ± 0.4% POST: −1.9 ± 0.4% DYNAMIC STRENGTH OF THE LOWER EXTREMITY Squat jump and countermovement jump heights: ↔ Wa vs. NWa at any time point ANAEROBIC POWER AND CAPACITY Anaerobic power, number of jumps, average jump height: ↔ Wa vs. NWa at any time point BASKETBALL PERFORMANCE FGA, FTA: ↔ Wa vs. NWa |
| Hoffman et al. 2012 [56] | Four-arm double-blind cross-over design | F/10 | 21.2 ± 1.6 | Four 40-min basketball games under distinct hydration conditions: (1) no drinking allowed—DHY (2) water allowed—W (3) water combined with L-alanyl-glutamine—1 g per 500 mL—AG1 (4) water combined with L-alanyl-glutamine—2 g per 500 mL—AG2 At conditions 2–3 fluid intake was adjusted to pre-evaluated fluid loss (during DHY condition) Environmental conditions Temperature: 22.6 ± 0.19 °C Relative humidity: 50.9 ± 3.1% | (1) 10-min dynamic typical warm-up (2) PRE-game testing battery (power [countermovement jump, CMJ], reaction [lower-body and hand-eye reaction time], basketball shooting circuit—5 shoots from 6 different locations on the court) (3) Game (HR, game load assessment) (4) POST-game testing battery: CMJ, reaction, and basketball shooting assessment | BM loss at DHY condition: ~−2.3% (−1.72 ± 0.42 kg) Fluid intake: ↔ W, AG1, AG2 BASKETBALL PERFORMANCE Δ POST-PRE in field goal shooting performance: ↓ DHY vs. AG1, ↓ W vs. AG1, ↔ between remaining conditions REACTION Δ POST-PRE in lower-body reaction (number of successful attempts): ↓ DHY vs. W, ↓ DHY vs. AG1, ↓ DHY vs. AG2, ↔ between remaining conditions Δ POST-PRE in visual reaction time: ↓ DHY vs. AG1, ↔ between remaining study conditions Δ POST-PRE in motor reaction time: ↔ between conditions Δ POST-PRE in physical reaction time: ↓ DHY vs. AG1, ↔ between remaining study conditions POWER Δ POST-PRE in peak and mean VJ power: ↔ between conditions Player load: ↑ AG2 vs. DHY, ↔ between remaining study conditions HR: ↔ between study conditions |
| Louis et al. 2018 [57] | Two-arm randomized cross-over trial | M/9 | 16.2 ± 0.7 | Two basketball trails under distinct hydration conditions: (1) EUH (2) DEH (~−2% BM) | (1) EUH/DEH obtaining procedure (60 min of low-intensity [90 ± 10 W] in an environmental chamber at 39 °C (2) 10-min rest (3) Habitual warm-up (4) Three-point shots test (success rate, shooting technique analysis, RPE) | Success rate and number of throws (per minute) in three-point shots test: ↔ DEH vs. EUH RPE: ↑ DEH vs. EUH Variables of body kinematics and ball release during three-point shots test: ↔ DEH vs. EUH |
| Minehan et al. 2002 [58] | Three-arm randomized cross-over design | M/8 F/7 | - | Nine training sessions under three distinct hydration conditions (3 trainings per condition): (1) WATER (2) CES (6.8% CHO, 1130 kJ∙L−1, 18.7 mmol∙L−1 Na, 2 mmol∙L−1 K) (3) low energy-electrolyte beverage (LKEB; 1% CHO, 170 kJ L−1, 18.7 mmol L−1 Na, 3 mmol L−1 K) | Ad libitum intake of WATER/CES/LKEB during nine trainings characterized by similar time and structure and undertaken in comparable environmental conditions (temperature 17.8 ± 0.9 °C, relative humidity 40.4 ± 8.1%). Evaluation of fluid intake, sweat loss, fluid balance | Fluid intake, sweat loss: ↔ between any fluids in M and F Fluid balance in M: ↑ CES vs. WATER, ↔ LKEB vs. WATER, ↔ CES vs. LKEB Fluid balance in F: ↑ CES vs. WATER, ↑ LKEB vs. WATER, ↔ CES vs. LKEB |
| Taim et al. 2021 [49] | Parallel group randomized between-subject design | M/18 | 23.1 ± 1.3 | 3 × 3 small-sided basketball game with participants divided into two groups consuming: (1) colourless, flavoured water (without CHO; sweetened with acesulfame K and sucralose, and containing negligible amounts of Na [less than 10 mg per 250 mL])—FW (2) plain water—PW Environmental conditions Temperature: 31.7 ± 0.5 °C Relative humidity: 62 ± 4% | (1) PRE-GAME evaluation (urine sampling, BM, fluid palatability, RPE, thirst) (2) standardized warm-up (3) 40-min GAME (HR, RPE, and thirst evaluation) (4) POST-GAME evaluation (BM, urine sampling) | PALATABILITY RATINGS Hedonic rating, sweetness, saltiness, sourness fluid: ↑ FW vs. PW HYDRATION STATE Fluid consumption, sweat rate: ↔ FW vs. PW BM loss: ↔ FW (−0.941 ± 0.524%) vs. PW (−0.534 ± 0.376%) HR: ↔ FW vs. PW RPE: ↔ FW vs. PW BASKETBALL PERFORMANCE 2-point and 3-point field-goal percentage, number of assists and defensive rebounds: ↔ FW vs. PW |
| Reference | Study Design | Gender/n | Age (years) | Dietary Intervention | Experimental Procedures † | Outcomes |
|---|---|---|---|---|---|---|
| Afman et al. 2014 [59] | Two-arm randomized cross-over counterbalanced placebo-controlled trial | M/10 | 20 ± 1 | Single acute ingestion of 75 g carbohydrate (CHO) as sucrose dissolved in 500 mL of sugar-free, orange-flavored, artificially sweetened beverage (CHO-SOL) 45 min before exercise vs. ingestion of volume, taste- and colour-matched placebo (PLA) | (1) BASELINE body mass (BM) measurement and blood sampling (glucose, lactate analyses) (2) CHO-SOL/PLA ingestion (3) 45-min rest (4) PRE-EXERCISE blood sampling (5) Basketball stimulation test—a modified version of Loughborough Intermittent Shuttle Test (LIST)—blood sampling and rate of perceived exertion (RPE) at 5 min of the 1st quarter (QR) and after the 1st QR, 2nd QR, 3rd QR, and 4th QR | BLOOD VARIABLES Glucose BASELINE: ↔ CHO-SOL vs. PLA PRE-EXERCISE: ↑ CHO-SOL vs. PLA at 5 min of 1st QR, 1st QR: ↓ CHO-SOL vs. PLA 2nd, 3rd, 4th QR: ↔ CHO-SOL vs. PLA Lactate: ↔ CHO-SOL vs. PLA at any time point BASKETBALL PERFORMANCE (LIST) Layup shooting 1st QR and overall mean: ↓ CHO-SOL vs. PLA 2nd, 3rd, 4th QR: ↔ CHO-SOL vs. PLA 20 m sprint time 1st QR: ↑ CHO-SOL vs. PLA 2nd, 3rd QR and overall mean: ↔ CHO-SOL vs. PLA 4th QR: ↓ CHO-SOL vs. PLA |
| Daniel et al. 2019 [60] | Two-arm randomized cross-over trial | M/9 | 18.0 ± 0.7 | Single ingestion of high-glycemic index (HGI; glycemic index [GI]) 71.8—74.9) versus low-glycemic index (LGI; GI 47.9–49.5) dinner and evening snacks across two consecutive days during competition Ad libitum intake of food during remaining meals | The day before competition: (1) 3-h fast (2) BEFORE DINNER (BD) evaluation of sleepiness (Epworth Sleepiness Scale [ESS], visual analogue scale [VAS]) and satiety (VAS); saliva sampling (melatonin, cortisol) (3) HGI/LGI dinner at 19:00 (4) blood sampling before (BD), 30 and 60 min after the start of HGI/LGI (5) AFTER DINNER (AD) evaluation of sleepiness and satiety (6) ~9:30 p.m. HGI/LGI evening snack (7) BEFORE SLEEP (BS) saliva sampling (8) Actigraph monitoring of sleep pattern Day of competition: (9) AFTER AWAKING (AA) evaluation of sleepiness, saliva sampling (free awaking between 7:00 and 8:00 a.m.) (10) BEFORE BREAKFAST (BB) evaluation of sleepiness and satiety, saliva sampling (11) Game (between 9:00 and 12:00 a.m.) | ENERGY AND MACRONUTRIENT INTAKE CHO (g∙kg−1): ↑ HGI vs. LGI Energy, protein (PRO), fat, CHO as % of energy intake (EI): ↔ HGI vs. LGI GLYCEMIC RESPONSE TO HGI/LGI DINNER Area under the curve: ↑ HGI vs. LGI SATIETY at BD, AD, BB: ↔ HGI vs. LGI SALIVA HORMONES Melatonin, cortisol at BD, BS, AA, BB: ↔ HGI vs. LGI SLEEPINESS Based on VAS at BD, AD, BB: ↔ HGI vs. LGI Based on ESS at BD, BB: ↔ HGI vs. LGI Sleep pattern (nocturnal and daytime sleep time, sleep latency, sleep efficiency, wake after sleep onset): ↔ HGI vs. LGI |
| Gentle et al. 2014 [61] | Two-arm randomized cross-over trial | M/10 | 22 ± 2 | Single ingestion of CHO (1gCHO∙kgBM−1) in conjunction with PRO (1gPRO∙kgBM −1; CHO-PRO) vs. CHO alone (2gCHO∙kgBM −1, CHO) 90 min before 87-min exercise protocol | (1) BASELINE fasting blood and saliva sampling (2) Ingestion of CHO-PRO/CHO meals (3) Anthropometric measurements (4) Heart rate (HR) recording during the entire protocol (5) PRE-EXERCISE urine and blood sampling, evaluation of gastrointestinal upset (GIU) and muscle soreness (MS) (6) Warm-up (7) Basketball performance protocol (4 QRs × 15 min with 15-min rest after 2nd QR) (8) DURING-EXERCISE (after 2nd QR) blood sampling, evaluation of RPE, MS, GIU (9) POST-EXERCISE blood, urine, and saliva sampling, evaluation of RPE, MS, GIU (10) 30-min post-exercise venous blood sampling (11) AFTER 24 h venous blood, urine, and saliva sampling, evaluation of RPE, MS, GIU | PHYSIOLOGICAL VARIABLES Mean and peak HR: ↔ CHO-PRO vs. CHO BLOOD VARIABLES Lactate PRE-, DURING-, POST-EXERCISE: ↔ CHO-PRO vs. CHO Glucose PRE-EXERCISE: ↔ CHO-PRO vs. CHO DURING-, POST-EXERCISE: ↑ CHO-PRO vs. CHO Mean Δ in creatine kinase (CK) activity BASELINE to POST-EXERCISE: ↓ CHO-PRO vs. CHO BASELINE to AFTER 24 h, POST-EXERCISE to AFTER 24 h: ↔ CHO-PRO vs. CHO SALIVA HORMONES CONCENTRATIONS Cortisol BASELINE, AFTER 24 h: ↔ CHO-PRO vs. CHO POST-EXERCISE: ↑ CHO-PRO vs. CHO Testosterone: ↔ CHO-PRO vs. CHO at any time point BASKETBALL PERFORMANCE Mean jump height, sprint time: ↔ CHO-PRO vs. CHO at any time point Mean success rate for the first two free throw attempts: ↑ CHO-PRO vs. CHO at 4th QR (no differences at any other time point) MS (upper, lower, and whole body): ↔ CHO-PRO vs. CHO at any time point GASTROINTESTINAL UPSET Increase in nausea and belching from BASELINE to DURING-EXERCISE: ↑ CHO-PRO vs. CHO Increase in nausea and stomach bloating from BASELINE to POST-EXERCISE: ↑ CHO-PRO vs. CHO RPE at 1st QR and 4th QR: ↑ CHO-PRO vs. CHO (no differences at any other time point) |
| Ghiasvand et al. 2010 [62] | Three-arm randomized double-blind placebo-controlled parallel group clinical trial | M/34 | 24 * (15–35) | 6 weeks of supplementation with: (1) 2 g of eicosapentaenoic acid (EPA) + 400 IU vitamin E—EPA + Vit E (n = 8) vs. (2) 2 g EPA + PLA (n = 9) vs. (3) 400 IU Vit E + PLA (n = 9) vs. (4) PLA + PLA (n = 8) | Venous blood samples (for interleukin 2 [IL-2], tumor necrosis factor alfa [TNF-α], and malonylodialdehyde [MDA] concentration, catalase, and glutathione reductase activity) between 5:00 and 6:00 p.m., after intensive endurance exercising for 2 h, at the BASELINE and AFTER 6-week supplementation | BASELINE vs. AFTER comparisons TNF-α in EPA + Vit E: ↓ AFTER vs. BASELINE MDA in EPA + PLA and Vit E + PLA: ↓ AFTER vs. BASELINE Glutathione reductase activity in EPA + Vit E and Vit E + PLA: ↓ AFTER vs. BASELINE No differences in the remaining groups and/or remaining variables BETWEEN GROUPS COMPARISONS AFTER SUPPLEMENTATION IL-2: ↑ EPA + Vit E vs. EPA + PLA; ↑ EPA + Vit E vs. Vit E + PLA; ↓ EPA + Vit E vs. PLA + PLA Glutathione reductase activity: ↓ EPA + PLA vs. Vit E + PLA No between-group differences in the remaining groups and/or variables |
| Ho et al. 2018 [63] | Two-arm randomized, placebo-controlled counterbalanced cross-over trial | -/15 | 18–20 | Single oral ingestion of 600 mL of high-PRO (36% PRO, 58% CHO, 6% FAT in total energy) versus low-PRO (12% PRO, 63% CHO, 25% FAT) isoenergetic drink (6.25 kcal∙kg−1) immediately after 1 h endurance cycling at 70% of maximal oxygen uptake (VO2max) | (1) PRE-exercise fasting blood sampling (2) 1 h endurance cycling at 70% VO2max (3) consumption of high-PRO/low-PRO drink (4) blood sampling immediately POST-ingestion of a drink and every 30 min until 2 h recovery completion (30, 60, 90, and 120 min) (5) Endurance time trial (TT) on the cycloergometer at 80% VO2max—simultaneous monitoring of cerebral hemodynamic response | Glucose PRE, POST, 30 min: ↔ high-PRO vs. low-PRO 60, 90, 120 min: ↓ high-PRO vs. low-PRO Insulin PRE, POST, 60, 90, 120 min: ↔ high-PRO vs. low-PRO 30 min: ↑ high-PRO vs. low-PRO Time to exhaustion in cycling TT: ↑ high-PRO vs. low-PRO Percent oxygen saturation in the frontal brain at 60, 120, 180, 240, and 300 s of cycling TT: ↑ high-PRO vs. low-PRO Blood perfusion (total hemoglobin) to the brain during cycling at 60, 120, 180, 240, and 300 s of cycling TT: ↓ high-PRO vs. low-PRO |
| Marques et al. 2015 [64] | Single-arm intervention | M/8 | 33.8 ± 8.3 | 30 days supplementation with 3 g of fish oil (1500 mg docosahexaenoic acid, 300 mg EPA, and 6 mg vitamin E) | Pre- (S0) and post-supplementation (S1) resting (REST) and after ACUTE EXERCISE blood sample analysis | LIPID PROFILE Total cholesterol S0: ↑ ACUTE EXERCISE vs. REST S1: ↔ ACUTE EXERCISE vs. REST LDL- and HDL-Chol, triglycerides: no effect of supplementation or exercise MUSCLE DAMAGE Lactate dehydrogenase activity S0: ↑ ACUTE EXERCISE vs. REST(55.4% increase) S1: ↔ ACUTE EXERCISE vs. REST CK activity: no effect of supplementation or exercise INFLAMMATORY MEDIATORS Interleukin 6 (IL-6), Interleukin 1ra (IL-1ra) S0: ↑ ACUTE EXERCISE vs. REST S1: ↔ ACUTE EXERCISE vs. REST ACUTE EXERCISE: ↓ S1 vs. S0 Interleukin 8 (IL-8) S0: ↔ ACUTE EXERCISE vs. REST S1: ↑ ACUTE EXERCISE vs. REST C-reactive protein, TNF-α, interleukin 1β (IL-1β), interleukin 4 (IL-4): no effect of supplementation or exercise NEUTROPHIL FUNCTION AND DEATH IL-6 production by lipopolysaccharide-stimulated neutrophils S0: ↓ ACUTE EXERCISE vs. REST S1: ↔ ACUTE EXERCISE vs. REST IL-8, TNF-α, IL-1ra, IL-1β, IL-4 production by unstimulated and stimulated neutrophils: no effect of supplementation or exercise Pathogenic capacity of neutrophils: S0: ↓ ACUTE EXERCISE vs. REST S1: ↔ ACUTE EXERCISE vs. REST REST: ↓ S1 vs. S0 ACUTE EXERCISE: ↔ S1 vs. S0 Percentage of cells with loss of membrane integrity: S0: ↑ ACUTE EXERCISE vs. REST S1: ↔ ACUTE EXERCISE vs. REST REST: ↑ S1 vs. S0 ACUTE EXERCISE: ↓ S1 vs. S0 Percentage of cells with phosphatidylserine externalization and with DNA fragmentation: no effect of supplementation or exercise Reactive oxygen species (ROS) production by unstimulated neutrophils: no effect of supplementation or exercise ROS production by stimulated neutrophils, accumulation of neutral lipids: S0: ↑ ACUTE EXERCISE vs. REST S1: ↔ ACUTE EXERCISE vs. REST REST: ↑ S1 vs. S0 ACUTE EXERCISE: ↔ S1 vs. S0 Mitochondrial membrane potential: S0: ↑ ACUTE EXERCISE vs. REST S1: ↔ ACUTE EXERCISE vs. REST REST: ↔ S1 vs. S0 ACUTE EXERCISE: ↓ S1 vs. S0 |
| Michalczyk et al. 2018 [65] | Single-arm intervention | M/11 | 24.27 ± 2.6 | 4 weeks of low CHO diet (LCD; ~10% EI from CHO, ~31% PRO and 59% FAT) followed by 7 days of CHO loading (Carbo-L; 75% CHO, ~16% PRO, ~9% FAT). Conventional diet (CD; ~54% CHO, ~15% PRO, ~31% FAT) 1 month prior to the experiment | Measurements taken before (CD) and after 4-week LCD, as well as after the 7-day Carbo-L | BODY MASS (BM) and BODY COMPOSITION BM, fat-free mass (FFM, kg): ↔ LCD vs. CD, ↔ Carbo-L vs. CD Body fat (%), fat mass (FM, kg): ↓ LCD vs. CD, ↓ Carbo-L vs. CD BLOOD VARIABLES Triglycerides: ↓ LCD vs. CD, ↑ Carbo-L vs. CD Glucose: ↔ LCD vs. CD, ↑ Carbo-L vs. CD Total-, HDL-, and LDL-cholesterol, insulin, homeostasis model assessment-estimated insulin resistance (HOMA-IR): ↔ LCD vs. CD, ↔ Carbo-L vs. CD |
| Michalczyk et al. 2019 [66] | Single-arm intervention | M/15 | 23.5 ± 2.2 | 4 weeks of low CHO diet (LCD; ~10% EI from CHO, ~31% PRO and 59% fat) followed by 7 days of CHO loading (Carbo-L; 75% CHO, ~16% PRO, ~9% FAT). Conventional diet (CD; ~54% CHO, ~15% PRO, ~31% FAT) 1 month prior to the experiment | Measurements taken before (CD) and after 4-week LCD, as well as after the 7-day Carbo-L Measurements taken at REST and POST-EXERCISE (after the 30 s Wingate Anaerobic Test for lower limbs) | BM and BODY COMPOSITION BM: ↓ LCD vs. CD, ↔ Carbo-L vs. CD, ↔ Carbo-L vs. LCD FFM (kg): ↔ LCD vs. CD, ↔ Carbo-L vs. CD, ↑ Carbo-L vs. LCD FM (%): ↓ LCD vs. CD, ↔ Carbo-L vs. CD, ↔ Carbo-L vs. LCD ANAEROBIC PERFORMANCE Peak power (PP), time to PP: no differences between any condition Total work: ↓ LCD vs. CD, ↔ Carbo-L vs. CD, ↑ Carbo-L vs. LCD BLOOD ACID-BASE BALANCE Lactate, pH at REST: ↓ LCD vs. CD, ↔ Carbo-L vs. CD, ↑ Carbo-L vs. LCD Lactate, pH at POST-EXERCISE: no differences between diets Bicarbonate at REST and POST-EXERCISE: no differences between diets β-HYDROXYBUTYRATE at REST: ↑ LCD vs. CD, ↔ Carbo-L vs. CD, ↓ Carbo-L vs. LCD HORMONES Testosterone: ↑ LCD vs. CD, ↑ Carbo-L vs. CD, ↔ Carbo-L vs. LCD Growth hormone: ↑ LCD vs. CD, ↔ Carbo-L vs. CD, ↓ Carbo-L vs. LCD Insulin: ↓ LCD vs. CD; ↔ Carbo-L vs. CD, ↑ Carbo-L vs. LCD Cortisol: no differences between diets |
| Ronghui 2015 [67] | Two-arm randomized parallel-group placebo-controlled study | M/10 | - | 30 days supplementation with 20 g whey PRO and 40 g oligosaccharides once every two days dissolved in 250 mL of whole milk 30 min before bed-time (NUTR) versus 250 mL of whole milk in the same manner (CTRL) | Measurements BEFORE and AFTER the supplementation period taken immediately after the incremental cycling test | Haemoglobin, red blood cells, haematocrit at AFTER: ↑ NUTR vs. CTRL Mean corpuscular volume at AFTER: ↓ NUTR vs. CTRL Mean corpuscular volume in CTRL: ↑ AFTER vs. BEFORE |
| Shi 2005 [68] | Two-arm parallel-group placebo-controlled study | M/10 | 19–23 | 10 days (or 9) supplementation with Weichuan high-octane solid beverage (CHO content 100g∙L−1, 500 mL every day, CHO-SOL, n = 5) versus no-energy, colour-, appearance- and taste-matched placebo solution (PLA, n = 5) Preparations were ingested in three parts (at 7:00, 12:30, and 19:00), each part diluted in 200 mL of water | Measurements BEFORE and AFTER supplementation period, before (PRE-EXERCISE), after exercise (POST-EXERCISE), and 30 min POST-EXERCISE | Δ in blood urea nitrogenAFTERsupplementation between PRE- and POST-EXERCISE: ↓ CHO-SOL vs. PLA Δ in CK activity AFTER supplementation between PRE- and POST-EXERCISE: ↓ CHO-SOL vs. PLA Glucose at AFTER: PRE-EXERCISE: ↔ CHO-SOL vs. PLA POST-EXERCISE: ↑ CHO-SOL vs. PLA 30 min POST-EXERCISE: ↔ CHO-SOL vs. PLA Lactate at AFTER: PRE-EXERCISE, 5 and 10 min POST-EXERCISE: ↔ CHO-SOL vs. PLA |
| Taylor et al. 2016 [69] | Two-arm randomized placebo-controlled double-blind parallel group study | F/14 | 20 ± 2 (WP) 21 ± 3 (MD) | 8 weeks of supplementation of 2 × 24 g whey PRO (WP; n = 8) versus maltodextrin (MD; n = 6) ingested immediately pre- and post-training (4 days/per week anaerobic and resistance training) during the pre-season part of training season | Measurements before (T1) and after (T2) supplementation (body composition, 1-repetition maximum [1RM] bench press [BP], 1RM leg press [LP], vertical jump [VJ], broad jump [BJ], 5-10-5 agility time) | BM and BODY COMPOSITION BM: no group, time, or group x time interaction Lean mass at T1 and T2: ↔ WP vs. MD Lean mass in WP: ↑ T2 vs. T1, lean mass in MD: ↔ T2 vs. T1 Increase in lean mass (T2–T1): ↑ WP vs. MD Fat mass at T1 and T2: ↔ WP vs. MD Fat mass in WP: ↓T2 vs. T1 Fat mass in MD: ↔ T2 vs. T1 PHYSICAL PERFORMANCE 1 RM BP, 1 RM LP, VJ, BJ, 5-10-5 agility drill at T1 and T2: ↔ WP vs. MD 1 RM BP, 1 RM LP, VJ in WP and MD: ↑ T2 vs. T1 Increase in 1 RM BP: ↑ WP vs. MD BJ in WP: ↑ T2 vs. T1, BJ in MP: ↔ T2 vs. T1 5-10-5 agility drill time in WP:↓ T2 vs. T1 and in MD: ↔ T2 vs. T1 |
| Wilborn et al. 2013 [70] | Two-arm randomized double-blind parallel group study | F/16 | 20.0 ± 1.9 (WP) 21.0 ± 2.8 (CP) | 8 week supplementation with 2 × 24 g whey PRO (WP; n = 8) versus 2 × 24 g casein PRO (CP; n = 8) 30 min before and immediately after training | Measurements before (T1) and after (T2) supplementation (body composition, 1RM BP, 1RM LP, VJ, BJ, 5-10-5 agility time) | BM and BODY COMPOSITION Body fat (%), FM (kg) in WP and CP: ↓ T2 vs. T1 Lean mass (kg) in WP and CP: ↑ T2 vs. T1 BASKETBALL PERFORMANCE 1RM BP, 1 RM LP, VJ, BJ in WP and CP:↑ T2 vs. T1 5-10-5 agility drill time in WP and CP: ↓ T2 vs. T1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowaczyk, P.M.; Adamczewski, J.; Durkalec-Michalski, K. Practical Application and Methodological Considerations on the Basics of Sports Nutrition in Basketball: A Comprehensive Systematic Review of Observational and Interventional Studies. Nutrients 2023, 15, 4484. https://doi.org/10.3390/nu15204484
Nowaczyk PM, Adamczewski J, Durkalec-Michalski K. Practical Application and Methodological Considerations on the Basics of Sports Nutrition in Basketball: A Comprehensive Systematic Review of Observational and Interventional Studies. Nutrients. 2023; 15(20):4484. https://doi.org/10.3390/nu15204484
Chicago/Turabian StyleNowaczyk, Paulina M., Jakub Adamczewski, and Krzysztof Durkalec-Michalski. 2023. "Practical Application and Methodological Considerations on the Basics of Sports Nutrition in Basketball: A Comprehensive Systematic Review of Observational and Interventional Studies" Nutrients 15, no. 20: 4484. https://doi.org/10.3390/nu15204484
APA StyleNowaczyk, P. M., Adamczewski, J., & Durkalec-Michalski, K. (2023). Practical Application and Methodological Considerations on the Basics of Sports Nutrition in Basketball: A Comprehensive Systematic Review of Observational and Interventional Studies. Nutrients, 15(20), 4484. https://doi.org/10.3390/nu15204484








