Foraging Wild Edibles: Dietary Diversity in Expanded Food Systems
Abstract
:1. Introduction
“The intentional use of perennial food-producing plants to improve the sustainability and resilience of urban communities. A wide range of activities and projects fit this definition, including community food forests, urban orchards, foraging in public urban forests, and community-wide gleaning programs. Even maintaining a small food forest on a private residential lot in a city is considered urban food forestry” (p. 6).
2. Antioxidants and Health
2.1. Diet-Related Chronic Disease and Food Supply
2.2. Chronic Disease and the Underlying Dysfunctions in Oxidative Stress, Inflammation, and Detoxification
2.3. Reducing Oxidative Stress and Chronic Inflammation
3. Method
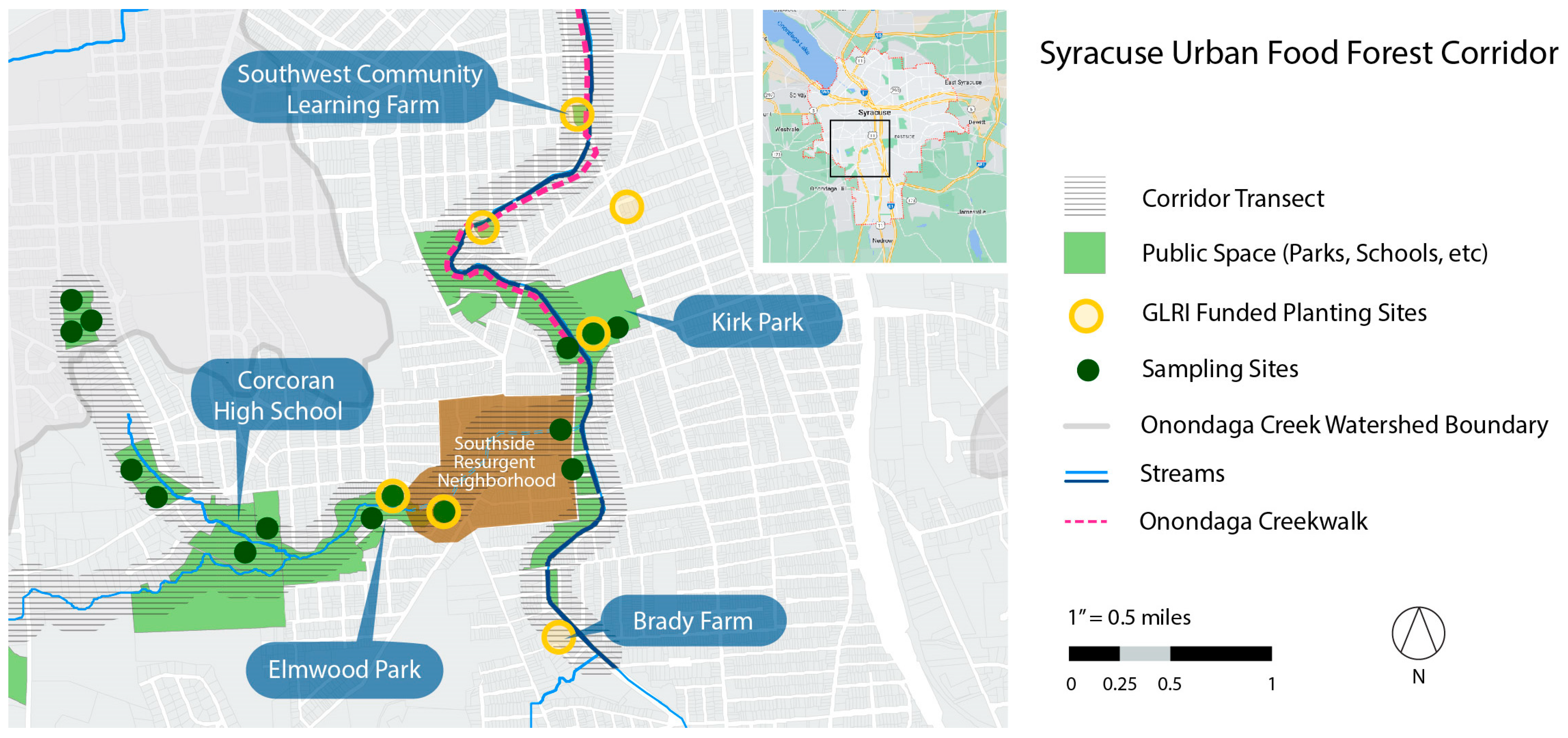
3.1. Case Study Background
3.1.1. Location
3.1.2. Poverty, Urban Food Infrastructure, and Health Nexus
3.1.3. Forageable Species Selection and Antioxidant Phytochemical Data Collection
3.1.4. Antioxidant Phytochemical Data Collection
3.2. Case Study Analysis
3.2.1. Stage 1 Analysis: Antioxidant Phytochemical Concentration and FDA Daily Value by Individual Plant Species
3.2.2. Stage 2 Analysis: Plants Ranked by Power of Composite (or “Packaged”) Antioxidant Scores
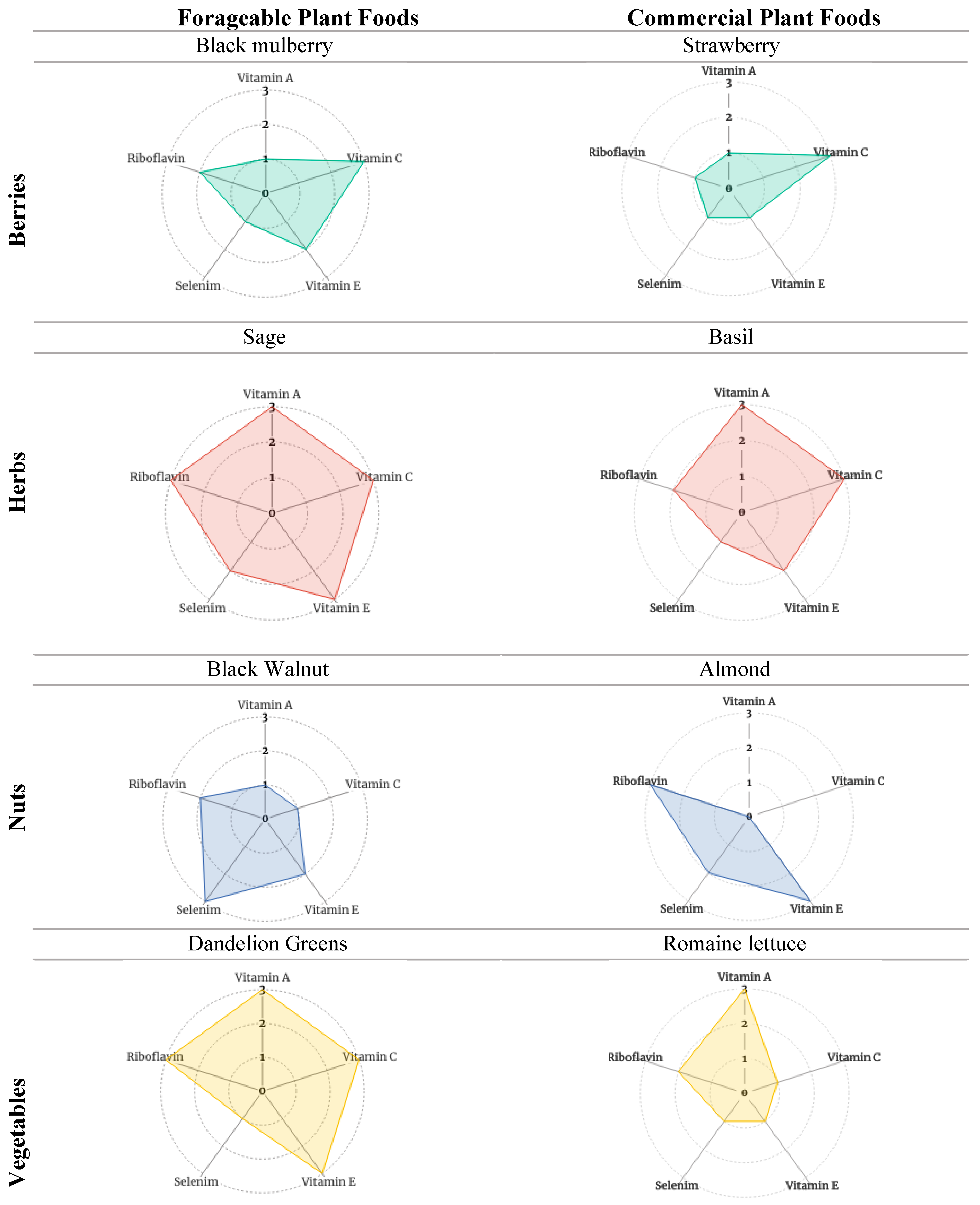
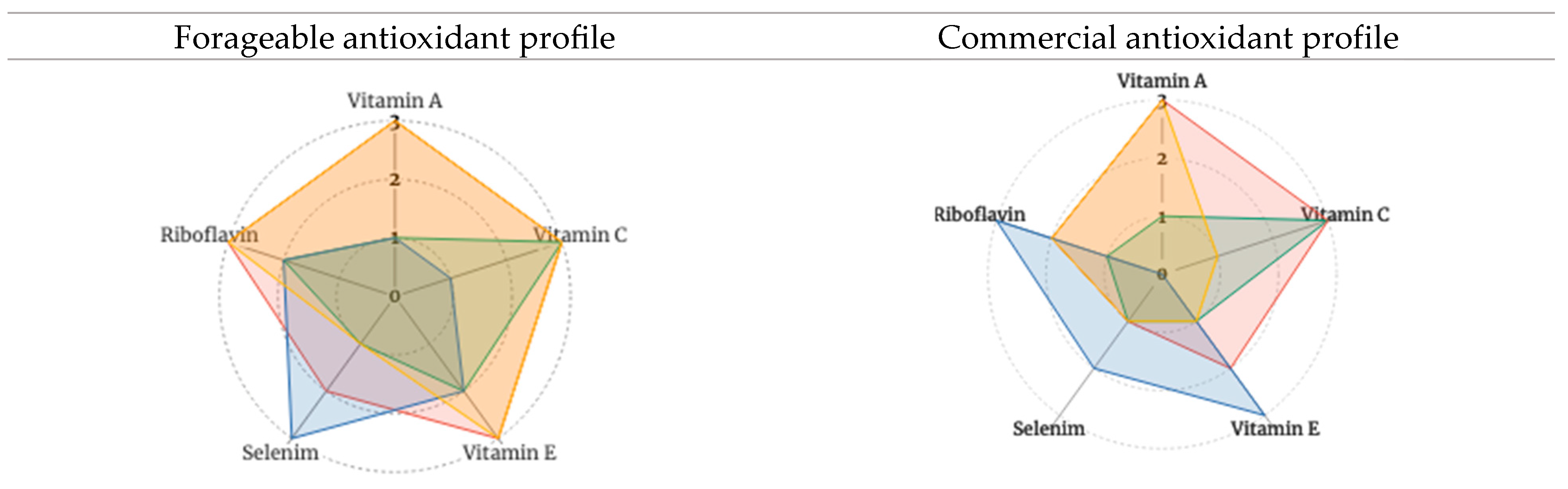
3.2.3. Stage 3 Analysis: Reflections on Antioxidant Phytochemical Packages of High-Value Forageables and Popular Commercial Plant Foods according to Food Type (Fruits, Herbs, Nuts, and Vegetables)
3.2.4. Limitations
4. Results
5. Discussion
5.1. Available Nutrition Data: Implications of What We Could Find
5.2. Non-Available Nutrition Data: Implications of What We Could Not Find
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Scientific Name | Common Name | Other Names | Edible Part | Vit A (RAE mcg) | Vit C (mg) | Vit E (mg) | Se (mcg) | Riboflavin (mg) | Source a |
|---|---|---|---|---|---|---|---|---|---|
| Berries | |||||||||
| Amelanchier alnifolia | saskatoon serviceberry | juneberry, shadbush | raw fruit | 10.91 | 3.55 | 1.12 | 3.54 | [127] | |
| Amelanchier arborea | downy serviceberry | sarvis-berry, shadblow, sugarplum, Indian cherry | fruit | ||||||
| Amelanchier canadensis | Canadian serviceberry | sarvice, wild currant | fruit | ||||||
| Amelanchier laevis | Allegheny serviceberry | fruit | |||||||
| Aronia melanocarpa | black chokeberry | fruit | |||||||
| Celtis occidentalis | hackberry | beaverwood, false elm, nettle tree, sugar berry | raw fruit | 18.3 | [128] | ||||
| Morus alba | white mulberry | raw fruit | 15.2 | 0.088 | [129] | ||||
| Morus nigra | black mulberry | raw fruit | 1 | 36.4 | 0.87 | 0.6 | 0.101 | FDC 169913 | |
| Morus rubra | red mulberry | fruit | |||||||
| Ribes nigrum | blackcurrant | raw fruit | 12 | 181 | 1 | 0.05 | FDC 173963 | ||
| Ribes rubrum | redcurrant | raw fruit | 2 | 41 | 0.1 | 0.6 | 0.05 | FDC 173964 | |
| Ribes uva-crispa | gooseberry | raw fruit | 15 | 27.7 | 0.37 | 0.6 | 0.03 | FDC 173030 | |
| Rubus caesius | European dewberry | fruit | |||||||
| Rubus fruticosus | blackberry | raw fruit | 11 | 21 | 1.17 | 0.4 | 0.026 | FDC 173946 | |
| Rubus idaeus | red raspberry | raw fruit | 2 | 26.4 | 0.57 | 0.08 | FDC 168997 | ||
| Rubus occidentalis | black raspberry | fruit | |||||||
| Sambucus canadensis | black elderberry | flor sauco, tree of music, Danewort, Walewort | raw fruit | 30 | 36 | 0.06 | 0.06 | FDC 171727 | |
| Other Fruits (excluding berries) | |||||||||
| Asimina triloba | pawpaw | false banana, custard apple, white plum | raw fruit | 8.6 | 18.3 | 0.09 | [130] | ||
| Chaenomeles speciosa | flowering quince | raw fruit | 72.15 | [131] | |||||
| Cornus kousa | kousa dogwood | Chinese/Korean/Japanese dogwood | raw fruit | 93 | [132] | ||||
| Diospyros virginiana | persimmon b | possumwood, American ebony, white ebony, bara-bara, boa-wood, butterwood | raw fruit | 66 | FDC 169943 | ||||
| Malus spp. | apple | raw fruit | 3 | 4.6 | 0.18 | 0 | 0.026 | FDC 171688 | |
| Malus spp. | crabapple | raw fruit | 2 | 8 | 0.02 | FDC 171721 | |||
| Podophyllum peltatum | mayapple | Indian apple, wild mandrake, pomme de mai, podophylle pelt | fruit | ||||||
| Prunus americana | American plum | wild plum, August plum, sand cherry, sloe | raw fruit | 173 | 10.3 | 0.53 | 0.042 | FDC 169820 | |
| Prunus avium | sweet cherry | wild cherry | raw fruit | 3 | 7 | 0.07 | 0 | 0.033 | FDC 171719 |
| Prunus tomentosa | nanking cherry | Korean/Chinese cherry | fruit | ||||||
| Pyrus spp. | pear | raw fruit | 1 | 4.3 | 0.12 | 0.1 | 0.026 | FDC 169118 | |
| Viburnum prunifolium | blackhaw | smooth blackhaw, stagbush | fruit | ||||||
| Vitis spp. | grape | raw fruit | 5 | 4 | 0.19 | 0.1 | 0.057 | FDC 174682 | |
| Herbs | |||||||||
| Achillea millefolium | yarrow c | western yarrow, milfoil | leaves | 0.95 | [133] | ||||
| Agastache foeniculum | anise hyssop | blue giant/lavender hyssop | leaves, flowers | ||||||
| Baptisia australis | blue false indigo | plains/rattlebush wild indigo, baptisia, rattlepod | leaves, roots | ||||||
| Ceanothus americanus | New Jersey tea | redroot | roots | ||||||
| Cercis canadensis | eastern redbud | Judas tree | flowers, seed pods | ||||||
| Echinacea purpurea | purple coneflower | Kansas snakeroot, scurvy root, Indian head, comb flower, hedge hog | leaves | ||||||
| Hamamelis ovalis | witch hazel | leaves, twigs | |||||||
| Juniperus spp. | juniper | fruit | |||||||
| Leonurus cardiaca | common motherwort | whole plant | |||||||
| Lindera benzoin | spicebush | wild allspice, Benjaminbush | leaves, twigs, fruits | ||||||
| Melissa officinalis | common balm | lemon balm | leaves | ||||||
| Mentha spicata | spearmint | fresh leaves | 203 | 13.3 | 0.175 | FDC 173475 | |||
| Monarda punctata | beebalm | spotted beebalm, horsemint | leaves, shoot tips, flowers | ||||||
| Picea abies | Norway spruce | needles | |||||||
| Picea glauca | white spruce | Canada spruce, skunk spruce | young tips gum | ||||||
| Picea pungens | blue spruce | white/silver spruce | needles | ||||||
| Rhus spp. | sumac | fruit | |||||||
| Salvia officinalis | sage | ground herb | 295 | 32.4 | 7.48 | 3.7 | 0.336 | FDC 170935 | |
| Symphytum spp. | comfrey | leaves, young shoots, roots | |||||||
| Nuts | |||||||||
| Carya cordiformis | bitternut hickory | nut | |||||||
| Carya glabra | pignut hickory | sweet hickory, smoothbark hickory, switch/switchbud hickory | nut | ||||||
| Carya illinoinensis | pecan | pecan hickory | raw nut | 3 | 1.1 | 1.4 | 3.8 | 0.13 | FDC 170182 |
| Carya laciniosa | shellbark hickory | bigleaf shagbark hickory, kingnut | nut | ||||||
| Carya ovata | shagbark hickory | nut | |||||||
| Castanea dentata | American chestnut | nut | |||||||
| Castanea pumila | Allegheny chinkapin | American/Ozark chinquapin, dwarf chestnut | nut | ||||||
| Corylus americana | hazelnut | American filbert | raw nut | 1 | 6.3 | 15 | 2.4 | 0.113 | FDC 170581 |
| Juglans ailantifolia | heartnut | nut | |||||||
| Juglans cinerea | butternut | white walnut, oilnut | dried nut | 6 | 3.2 | 17.2 | 0.148 | FDC 170570 | |
| Juglans nigra | black walnut | dried nut | 2 | 1.7 | 2.08 | 17 | 0.13 | FDC 170186 | |
| Juglans regia | English walnut | dried nut | 1 | 1.3 | 0.7 | 4.9 | 0.15 | FDC 170187 | |
| Quercus alba | white oak | nut | |||||||
| Quercus coccinea | scarlet oak | nut | |||||||
| Quercus macrocarpa | bur oak | blue oak | nut | ||||||
| Quercus muehlenbergii | chinkapin oak | chestnut/rock chestnut oak | nut | ||||||
| Quercus palustris | pin oak | nut | |||||||
| Quercus rubra | northern red oak | red oak | nut | ||||||
| Quercus velutina | black oak | nut | |||||||
| Vegetables | |||||||||
| Alliaria petiolata | garlic mustard | fresh leaves | 26.1 | [134] | |||||
| Allium tricoccum | ramps | wild leeks | leaves, bulbs | 80 | [135] | ||||
| Amaranthus albus | prostrate pigweed | leaves, stems | |||||||
| Asclepias tuberosa | butterfly milkweed | pleurisy root, orange milkweed, chigger flower | flowers, flower buds, seed pods, root, seed oil | ||||||
| Chenopodium album | lambsquarters | raw vegetable | 580 | 80 | 0.9 | 0.44 | FDC 169244 | ||
| Matteuccia struthiopteris | ostrich fern | fiddlehead fern | raw fronds | 181 | 26.6 | 0.21 | FDC 169405 | ||
| Nasturtium officinale | watercress | fresh leaves | 160 | 43 | 1 | 0.9 | 0.12 | FDC 170068 | |
| Polygonum cuspidatum | Japanese knotweed | shoots, leaves, roots | |||||||
| Portulaca oleracea | purslane | little hogweed | raw vegetable | 21 | 0.9 | 0.112 | FDC 169274 | ||
| Taraxacum spp. | dandelion | raw greens | 508 | 35 | 3.44 | 0.5 | 0.26 | FDC 169226 | |
| Typha latifolia | broadleaf cattail | flags, rushes, bulbrushes | young shoots | 1 | 0.7 | 0.6 | 0.025 | FDC 168994 | |
| Vitis spp. | grape | leaves | 1380 | 11.1 | 2 | 0.9 | 0.354 | FDC 168575 | |
| Other | |||||||||
| Acer saccharum | sugar maple | rock maple, hard maple | sap | 0 | 0 | 0 | 0.6 | 1.27 | FDC 169661 |
| Asarum canadense | wild ginger | Canadian wild ginger, Canadian snakeroot | roots | ||||||
| Robinia pseudoacacia | black locust | false acacia; yellow locust | flowers | ||||||
| Viola spp. | violet | flowers | |||||||
References
- Boushey, C.J. Dietary Guidelines for Americans Committee: Process and Updates. Dietary Guidelines for Americans 2020–2025. 2020. Available online: https://mchnutritionpartners.ucla.edu/wp-content/uploads/2021/04/NLN-DGA-Boushey.pdf (accessed on 23 August 2023).
- Nelson, M.E.; Hamm, M.W.; Hu, F.B.; Abrams, S.A.; Griffin, T.S. Alignment of Healthy Dietary Patterns and Environmental Sustainability: A Systematic Review. Adv. Nutr. 2016, 7, 1005–1025. [Google Scholar] [CrossRef]
- Chen, C.; Chaudhary, A.; Mathys, A. Dietary Change Scenarios and Implications for Environmental, Nutrition, Human Health and Economic Dimensions of Food Sustainability. Nutrients 2019, 11, 856. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture (USDA); U.S. Department of Health and Human Services (HHS. Dietary Guidelines for Americans, 2020–2025. 2020. Available online: https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf (accessed on 23 August 2023).
- U.S. Department of Agriculture (USDA); Food and Nutrition Service & Center for Nutrition Policy and Promotion. Average Healthy Eating Index-2015 Scores for All Americans by Age Groups. What We Eat in America, NHANES 2017–2018. 2022. Available online: https://fns-prod.azureedge.us/sites/default/files/media/file/HEIScores_AllAmericans_byAgeGroup_NHANES2017-2018.pdf (accessed on 23 August 2023).
- Fanzo, J.; Davis, C. Global Food Systems, Diets, and Nutrition: Linking Science, Economics, and Policy; Palgrave Macmillan: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Bai, Y. Quantifying the Cost of Nutritious Diets and Dietary Impacts on Health: Economic Approaches to Global Food Systems and Nutrition Transition. Ph.D. Thesis, Tufts University, Gerald J. and Dorothy R. Friedman School of Nutrition Science and Policy, Somerville, MA, USA, 2021. Available online: https://www.proquest.com/docview/2489532200/abstract/30F0C8C12DC64F34PQ/1 (accessed on 23 August 2023).
- Mason, P.; Lang, T. Sustainable Diets: How Ecological Nutrition Can Transform Consumption and the Food System; Routledge, Taylor & Francis Group: London, UK, 2017. [Google Scholar]
- Morland, K.B. (Ed.) Local Food Environments: Food Access in America, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Kimmerer, R.W. Braiding Sweetgrass: Indigenous Wisdom, Scientific Knowledge and the Teachings of Plants, 1st ed.; Milkweed Editions: Minneapolis, MN, USA, 2013. [Google Scholar]
- Khan, S.; Hussain, W.; Sulaiman Shah, S.; Hussain, H.; Altyar, A.E.; Ashour, M.L.; Pieroni, A. Overcoming Tribal Boundaries: The Biocultural Heritage of Foraging and Cooking Wild Vegetables among Four Pathan Groups in the Gadoon Valley, NW Pakistan. Biology 2021, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, C.; Lestrat, K.E.; Vatanparast, H. Food Security Interventions among Refugees around the Globe: A Scoping Review. Nutrients 2022, 14, 522. [Google Scholar] [CrossRef] [PubMed]
- Potteiger, M. Eating Ecologies: Integraging productive ecologies and foraging at the landscape scale. In Localizing Urban Food Strategies. Farming Cities and Performing Rurality; Cinà, G., Dansero, E., Eds.; Politecnico di Torino: Torino, Italy, 2015; pp. 131–145. Available online: http://www.aesoptorino2015.it/content/download/408/2229/version/1/file/10_T1C_672_potteiger_A.pdf (accessed on 23 August 2023).
- McGrath, N. Foraging for Food Security. Thought for Food Blog. 2014. Available online: https://www.ifis.org/blog/2014/food-science-and-technology/foraging-for-food-security (accessed on 23 August 2023).
- Shackleton, C.M.; Hurley, P.T.; Dahlberg, A.C.; Emery, M.R.; Nagendra, H. Urban Foraging: A Ubiquitous Human Practice Overlooked by Urban Planners, Policy, and Research. Sustainability 2017, 9, 10. [Google Scholar] [CrossRef]
- Bukowski, C.; Munsell, J.F. The Community Food Forest Handbook: How to Plan, Organize, and Nurture Edible Gathering Places; Chelsea Green Publishing: London, UK, 2018. [Google Scholar]
- Yoo, S.; Kim, K.; Nam, H.; Lee, D. Discovering Health Benefits of Phytochemicals with Integrated Analysis of the Molecular Network, Chemical Properties and Ethnopharmacological Evidence. Nutrients 2018, 10, 1042. [Google Scholar] [CrossRef] [PubMed]
- Wallinga, D.; Maizes, V. Foraging for healthy food in the global economy: Ten steps we can all take. Explore 2008, 4, 385–388. [Google Scholar] [CrossRef]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 12. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Author Correction: Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 8. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit. Rev. Food Sci. Nutr. 2022, 62, 5658–5677. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Habtamu, F.G.; Negussie, R. Antinutritional Factors in Plant Foods: Potential Health Benefits and Adverse Effects. Int. J. Nutr. Food Sci. 2014, 3, 284–289. [Google Scholar] [CrossRef]
- Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process Nutr. 2020, 6, 2. [Google Scholar] [CrossRef]
- Säumel, I.; Kotsyuk, I.; Hölscher, M.; Lenkereit, C.; Weber, F.; Kowarik, I. How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighbourhoods in Berlin, Germany. Environ. Pollut. 2012, 165, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.M.; Felix, D.; Alcantara, F.; Zaslavsky, I.; Work, A.; Watson, P.L.; Pezzoli, K.; Yu, Q.; Zhu, D.; Scavo, A.J.; et al. Monitoring and mitigation of toxic heavy metals and arsenic accumulation in food crops: A case study of an urban community garden. Plant Direct 2020, 4, e00198. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijević, M.D.; Nujkić, M.M.; Alagić, S.Č.; Milić, S.M.; Tošić, S.B. Heavy metal contamination of topsoil and parts of peach-tree growing at different distances from a smelting complex. Int. J. Environ. Sci. Technol. 2016, 13, 615–630. [Google Scholar] [CrossRef]
- Stark, P.B.; Miller, D.; Carlson, T.J.; De Vasquez, K.R. Open-source food: Nutrition, toxicology, and availability of wild edible greens in the East Bay. PLoS ONE 2019, 14, e0202450. [Google Scholar] [CrossRef]
- Guil, J.L.; Rodrigues-Garcia, I.; Torija, E. Nutritional and toxic factors in selected wild edible plants. Plant Foods Hum. Nutr. 1997, 51, 99–107. [Google Scholar] [CrossRef]
- Samsøe-Petersen, L.; Larsen, E.H.; Larsen, P.B.; Bruun, P. Uptake of Trace Elements and PAHs by Fruit and Vegetables from Contaminated Soils. Environ. Sci. Technol. 2002, 36, 3057–3063. [Google Scholar] [CrossRef]
- Von Hoffen, L.P.; Säumel, I. Orchards for edible cities: Cadmium and lead content in nuts, berries, pome and stone fruits harvested within the inner city neighbourhoods in Berlin, Germany. Ecotoxicol. Environ. Saf. 2014, 101, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Zeiner, M.; Cindrić, I. Harmful Elements (Al, Cd, Cr, Ni, and Pb) in Wild Berries and Fruits Collected in Croatia. Toxics 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Dabady, S.; Stark, P.B. Urban Foraging in Municipal Parks and Public Schools: Opportunities for Policymakers. Berkley Food Institute. 2017. Available online: https://forage.berkeley.edu/wp-content/uploads/2017/08/UrbanForagingPolicyBrief-2017-08-29.pdf (accessed on 4 October 2023).
- Di Renzo, L.; Gualtieri, P.; De Lorenzo, A. Diet, Nutrition and Chronic Degenerative Diseases. Nutrients 2021, 13, 1372. [Google Scholar] [CrossRef]
- Bilali, H.E.; Allahyari, M.S. Transition towards sustainability in agriculture and food systems: Role of information and communication technologies. Inf. Process. Agric. 2018, 5, 456–464. [Google Scholar] [CrossRef]
- Hawkes, C. Uneven dietary development: Linking the policies and processes of globalization with the nutrition transition, obesity and diet-related chronic diseases. Glob. Health 2006, 2, 4. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G. The Impact of Transnational “Big Food” Companies on the South: A View from Brazil. PLoS Med. 2012, 9, e1001252. [Google Scholar] [CrossRef]
- Baker, P.; Friel, S. Food systems transformations, ultra-processed food markets and the nutrition transition in Asia. Glob. Health 2016, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Willey, J.; Wakefield, M.; Silver, H.J. Exploring the Diets of Adults with Obesity and Type II Diabetes from Nine Diverse Countries: Dietary Intakes, Patterns, and Quality. Nutrients 2020, 12, 2027. [Google Scholar] [CrossRef]
- Raj, S. Influences of the Nutrition Transition on Chronic Disease. In Integrative and Functional Medical Nutrition Therapy: Principles and Practices; Noland, D., Drisko, J.A., Wagner, L., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 17–29. [Google Scholar] [CrossRef]
- Mapesi, H.; Paris, D.H. Non-Communicable Diseases on the Rise in Sub-Saharan Africa, the Underappreciated Threat of a Dual Disease Burden. Praxis 2019, 108, 997–1005. [Google Scholar] [CrossRef]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Lechner, K.; von Schacky, C.; McKenzie, A.L.; Worm, N.; Nixdorff, U.; Lechner, B.; Kränkel, N.; Halle, M.; Krauss, R.M.; Scherr, J. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 2020, 27, 394–406. [Google Scholar] [CrossRef]
- Perrone, S.; Tataranno, M.L.; Santacroce, A.; Bracciali, C.; Riccitelli, M.; Alagna, M.G.; Longini, M.; Belvisi, E.; Bazzini, F.; Buonocore, G. Fetal Programming, Maternal Nutrition, and Oxidative Stress Hypothesis. J. Pediatr. Biochem. 2016, 6, 96–102. [Google Scholar] [CrossRef]
- Kuiper-Makris, C.; Selle, J.; Nüsken, E.; Dötsch, J.; Alejandre Alcazar, M.A. Perinatal Nutritional and Metabolic Pathways: Early Origins of Chronic Lung Diseases. Front. Med. 2021, 8, 667315. [Google Scholar] [CrossRef] [PubMed]
- Carpinello, O.J.; DeCherney, A.H.; Hill, M.J. Developmental Origins of Health and Disease: The History of the Barker Hypothesis and Assisted Reproductive Technology. Semin. Reprod. Med. 2018, 36, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, V.; Cardoso, R.C.; Puttabyatappa, M. Developmental Programming, a Pathway to Disease. Endocrinology 2016, 157, 1328–1340. [Google Scholar] [CrossRef]
- MacDonald Baker, S. The Metaphor of Oceanic Disease. Integr. Med. 2008, 7, 40–45. [Google Scholar]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.M.; Dayem, A.A.; Cho, S.G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Bosma-den Boer, M.M.; van Wetten, M.-L.; Pruimboom, L. Chronic inflammatory diseases are stimulated by current lifestyle: How diet, stress levels and medication prevent our body from recovering. Nutr. Metab. 2012, 9, 32. [Google Scholar] [CrossRef]
- Li, Q.-F.; Hao, H.; Tu, W.-S.; Guo, N.; Zhou, X.-Y. Maresins: Anti-inflammatory pro-resolving mediators with therapeutic potential. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7442–7453. [Google Scholar] [CrossRef] [PubMed]
- Liska, D.J. The detoxification enzyme systems. Altern. Med. Rev. A J. Clin. Ther. 1998, 3, 187–198. [Google Scholar]
- Palrasu, M.; Siddavaram, N. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 18, 38–54. [Google Scholar] [CrossRef]
- Pingitore, A.; Lima, G.P.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R.; Gross, M.D.; Tapsell, L.C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009, 89, 1543S–1548S. [Google Scholar] [CrossRef] [PubMed]
- Barba, F. (Ed.) Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Woodhead Publishing: Sawston, UK, 2019. [Google Scholar]
- Petroski, W.; Minich, D.M. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 2020, 12, E2929. [Google Scholar] [CrossRef]
- Foyer, C.H.; Kyndt, T.; Hancock, R.D. Vitamin C in Plants: Novel Concepts, New Perspectives, and Outstanding Issues. Antioxid. Redox Signal. 2020, 32, 463–485. [Google Scholar] [CrossRef]
- Webb, G.P. Nutritional supplements and conventional medicine; what should the physician know? Proc. Nutr. Soc. 2007, 66, 471–478. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I. Functional Foods for Health: The Interrelated Antioxidant and Anti-Inflammatory Role of Fruits, Vegetables, Herbs, Spices and Cocoa in Humans. Curr. Pharm. Des. 2016, 22, 6701–6715. [Google Scholar] [CrossRef]
- Augimeri, G.; Montalto, F.I.; Giordano, C.; Barone, I.; Lanzino, M.; Catalano, S.; Andò, S.; De Amicis, F.; Bonofiglio, D. Nutraceuticals in the Mediterranean Diet: Potential Avenues for Breast Cancer Treatment. Nutrients 2021, 13, 2557. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G. Dietary Antioxidants and Prevention of Non-Communicable Diseases. Antioxidants 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, N.; Victor, V.M. Molecular strategies for targeting antioxidants to mitochondria: Therapeutic implications. Antioxid. Redox Signal. 2015, 22, 686–729. [Google Scholar] [CrossRef] [PubMed]
- Kiełczykowska, M.; Kocot, J.; Paździor, M.; Musik, I. Selenium—A fascinating antioxidant of protective properties. Adv. Clin. Exp. Med. Off. Organ. Wroc. Med. Univ. 2018, 27, 245–255. [Google Scholar] [CrossRef]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, E950. [Google Scholar] [CrossRef]
- Our City. In 2020 State of The City, Syracuse Mayor Ben Walsh Sees “Window of Opportunity” to Revitalize Neighborhoods and Continue Progress on Syracuse Surge. Our City. 2020. Available online: https://ourcity.syrgov.net/2020/01/in-2020-state-of-the-city-syracuse-mayor-ben-walsh-sees-window-of-opportunity-to-revitalize-neighborhoods-and-continue-progress-on-syracuse-surge/ (accessed on 23 August 2023).
- U.S. Census Bureau. 2020 Census Redistricting Data (Public Law 94-171). United States Census Bureau. 2020. Available online: https://data.census.gov/cedsci/table?q=Syracuse%20city,%20New%20York&tid=DECENNIALPL2020.P1 (accessed on 23 August 2023).
- U.S. Census Bureau. 2020 American Community Survey 5-Year Estimates. United States Census Bureau. 2020. Available online: https://data.census.gov/cedsci/all?q=american%20community%20survey (accessed on 23 August 2023).
- Jargowsky, P.A. The Architecture of Segregation: Civil Unrest, the Concentration of Poverty, and Public Policy. The Century Foundation. 2015. Available online: https://tcf.org/content/report/architecture-of-segregation/ (accessed on 23 August 2023).
- Davey Resource Group, Inc. Syracuse Urban Forest Master Plan; Forestry Department City of Syracuse: Syracuse, NY, USA, 2020.
- Pine, A. Ambient struggling: Food, chronic disease, and spatial isolation among the urban poor. Agric. Hum. Values 2022, 40, 1105–1116. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. PLACES: Local Data for Better Health. 2022. Available online: https://www.cdc.gov/places/index.html (accessed on 23 August 2023).
- Potteiger, M.; Weissman, E. FoodPlanCNY. 2021. Available online: https://agriculture.ongov.net/wp-content/uploads/2021/03/Food-Plan-CNY.pdf (accessed on 23 August 2023).
- Economic Research Service (ERS); U.S. Department of Agriculture (USDA). Food Access Research Atlas. 2019. Available online: https://www.ers.usda.gov/data-products/food-access-research-atlas/ (accessed on 23 August 2023).
- United States Department of Agriculture. USDA Plant Hardiness Zone Map. USDA. 2012. Available online: https://planthardiness.ars.usda.gov/ (accessed on 23 August 2023).
- United States Department of Agriculture, Agricultural Research Service. FoodData Central. 2019. Available online: https://fdc.nal.usda.gov/ (accessed on 23 August 2023).
- National Institutes of Health Office of Dietary Supplements. Nutrient Recommendations and Databases. National Institutes of Health, n.d. Available online: https://ods.od.nih.gov/HealthInformation/nutrientrecommendations.aspx (accessed on 23 August 2023).
- Food and Drug Administration. Food Labeling: Revision of the Nutrition and Supplement Facts Labels and Serving Sizes of Foods That Can Reasonably Be Consumed at One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments; Proposed Extension of Compliance Dates. 2017. Available online: https://www.federalregister.gov/documents/2017/10/02/2017-21019/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels-and-serving-sizes-of-foods-that (accessed on 23 August 2023).
- Center for Food Safety and Applied Nutrition. Daily Value on the New Nutrition and Supplement Facts Labels. U.S. Food and Drug Administration. FDA. 2022. Available online: https://www.fda.gov/food/new-nutrition-facts-label/daily-value-new-nutrition-and-supplement-facts-labels (accessed on 23 August 2023).
- National Research Council (US) Subcommittee on Selenium. Distribution. In Selenium in Nutrition: Revised Edition; National Academies Press (US): Washington, DC, USA, 1983. Available online: https://www.ncbi.nlm.nih.gov/books/NBK216733/ (accessed on 23 August 2023).
- National Institutes of Health Office of Dietary Supplements. Selenium: Fact Sheet for Health Professionals. National Institutes of Health. 2021. Available online: https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/ (accessed on 23 August 2023).
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. Available online: https://www.frontiersin.org/articles/10.3389/fpls.2016.02074 (accessed on 23 August 2023). [CrossRef]
- United States Geological Survey. Average concentrations of elements in Onondaga County, New York. United States Geological Survey. 2008. Available online: https://mrdata.usgs.gov/geochem/county.php?place=f36067&el=Se&rf=northeastern (accessed on 23 August 2023).
- Economic Research Service (ERS). Vegetables, Fresh Market (Including Mushrooms): Selected U.S. Per Capita Availability; [Data set]; USDA Economic Research Service: Washington, DC, USA, 2022.
- Economic Research Service (ERS). U.S. Per Capita Use of Selected, Commercially Produced, Fresh, and Processing Fruit and Tree Nuts, 1976 to Date; [Data set]; USDA Economic Research Service: Washington, DC, USA, 2022.
- National Institutes of Health Office of Dietary Supplements. Vitamin A and Carotenoids: Fact Sheet for Health Professionals. National Institutes of Health. 2022. Available online: https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/ (accessed on 23 August 2023).
- National Institutes of Health Office of Dietary Supplements. Vitamin C: Fact Sheet for Health Professionals. National Institutes of Health. 2021. Available online: https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/ (accessed on 23 August 2023).
- National Institutes of Health Office of Dietary Supplements. Vitamin E: Fact Sheet for Health Professionals. National Institutes of Health. 2021. Available online: https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/ (accessed on 23 August 2023).
- National Institutes of Health Office of Dietary Supplements. Riboflavin: Fact Sheet for Health Professionals. National Institutes of Health. 2022. Available online: https://ods.od.nih.gov/factsheets/Riboflavin-HealthProfessional/ (accessed on 23 August 2023).
- Ao, T.; Deb, C.R. Nutritional and antioxidant potential of some wild edible mushrooms of Nagaland, India. J. Food Sci. Technol. 2019, 56, 1084–1089. [Google Scholar] [CrossRef]
- Duguma, H.T. Wild Edible Plant Nutritional Contribution and Consumer Perception in Ethiopia. Int. J. Food Sci. 2020, 2020, e2958623. [Google Scholar] [CrossRef]
- Motti, R. Wild Edible Plants: A Challenge for Future Diet and Health. Plants 2022, 11, 344. [Google Scholar] [CrossRef]
- Pinela, J.; Carvalho, A.M.; Ferreira, I.C.F.R. Wild edible plants: Nutritional and toxicological characteristics, retrieval strategies and importance for today’s society. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 110, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Queirós, B.; Barreira, J.C.M.; Sarmento, A.C.; Ferreira, I.C.F.R. In search of synergistic effects in antioxidant capacity of combined edible mushrooms. Int. J. Food Sci. Nutr. 2009, 60, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Tharmabalan, R.T. Nutritional Analysis of Five Wild Edible Vegetables Traditionally Consumed by the Orang Asli in Perak. Int. J. Food Sci. 2021, 8823565. [Google Scholar] [CrossRef]
- Ullah, I.; Gul, S.; Rehman, H.U.; Ahmad, N.; Ullah, I.; Jawad, S.M.; Ali, J.; Ahmad, A.; Akbar, M.U. Analysis of nutrients and minerals of some wild edible plants. Int. J. Fauna Biol. Stud. 2017, 4, 35–39. [Google Scholar]
- Monteiro, C.A. Nutrition and health. The issue is not food, nor nutrients, so much as processing. Public. Health Nutr. 2009, 12, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Crowe, K. Designing Functional Foods with Bioactive Polyphenols: Highlighting Lessons Learned from Original Plant Matrices. 2014. Available online: https://www.semanticscholar.org/paper/Designing-Functional-Foods-with-Bioactive-%3A-Lessons-Crowe/dea27cdd60e5ebf8319d3651dac58e298aafb033 (accessed on 23 August 2023).
- Aguilera, J.M. The food matrix: Implications in processing, nutrition and health. Crit. Rev. Food Sci. Nutr. 2019, 59, 3612–3629. [Google Scholar] [CrossRef]
- Bolling, B.W.; Chen, C.-Y.O.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef]
- Virgen-Carrillo, C.A.; Martínez Moreno, A.G.; Valdés Miramontes, E.H. Potential Hypoglycemic Effect of Pomegranate Juice and Its Mechanism of Action: A Systematic Review. J. Med. Food 2020, 23, 1–11. [Google Scholar] [CrossRef]
- Bellows, A.C.; Valente, F.L.S.; Lemke, S.; de Lara, M.D.N.B. (Eds.) Gender, Nutrition, and the Human Right to Adequate Food: Toward an Inclusive Framework; Routledge: London, UK, 2017. [Google Scholar] [CrossRef]
- Kurashima, N.; Jeremiah, J.; Ticktin, T.; Ka Wā, I. Ma Mua: The Value of a Historical Ecology Approach to Ecological Restoration in Hawaii 1. Pac. Sci. 2017, 71, 437–456. [Google Scholar] [CrossRef]
- Waldman, J. On natural abundances: Remembering not to forget. In Running Silver: Restoring Atlantic Rivers and Their Great Fish Migrations, 1st ed.; Lyons Press: Lyon, France, 2013; pp. 63–71. [Google Scholar]
- Ojha, S.N.; Anand, A.; Sundriyal, R.C.; Arya, D. Traditional Dietary Knowledge of a Marginal Hill Community in the Central Himalaya: Implications for Food, Nutrition, and Medicinal Security. Front. Pharmacol. 2021, 12, 789360. [Google Scholar] [CrossRef]
- Johns, T.; Sthapit, B.R. Biocultural Diversity in the Sustainability of Developing-Country Food Systems. Food Nutr. Bull. 2004, 25, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Spring, A.; Neyelle, M.; Bezha, W.; Simmons, D.; Blay-Palmer, A. Learning from the past to deal with the future: Using different knowledges to ensure food security in the Tsá Tué biosphere reserve (Northwest Territories, Canada). Front. Sustain. Food Syst. 2023, 6, 984290. [Google Scholar] [CrossRef]
- Biedenweg, K.; Harguth, H.; Stiles, K. The science and politics of human well-being: A case study in cocreating indicators for Puget Sound restoration. Ecol. Soc. 2017, 22, 11. [Google Scholar] [CrossRef]
- Reyes-García, V.; Paneque-Gálvez, J.; Luz, A.C.; Gueze, M.; Macía, M.J.; Orta-Martínez, M.; Pino, J. Cultural change and traditional ecological knowledge. An empirical analysis from the Tsimane’ in the Bolivian Amazon. Hum. Organ. 2014, 73, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Pape, T. Futuristic restoration as a policy tool for environmental justice objectives. Restor. Ecol. 2022, 30, e13629. [Google Scholar] [CrossRef]
- Pilgrim, S.; Samson, C.; Pretty, J. Rebuilding Lost Connections: How Revitalisation Projects Contribute to Cultural Continuity and Improve the Environment; Semantic Scholar: Seattle, WA, USA, 2023. [Google Scholar]
- Maillard, K.N. On Remote Farms and in City Gardens, a Native American Movement Grows. The New York Times. 26 August 2022. Available online: https://www.nytimes.com/2022/08/26/dining/native-american-agriculture.html (accessed on 23 August 2023).
- Lang, M. Buen vivir as a territorial practice. Building a more just and sustainable life through interculturality. Sustain. Sci. 2022, 17, 1287–1299. [Google Scholar] [CrossRef]
- Kimmerer, R.W. Restoration and Reciprocity: The Contributions of Traditional Ecological Knowledge to the Philosophy and Practice of Ecological Restoration. In Human Dimensions of Ecological Restoration: Integrating Science, Nature, and Culture; Egan, D., Hjerpe, E.E., Abrams, J., Eds.; Island Press: Washington, DC, USA, 2011; pp. 257–276. [Google Scholar]
- Gingell, T.; Murray, K.; Correa-Velez, I.; Gallegos, D. Determinants of food security among people from refugee backgrounds resettled in high-income countries: A systematic review and thematic synthesis. PLoS ONE 2022, 17, e0268830. [Google Scholar] [CrossRef]
- Pasquier Merino, A.G.; Torres Salcido, G.; Monachon, D.S.; Villatoro Hernández, J.G. Alternative Food Networks, Social Capital, and Public Policy in Mexico City. Sustainability 2022, 14, 23. [Google Scholar] [CrossRef]
- Lyson, T.A. Civic Agriculture: Reconnecting Farm, Food, and Community; University Press of New England: Lebanon, NH, USA, 2004; Available online: https://press.uchicago.edu/ucp/books/book/distributed/C/bo44309143.html (accessed on 23 August 2023).
- Tidball, M.M.; Tidball, K.G.; Curtis, P. The Absence of Wild Game and Fish Species from the USDA National Nutrient Database for Standard Reference: Addressing Information Gaps in Wild Caught Foods. Ecol. Food Nutr. 2014, 53, 142–148. [Google Scholar] [CrossRef]
- Little Bear, L. Jagged Worldviews Colliding. In Reclaiming Indigenous Voice and Vision; Battiste, M., Ed.; University of British Columbia Press: Vancouver, BC, Canada, 2000; Available online: https://press.uchicago.edu/ucp/books/book/distributed/R/bo69994495.html (accessed on 23 August 2023).
- Nestle, M. Unsavory Truth, 1st ed.; Basic Books: New York, NY, USA, 2018. [Google Scholar]
- Scrinis, G. Nutritionism: The Science and Politics of Dietary Advice. In Nutritionism; Columbia University Press: Vancouver, BC, Canada, 2013. [Google Scholar] [CrossRef]
- Scrinis, G. Ultra-processed foods and the corporate capture of nutrition-an essay by Gyorgy Scrinis. BMJ 2020, 371, m4601. [Google Scholar] [CrossRef]
- Mazza, G. Compositional and Functional Properties of Saskatoon Berry and Blueberry. Int. J. Fruit. Sci. 2005, 5, 101–120. [Google Scholar] [CrossRef]
- Demır, F.; Doğan, H.; Özcan, M.; Haciseferoğullari, H. Nutritional and physical properties of hackberry (Celtis australis L.). J. Food Eng. 2002, 54, 241–247. [Google Scholar] [CrossRef]
- Imran, M.; Khan, H.; Shah, M.; Khan, R.; Khan, F. Chemical composition and antioxidant activity of certain Morus species. J. Zhejiang Univ. Sci. B 2010, 11, 973–980. [Google Scholar] [CrossRef]
- Peterson, R.N.; Cherry, J.P.; Simmons, J.G. Composition of pawpaw (Asimina triloba) fruit [Flavor and aroma, eastern United States]. Annu. Rep. North. Nut Grow. Assoc. 1982, 77, 97–106. [Google Scholar]
- Yang, L.; Ahmed, S.; Stepp, J.R.; Zhao, Y.; Zeng, M.J.; Pei, S.; Xue, D.; Xu, G. Cultural Uses, Ecosystem Services, and Nutrient Profile of Flowering Quince (Chaenomeles speciosa) in the Highlands of Western Yunnan, China. Econ. Bot. 2015, 69, 273–283. [Google Scholar] [CrossRef]
- Schmitzer, V.; Sircelj, H.; Stampar, F.; Slatnar, A. Physico-chemical characterization of Cornus kousa Burg. Fruit: Determining optimal maturity for fresh consumption. J. Sci. Food Agric. 2021, 101, 778–785. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L.; Giménez-Martínez, J.J.; Torija-Isasa, M.E. Nutritional Composition of Wild Edible Crucifer Species. J. Food Biochem. 1999, 23, 283–294. [Google Scholar] [CrossRef]
- Zennie, T.; Ogzewalla, D. Ascorbic acid and Vitamin A content of edible wild plants of Ohio and Kentucky. Econ. Bot. 1977, 31, 76–79. [Google Scholar] [CrossRef]
| Location | Cancer a (%) | Coronary Heart Disease (%) | Diabetes (%) | High Blood Pressure (%) | Kidney Disease (%) | Obesity (%) | Food Insecurity b LI = Low-Income LA = Low-Access c | |
|---|---|---|---|---|---|---|---|---|
| United States | 7.5 | 8.2 | 12.7 | 37.1 | 3.5 | 36.0 | ||
| Onondaga County | 7.4 | 6.3 | 9.7 | 30.7 | 2.9 | 31.9 | ---- | |
| City of Syracuse | 5.5 | 6.4 | 10.9 | 30.9 | 3.2 | 35.9 | ---- | |
| Southside (mean of 4 Census Tracts) | 5.2 | 8.9 | 19.0 | 44.1 | 5.2 | 49.1 | ---- | |
| Census Tract 42 | 4.3 | 9.1 | 20.1 | 44.0 | 5.7 | 52.3 | Not LI and/or LA | |
| Census Tract 53 | 6.4 | 11.0 | 20.3 | 46.7 | 5.8 | 47.9 | LI and LA at 0.5 miles | |
| Census Tract 54 | 5.2 | 7.7 | 17.7 | 42.6 | 4.6 | 47.3 | LI and LA at 0.5 miles | |
| Census Tract 58 | 5.0 | 7.6 | 17.9 | 43.2 | 4.6 | 48.9 | LI and LA at 0.5 miles | |
| Assigned Score | Vitamin A (DV = 900 mcg) | Vitamin C (DV = 90 mg) | Vitamin E (DV = 15 mg) | Selenium (DV = 55 mcg) | Riboflavin (DV = 1.3 mg) | |
|---|---|---|---|---|---|---|
| High source (≥20% DV) | 3 | ≥180 mcg | ≥18 mg | ≥3 mg | ≥11 mcg | ≥0.26 mg |
| Medium source (20–5% DV) | 2 | 45–180 mcg | 4.5–18 mg | 0.75– 3 mg | 2.75–11mcg | 0.065–0.26 mg |
| Low source (≤5% DV) | 1 | ≤45 mcg | ≤4.5 mg | ≤0.75 mg | ≤2.75 mcg | ≤0.065 mg |
| No value | 0 | 0 | 0 | 0 | 0 | 0 |
| Scientific Name | Common Name | Edible Part | Vitamin A (RAE mcg) | Vitamin C (mg) | Vitamin E (mg) | Se (mcg) | Riboflavin (mg) |
|---|---|---|---|---|---|---|---|
| Fruits | |||||||
| Amelanchier alnifolia | Saskatoon serviceberry | raw fruit | 10.91 | 3.55 | 1.12 | 3.54 | |
| Asimina triloba | pawpaw | raw fruit | 8.6 | 18.3 | 0.09 | ||
| Celtis occidentalis | hackberry | raw fruit | 18.3 | ||||
| Chaenomeles speciosa | flowering quince | raw fruit | 72.15 | ||||
| Cornus kousa | kousa dogwood | raw fruit | 93 | ||||
| Diospyros virginiana | persimmon b | raw fruit | 66 | ||||
| Malus spp. | apple | raw fruit | 3 | 4.6 | 0.18 | 0 | 0.026 |
| Malus spp. | crabapple | raw fruit | 2 | 8 | 0.02 | ||
| Morus alba | white mulberry | raw fruit | 15.2 | 0.088 | |||
| Morus nigra | black mulberry | raw fruit | 1 | 36.4 | 0.87 | 0.6 | 0.101 |
| Prunus americana | American plum | raw fruit | 173 | 10.3 | 0.53 | 0.042 | |
| Prunus avium | sweet cherry | raw fruit | 3 | 7 | 0.07 | 0 | 0.033 |
| Pyrus spp. | pear | raw fruit | 1 | 4.3 | 0.12 | 0.1 | 0.026 |
| Ribes nigrum | blackcurrant | raw fruit | 12 | 181 | 1 | 0.05 | |
| Ribes rubrum | redcurrant | raw fruit | 2 | 41 | 0.1 | 0.6 | 0.05 |
| Ribes uva-crispa | gooseberry | raw fruit | 15 | 27.7 | 0.37 | 0.6 | 0.03 |
| Rubus fruticosus | blackberry | raw fruit | 11 | 21 | 1.17 | 0.4 | 0.026 |
| Rubus idaeus | red raspberry | raw fruit | 2 | 26.4 | 0.57 | 0.08 | |
| Sambucus canadensis | black elderberry | raw fruit | 30 | 36 | 0.06 | 0.06 | |
| Vitis spp. | grape | raw fruit | 5 | 4 | 0.19 | 0.1 | 0.057 |
| Herbs | |||||||
| Achillea millefolium | yarrow c | leaves | 0.95 | ||||
| Mentha spicata | spearmint | fresh leaves | 203 | 13.3 | 0.175 | ||
| Salvia officinalis | sage | ground herb | 295 | 32.4 | 7.48 | 3.7 | 0.336 |
| Nuts | |||||||
| Carya illinoinensis | pecan | raw nut | 3 | 1.1 | 1.4 | 3.8 | 0.13 |
| Corylus americana | hazelnut | raw nut | 1 | 6.3 | 15 | 2.4 | 0.113 |
| Juglans cinerea | butternut | dried nut | 6 | 3.2 | 17.2 | 0.148 | |
| Juglans nigra | black walnut | dried nut | 2 | 1.7 | 2.08 | 17 | 0.13 |
| Juglans regia | English walnut | dried nut | 1 | 1.3 | 0.7 | 4.9 | 0.15 |
| Vegetables | |||||||
| Alliaria petiolata | garlic mustard | fresh leaves | 26.1 | ||||
| Allium tricoccum | ramps | whole plant | 80 | ||||
| Chenopodium album | lambsquarters | raw vegetable | 580 | 80 | 0.9 | 0.44 | |
| Matteuccia struthiopteris | ostrich fern | raw fronds | 181 | 26.6 | 0.21 | ||
| Nasturtium officinale | watercress | fresh leaves | 160 | 43 | 1 | 0.9 | 0.12 |
| Portulaca oleracea | purslane | raw vegetable | 21 | 0.9 | 0.112 | ||
| Taraxacum spp. | dandelion | raw greens | 508 | 35 | 3.44 | 0.5 | 0.26 |
| Typha latifolia | broadleaf cattail | young shoots | 1 | 0.7 | 0.6 | 0.025 | |
| Vitis spp. | grape | leaves | 1380 | 11.1 | 2 | 0.9 | 0.354 |
| Other | |||||||
| Acer saccharum | sugar maple | sap | 0 | 0 | 0 | 0.6 | 1.27 |
| Scientific Name | Common Name | Edible Part | Vit A Score | Vit C Score | Vit E Score | Se Score | Riboflavin Score | Composite Score |
|---|---|---|---|---|---|---|---|---|
| Fruits | ||||||||
| Morus nigra | black mulberry | raw fruit | 1 | 3 | 2 | 1 | 2 | 9 |
| Rubus fruticosus | blackberry | raw fruit | 1 | 3 | 2 | 1 | 1 | 8 |
| Ribes uva | gooseberry | raw fruit | 1 | 3 | 1 | 1 | 1 | 7 |
| Ribes rubrum | redcurrant | raw fruit | 1 | 3 | 1 | 1 | 1 | 7 |
| Prunus avium | sweet cherry | raw fruit | 1 | 2 | 1 | 0 | 1 | 5 |
| Pyrus spp. | pear | raw fruit | 1 | 1 | 1 | 1 | 1 | 5 |
| Vitis spp. | grape | raw fruit | 1 | 1 | 1 | 1 | 1 | 5 |
| Malus spp. | apple | raw fruit | 1 | 1 | 1 | 0 | 1 | 4 |
| Herbs | ||||||||
| Salvia officinalis | sage | ground herb | 3 | 3 | 3 | 2 | 3 | 14 |
| Nuts | ||||||||
| Juglans nigra | black walnut | dried nut | 1 | 1 | 2 | 3 | 2 | 9 |
| Corylus americana | hazelnut | raw nut | 1 | 2 | 3 | 1 | 2 | 9 |
| Carya illinoinensis | pecan | raw nut | 1 | 1 | 2 | 2 | 2 | 8 |
| Juglans regia | English walnut | dried nut | 1 | 1 | 1 | 2 | 2 | 7 |
| Vegetables | ||||||||
| Taraxacum spp. | dandelion | raw greens | 3 | 3 | 3 | 1 | 3 | 13 |
| Vitis spp. | grape | leaves | 3 | 2 | 2 | 1 | 3 | 11 |
| Nasturtium officinale | watercress | fresh leaves | 2 | 3 | 2 | 1 | 2 | 10 |
| Other | ||||||||
| Acer saccharum | sugar maple | sap | 0 | 0 | 0 | 1 | 3 | 4 |
| Scientific Name | Common Name | Edible Part | Vit A Score | Vit C Score | Vit E Score | Se Score | Riboflavin Score | Composite Score |
|---|---|---|---|---|---|---|---|---|
| Fruits | ||||||||
| Morus nigra | black mulberry | raw fruit | 1 | 3 | 2 | 1 | 2 | 9 |
| Fragaria spp. | strawberry a | raw fruit | 1 | 3 | 1 | 1 | 1 | 7 |
| (1 mcg/100 g) | (58.8 mg/100 g) | (0.29 mg/100 g) | (0.4 mcg/100 g) | (0.022 mg/100 g) | ||||
| Herbs | ||||||||
| Salvia officinalis | sage | ground herb | 3 | 3 | 3 | 2 | 3 | 14 |
| Ocimum basilicum | basil b | fresh herb | 3 | 3 | 2 | 1 | 2 | 11 |
| (264 mcg/100 g) | (18 mg/100 g) | (0.8 mg/100 g) | (0.3 mcg/100 g) | (0.076 mg/100 g) | ||||
| Nuts | ||||||||
| Juglans nigra | black walnut | dried nut | 1 | 1 | 2 | 3 | 2 | 9 |
| Prunus dulcis | almond c | raw nut | 0 | 0 | 3 | 2 | 3 | 8 |
| (0 mcg/100 g) | (0 mg/100 g) | (25.6 mg/100 g) | (4.1 mcg/100 g) | (1.14 mg/100 g) | ||||
| Vegetables | ||||||||
| Taraxacum spp. | dandelion | raw greens | 3 | 3 | 3 | 1 | 3 | 13 |
| Lactuca sativa | romaine lettuce d | raw leaves | 3 | 1 | 1 | 1 | 2 | 8 |
| (436 mcg/100 g) | (4 mg/100 g) | (0.13 mg/100 g) | (0.4 mcg/100 g) | (0.067 mg/100 g) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellows, A.C.; Raj, S.; Pitstick, E.; Potteiger, M.R.; Diemont, S.A.W. Foraging Wild Edibles: Dietary Diversity in Expanded Food Systems. Nutrients 2023, 15, 4630. https://doi.org/10.3390/nu15214630
Bellows AC, Raj S, Pitstick E, Potteiger MR, Diemont SAW. Foraging Wild Edibles: Dietary Diversity in Expanded Food Systems. Nutrients. 2023; 15(21):4630. https://doi.org/10.3390/nu15214630
Chicago/Turabian StyleBellows, Anne C., Sudha Raj, Ellen Pitstick, Matthew R. Potteiger, and Stewart A. W. Diemont. 2023. "Foraging Wild Edibles: Dietary Diversity in Expanded Food Systems" Nutrients 15, no. 21: 4630. https://doi.org/10.3390/nu15214630
APA StyleBellows, A. C., Raj, S., Pitstick, E., Potteiger, M. R., & Diemont, S. A. W. (2023). Foraging Wild Edibles: Dietary Diversity in Expanded Food Systems. Nutrients, 15(21), 4630. https://doi.org/10.3390/nu15214630






