The Role of Human Milk Oligosaccharides in Myelination, Socio-Emotional and Language Development: Observational Data from Breast-Fed Infants in the United States of America
Abstract
:1. Introduction
The Current Study
2. Materials and Methods
2.1. Study Population and Data
2.2. Human Milk Samples
2.3. Cognitive Assessments
2.4. Neuroimaging
2.5. Analytical Methods for Human Milk Quantification
2.6. Statistical Analysis
3. Results
3.1. Population and Demographics
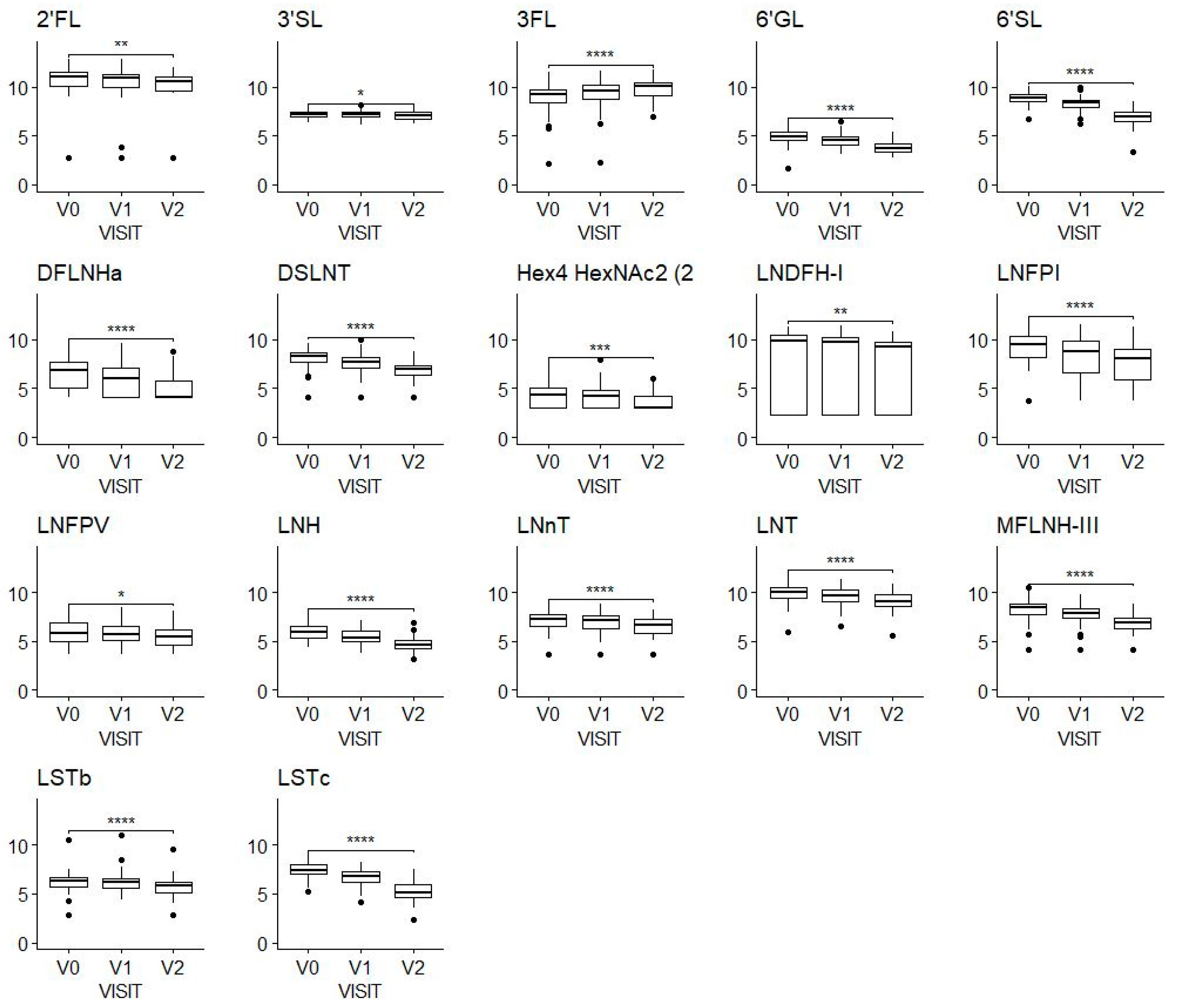
3.2. HMOs
3.3. Correlations between Human Milk HMO Concentration and ASQ-SE:2
3.4. Correlations between Human Milk HMO Concentration and Myelination
3.5. Does Myelination Mediate the Relationship between 6′SL and Social-Emotional Development?
3.6. Correlations between Human Milk HMO Concentration and BAYLEY-III Scores
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, M.H. Functional Brain Development in Humans. Nat. Rev. Neurosci. 2001, 2, 475–483. [Google Scholar] [CrossRef]
- Belfort, M.B.; Anderson, P.J.; Nowak, V.A.; Lee, K.J.; Molesworth, C.; Thompson, D.K.; Doyle, L.W.; Inder, T.E. Breast Milk Feeding, Brain Development, and Neurocognitive Outcomes: A 7-Year Longitudinal Study in Infants Born at Less Than 30 Weeks’ Gestation. J. Pediatr. 2016, 177, 133–139.e1. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.J.; Tang, J. A Review on the Relationship between Breast Milk Nutrients and Brain Development in Preterm Infants. Chin. J. Contemp. Pediatr. 2019, 21, 607–612. [Google Scholar]
- Hobbs, M.; Jahan, M.; Ghorashi, S.A.; Wang, B. Current Perspective of Sialylated Milk Oligosaccharides in Mammalian Milk: Implications for Brain and Gut Health of Newborns. Foods 2021, 10, 473. [Google Scholar] [CrossRef]
- Krol, K.M.; Rajhans, P.; Missana, M.; Grossmann, T. Duration of Exclusive Breastfeeding Is Associated with Differences in Infants’ Brain Responses to Emotional Body Expressions. Front. Behav. Neurosci. 2015, 8, 459. [Google Scholar] [CrossRef] [PubMed]
- Daniels, M.C.; Adair, L.S. Breast-Feeding Influences Cognitive Development in Filipino Children. J. Nutr. 2005, 135, 2589–2595. [Google Scholar] [CrossRef]
- Deoni, S.; Dean, D.; Joelson, S.; O’Regan, J.; Schneider, N. Early Nutrition Influences Developmental Myelination and Cognition in Infants and Young Children. Neuroimage 2018, 178, 649–659. [Google Scholar] [CrossRef]
- Schneider, N.; Hauser, J.; Oliveira, M.; Cazaubon, E.; Mottaz, S.C.; O’Neill, B.V.; Steiner, P.; Deoni, S.C.L. Sphingomyelin in Brain and Cognitive Development: Preliminary Data. eNeuro 2019, 6, 1–13. [Google Scholar] [CrossRef]
- Bode, L.; Jantscher-Krenn, E. Structure-Function Relationships of Human Milk Oligosaccharides. Adv. Nutr. 2012, 3, 383S–391S. [Google Scholar] [CrossRef]
- Donovan, S.M.; Comstock, S.S. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann. Nutr. Metab. 2017, 69, 41–51. [Google Scholar] [CrossRef]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Impact of Human Milk Bacteria and Oligosaccharides on Neonatal Gut Microbiota Establishment and Gut Health. Nutr. Rev. 2015, 73, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Sugawara, M.; Kawakami, H. Sialic Acid in Human Milk: Composition and Functions. Acta Paediatr. Taiwanica 2001, 42, 11–17. [Google Scholar]
- Clouard, C.; Reimert, I.; Fleming, S.A.; Koopmans, S.J.; Schuurman, T.; Hauser, J. Dietary Sialylated Oligosaccharides in Early-Life May Promote Cognitive Flexibility during Development in Context of Obesogenic Dietary Intake. Nutr. Neurosci. 2022, 25, 2461–2478. [Google Scholar] [CrossRef] [PubMed]
- Hauser, J.; Pisa, E.; Arias Vásquez, A.; Tomasi, F.; Traversa, A.; Chiodi, V.; Martin, F.P.; Sprenger, N.; Lukjancenko, O.; Zollinger, A.; et al. Sialylated Human Milk Oligosaccharides Program Cognitive Development through a Non-Genomic Transmission Mode. Mol. Psychiatry 2021, 26, 2854–2871. [Google Scholar] [CrossRef]
- Oliveros, E.; Ramirez, M.; Vazquez, E.; Barranco, A.; Gruart, A.; Delgado-Garcia, J.M.; Buck, R.; Rueda, R.; Martin, M.J. Oral Supplementation of 2’-Fucosyllactose during Lactation Improves Memory and Learning in Rats. J. Nutr. Biochem. 2016, 31, 20–27. [Google Scholar] [CrossRef]
- Pisa, E.; Martire, A.; Chiodi, V.; Traversa, A.; Caputo, V.; Hauser, J.; Macrì, S. Exposure to 3′sialyllactose-poor Milk during Lactation Impairs Cognitive Capabilities in Adulthood. Nutrients 2021, 13, 4191. [Google Scholar] [CrossRef]
- Tarr, A.J.; Galley, J.D.; Fisher, S.E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The Prebiotics 3’Sialyllactose and 6′Sialyllactose Diminish Stressor-Induced Anxiety-like Behavior and Colonic Microbiota Alterations: Evidence for Effects on the Gut-Brain Axis. Brain Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef]
- Berger, P.K.; Plows, J.F.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Poulsen, M.; Ryoo, J.H.; Peterson, B.S.; Bode, L.; Goran, M.I. Human Milk Oligosaccharide 2’-Fucosyllactose Links Feedings at 1 Month to Cognitive Development at 24 Months in Infants of Normal and Overweight Mothers. PLoS ONE 2020, 15, e0228323. [Google Scholar] [CrossRef]
- Berger, P.; Bansal, R. Associations of Human Milk Oligosaccharides with Infant Brain Tissue Organization and Regional Blood Flow at 1 Month of Age. Nutrients 2022, 14, 3820. [Google Scholar] [CrossRef]
- Cho, S.; Zhu, Z.; Li, T.; Baluyot, K.; Howell, B.R.; Hazlett, H.C.; Elison, J.T.; Hauser, J.; Sprenger, N.; Wu, D.; et al. Human Milk 3’-Sialyllactose Is Positively Associated with Language Development during Infancy. Am. J. Clin. Nutr. 2021, 114, 588–597. [Google Scholar] [CrossRef]
- Oliveros, E.; Martín, M.J.; Torres-Espínola, F.J.; Segura-Moreno, T.; Ramírez, M.; Santos, A.; Buck, R.; Rueda, R.; Escudero, M.; Catena, A.; et al. Human Milk Levels of 2’-Fucosyllactose and 6′-Sialyllactose Are Positively Associated with Infant Neurodevelopment and Are Not Impacted by Maternal BMI or Diabetic Status. J. Nutr. Food Sci. 2021, 4, 100024. [Google Scholar]
- Ferreira, A.L.L.; Alves-Santos, N.H.; Freitas-Costa, N.C.; Santos, P.P.T.; Batalha, M.A.; Figueiredo, A.C.C.; Yonemitsu, C.; Manivong, N.; Furst, A.; Bode, L.; et al. Associations between Human Milk Oligosaccharides at 1 Month and Infant Development throughout the First Year of Life in a Brazilian Cohort. J. Nutr. 2021, 151, 3543–3554. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, Y.; de Weerth, C. Fucosylated Human Milk Oligosaccharides during the First 12 Postnatal Weeks Are Associated with Better Executive Functions in Toddlers. Nutrients 2023, 15, 1463. [Google Scholar] [CrossRef] [PubMed]
- Schnaar, R.L. Gangliosides as Siglec Ligands. Glycoconj. J. 2023, 40, 159–167. [Google Scholar] [CrossRef]
- Deoni, S.C.L.; O’Muircheartaigh, J.; Elison, J.T.; Walker, L.; Doernberg, E.; Waskiewicz, N.; Dirks, H.; Piryatinsky, I.; Dean, D.C.; Jumbe, N.L. White Matter Maturation Profiles through Early Childhood Predict General Cognitive Ability. Brain Struct. Funct. 2016, 221, 1189–1203. [Google Scholar] [CrossRef]
- Chevalier, N.; Kurth, S.; Doucette, M.R.; Wiseheart, M.; Deoni, S.C.L.; Dean, D.C.; O’Muircheartaigh, J.; Blackwell, K.A.; Munakata, Y.; LeBourgeois, M.K. Myelination Is Associated with Processing Speed in Early Childhood: Preliminary Insights. PLoS ONE 2015, 10, e0139897. [Google Scholar] [CrossRef]
- Su, P.; Kuan, C.C.; Kaga, K.; Sano, M.; Mima, K. Myelination Progression in Language-Correlated Regions in Brain of Normal Children Determined by Quantitative MRI Assessment. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 1751–1763. [Google Scholar] [CrossRef]
- McKenzie, I.A.; Ohayon, D.; Li, H.; De Faria, J.P.; Emery, B.; Tohyama, K.; Richardson, W.D. Motor Skill Learning Requires Active Central Myelination. Science 2014, 346, 318–322. [Google Scholar] [CrossRef]
- Schneider, N.; Greenstreet, E.; Deoni, S.C.L. Connecting inside out: Development of the Social Brain in Infants and Toddlers with a Focus on Myelination as a Marker of Brain Maturation. Child. Dev. 2022, 93, 359–371. [Google Scholar] [CrossRef]
- Schneider, N.; Bruchhage, M.M.K.; O’Neill, B.V.; Hartweg, M.; Tanguy, J.; Steiner, P.; Mutungi, G.; O’Regan, J.; Mcsweeney, S.; D’Sa, V.; et al. A Nutrient Formulation Affects Developmental Myelination in Term Infants: A Randomized Clinical Trial. Front. Nutr. 2022, 9, 823893. [Google Scholar] [CrossRef]
- Deoni, S.C.L.; Matthews, L.; Kolind, S.H. One Component? Two Components? Three? The Effect of Including a Nonexchanging “Free” Water Component in Multicomponent Driven Equilibrium Single Pulse Observation of T1 and T2. Magn. Reson. Med. 2013, 70, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Bruchhage, M.M.K.; Ngo, G.C.; Schneider, N.; D’Sa, V.; Deoni, S.C.L. Functional Connectivity Correlates of Infant and Early Childhood Cognitive Development. Brain Struct. Funct. 2020, 225, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Deoni, S.C.L.; Dean, D.C.; O’Muircheartaigh, J.; Dirks, H.; Jerskey, B.A. Investigating White Matter Development in Infancy and Early Childhood Using Myelin Water Faction and Relaxation Time Mapping. Neuroimage 2012, 63, 1038–1053. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; Bénet, T. Quantitative Determination of Non-Lactose Milk Oligosaccharides. Anal. Chim. Acta 2018, 1010, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Tingley, D.; Yamamoto, T.; Hirose, K.; Keele, L.; Imai, K. Mediation: R Package for Causal Mediation Analysis. J. Stat. Softw. 2014, 59, 1–38. [Google Scholar] [CrossRef]
- Huber, L.; Plötner, M.; Schmitz, J. Behavioral Observation of Prosocial Behavior and Social Initiative Is Related to Preschoolers’ Psychopathological Symptoms. PLoS ONE 2019, 14, e0225274. [Google Scholar] [CrossRef]
- Denham, S.A.; Wyatt, T.M.; Bassett, H.H.; Echeverria, D.; Knox, S.S. Assessing Social-Emotional Development in Children from a Longitudinal Perspective. J. Epidemiol. Community Health 2009, 63, i37–i52. [Google Scholar] [CrossRef]
- Alduncin, N.; Huffman, L.C.; Feldman, H.M.; Loe, I.M. Executive Function Is Associated with Social Competence in Preschool-Aged Children Born Preterm or Full Term. Early Hum. Dev. 2014, 90, 299–306. [Google Scholar] [CrossRef]
- Scharf, R.J.; Scharf, G.J.; Stroustrup, A. Developmental Milestones. Pediatr. Rev. 2016, 37. [Google Scholar] [CrossRef]
- Blakemore, S.J. The Social Brain in Adolescence. Nat. Rev. Neurosci. 2008, 9, 267–277. [Google Scholar] [CrossRef]
- Crafa, D. Neural Correlates of Social Development. In International Encyclopedia of the Social & Behavioral Sciences, 2nd ed.; Pergamon: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the Social Brain. Science 2019, 366, 587. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sosa, S.; Martín, M.J.; García-Pardo, L.A.; Hueso, P. Sialyloligosaccharides in Human and Bovine Milk and in Infant Formulas: Variations with the Progression of Lactation. J. Dairy Sci. 2003, 86, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Molecular Mechanism Underlying Sialic Acid as an Essential Nutrient for Brain Development and Cognition. Adv. Nutr. 2012, 3, 465S–472S. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, S.K.; Yatsunenko, T.; Li, D.; Dasgupta, S.; Yu, R.K.; Berg, B.M.; Chichlowski, M.; Odle, J. Dietary Isomers of Sialyllactose Increase Ganglioside Sialic Acid Concentrations in the Corpus Callosum and Cerebellum and Modulate the Colonic Microbiota of Formula-Fed Piglets. J. Nutr. 2016, 146, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Sialic Acid Is an Essential Nutrient for Brain Development and Cognition. Annu. Rev. Nutr. 2009, 29, 177–222. [Google Scholar] [CrossRef]
- Yoo, S.W.; Motari, M.G.; Susuki, K.; Prendergast, J.; Mountney, A.; Hurtado, A.; Schnaar, R.L. Sialylation Regulates Brain Structure and Function. FASEB J. 2015, 29, 3040–3053. [Google Scholar] [CrossRef]
- Berger, P.K.; Ong, M.L.; Bode, L.; Belfort, M.B. Human Milk Oligosaccharides and Infant Neurodevelopment: A Narrative Review. Nutrients 2023, 15, 719. [Google Scholar] [CrossRef]
- Macdonald, J.; McGurk, H. Visual Influences on Speech Perception Processes. Percept. Psychophys. 1978, 24, 253–257. [Google Scholar] [CrossRef]
- Jorgensen, J.M.; Young, R.; Ashorn, P.; Ashorn, U.; Chaima, D.; Davis, J.C.C.; Goonatilleke, E.; Kumwenda, C.; Lebrilla, C.B.; Maleta, K.; et al. Associations of Human Milk Oligosaccharides and Bioactive Proteins with Infant Growth and Development among Malawian Mother-Infant Dyads. Am. J. Clin. Nutr. 2021, 113, 209–220. [Google Scholar] [CrossRef]
- Lefebvre, G.; Shevlyakova, M.; Charpagne, A.; Marquis, J.; Vogel, M.; Kirsten, T.; Kiess, W.; Austin, S.; Sprenger, N.; Binia, A. Time of Lactation and Maternal Fucosyltransferase Genetic Polymorphisms Determine the Variability in Human Milk Oligosaccharides. Front. Nutr. 2020, 7, 574459. [Google Scholar] [CrossRef]
- Samuel, T.M.; Binia, A.; de Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Costeira, M.J.; Marchini, G.; Martínez-Costa, C.; et al. Impact of Maternal Characteristics on Human Milk Oligosaccharide Composition over the First 4 Months of Lactation in a Cohort of Healthy European Mothers. Sci. Rep. 2019, 9, 11767. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, N.M.; Yarnykh, V.L.; Huber, E.; Zhao, T.C.; Kuhl, P.K. Brain Myelination at 7 Months of Age Predicts Later Language Development. Neuroimage 2022, 263, 119641. [Google Scholar] [CrossRef] [PubMed]

| Ethnicity | n | % |
|---|---|---|
| Caucasian, White | 77 | 71 |
| African American, Black | 8 | 7 |
| Mixed race | 8 | 7 |
| Hispanic + Latino | 7 | 7 |
| Asian | 3 | 3 |
| Other | 6 | 5 |
| Income | n | % |
| 200,000 USD or more | 7 | 6 |
| 150,000–199,999 USD | 10 | 9 |
| 110,000–149,999 USD | 17 | 16 |
| 90,000–109,999 USD | 9 | 8 |
| 70,000–89,999 USD | 11 | 10 |
| 50,000–69,999 USD | 10 | 9 |
| 30,000–49,999 USD | 10 | 9 |
| 10,000–29,999 USD | 10 | 9 |
| Missing | 25 | 24 |
| Number of siblings at birth | n | % |
| 0 | 41 | 38 |
| 1 | 44 | 40 |
| 2 | 14 | 13 |
| 3 | 7 | 6 |
| 4 | 1 | 1 |
| 5 | 2 | 2 |
| Body measures | Mean (SD) | [Min, Max] |
| BMI before pregnancy | 27.3 (5.8) | [19, 39.9] |
| Gestational Age | Mean (SD) | [Min, Max] |
| Weeks | 39.3 (1.08) | [37.0, 41.0] |
| Maternal age at recruitment | Mean (SD) | [Min, Max] |
| Years | 31 (5) | [19, 43] |
| Mode of delivery | n | % |
| Vaginal | 77 | 72 |
| Maternal education | n | % |
| Partial high school (10th or 11th grade) | 2 | 2 |
| High school graduate | 8 | 7 |
| Partial college/university | 24 | 22 |
| Standard college/university graduate/bachelor’s degree | 30 | 28 |
| Graduate degree/master’s degree/doctorate/MBA | 43 | 40 |
| Maternal IQ | Mean (SD) | [Min, Max] |
| Full-scale IQ | 104 (13.6) | [80, 141] |
| Perceptual Reasoning Index | 103 (13.4) | [75, 138] |
| Verbal Comprehension Index | 103 (13.4) | [74, 151] |
| Maternal Edinburgh Post-Natal Depression Score | Mean (SD) | % above cut-off |
| Total score (last trimester of pregnancy, n = 76) | 4.4 (4.1) | 4 |
| Total score (V1, n = 101) | 4.3 (4.6) | 5 |
| Total score (V2, n = 86) | 3.5 (3.5) | 1 |
| Number of feedings per day a | Mean (SD) | [Min, Max] |
| V0 (N = 107) | 10 (3.3) | [1, 30] |
| V1 (N = 101) | 9 (2.5) | [2, 20] |
| V2 (N = 86) | 8 (2.3) | [1, 14] |
| Scale | Visit | Mean (SD) | Median [Min, Max] |
|---|---|---|---|
| ASQ:SE-2 | 3 mo (N = 87) | 18.7 (12.7) | 15 [0, 55] |
| 6 mo (N = 78) | 18.8 (13.1) | 20 [0, 75] | |
| 12 mo (N = 61) | 26.7 (18.1) | 25 [0, 85] | |
| 18 mo (N = 60) | 28.1 (25.3) | 20 [0, 140] | |
| 24 mo (N = 55) | 30.0 (22.3) | 30 [0, 95] |
| ASQ [12 Months] | |||
|---|---|---|---|
| Predictors | Estimates | CI | p |
| (Intercept) | 63.48 | 43.02–83.93 | <0.001 |
| VISITV1 | 0.39 | −15.68–16.45 | 0.962 |
| VISITV2 | −6.87 | 21.89–8.15 | 0.368 |
| 6′SL | −0.04 | −0.06–−0.02 | 0.001 |
| Mother edu4 | −19.76 | -41.63–2.11 | 0.076 |
| Mother edu5 | −8.68 | −27.86–10.50 | 0.373 |
| Mother edu6 | −18.35 | −36.65–−0.06 | 0.049 |
| Mother edu7 | −22.34 | −40.49–−4.18 | 0.016 |
| VISITV1:6′SL | −0.02 | −0.06–0.02 | 0.234 |
| VISITV2:6′SL | −0.05 | −0.11–0.02 | 0.147 |
| Observations | 165 | ||
| R2/R2 adjusted | 0.241/0.197 | ||
| Independent Component | Proportion Mediated | Total Effect | ACME |
|---|---|---|---|
| IC_5 | 0.3 *** | −0.0559 *** | −0.0166 *** |
| IC_24 | 0.3 *** | −0.058 *** | −0.0193 *** |
| IC_67 | 0.6 *** | −0.0553 *** | −0.0322 *** |
| IC_74 | 0.3 * | −0.0552 | −0.0153 ** |
| IC_150 | 0.4 *** | −0.0564 *** | −0.0217 *** |
| IC_175 | 0.6 *** | −0.0574 *** | −0.0274 *** |
| Correlation | HMO [mg/L] | Outcome | Time Point Milk Sample | Time Point Bayley | N | p.adj |
|---|---|---|---|---|---|---|
| 0.42 | 3FL | Language | 2–5 weeks | 12 months | 54 | 0.02 |
| 0.45 | 3FL | Language | 6 weeks | 12 months | 51 | 0.02 |
| 0.44 | 3FL | Language | 2–5 weeks | 12 months | 48 | 0.04 |
| 0.43 | LNFP-II | Language | 2–5 weeks | 12 months | 54 | 0.02 |
| 0.41 | LNFP-V | Language | 2–5 weeks | 12 months | 54 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajhans, P.; Mainardi, F.; Austin, S.; Sprenger, N.; Deoni, S.; Hauser, J.; Schneider, N. The Role of Human Milk Oligosaccharides in Myelination, Socio-Emotional and Language Development: Observational Data from Breast-Fed Infants in the United States of America. Nutrients 2023, 15, 4624. https://doi.org/10.3390/nu15214624
Rajhans P, Mainardi F, Austin S, Sprenger N, Deoni S, Hauser J, Schneider N. The Role of Human Milk Oligosaccharides in Myelination, Socio-Emotional and Language Development: Observational Data from Breast-Fed Infants in the United States of America. Nutrients. 2023; 15(21):4624. https://doi.org/10.3390/nu15214624
Chicago/Turabian StyleRajhans, Purva, Fabio Mainardi, Sean Austin, Norbert Sprenger, Sean Deoni, Jonas Hauser, and Nora Schneider. 2023. "The Role of Human Milk Oligosaccharides in Myelination, Socio-Emotional and Language Development: Observational Data from Breast-Fed Infants in the United States of America" Nutrients 15, no. 21: 4624. https://doi.org/10.3390/nu15214624
APA StyleRajhans, P., Mainardi, F., Austin, S., Sprenger, N., Deoni, S., Hauser, J., & Schneider, N. (2023). The Role of Human Milk Oligosaccharides in Myelination, Socio-Emotional and Language Development: Observational Data from Breast-Fed Infants in the United States of America. Nutrients, 15(21), 4624. https://doi.org/10.3390/nu15214624





