Microbiota-Derived Extracellular Vesicles Promote Immunity and Intestinal Maturation in Suckling Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Isolation of EVs
2.2. Animals
2.3. Experimental Design
2.4. Sample Collection
2.5. Quantification of Immunoglobulins
2.6. Gene Expression Analysis by Reverse Transcription Quantitative PCR (RT-qPCR)
2.7. Lymphocyte Isolation from Spleen
2.8. Lymphocyte Phenotypic Analysis by Immunofluoroscence Staining and Flow Cytometry
2.9. Histomorphometry Analysis
2.10. Statistical Analysis
3. Results
3.1. Growth and Morphometry Variables
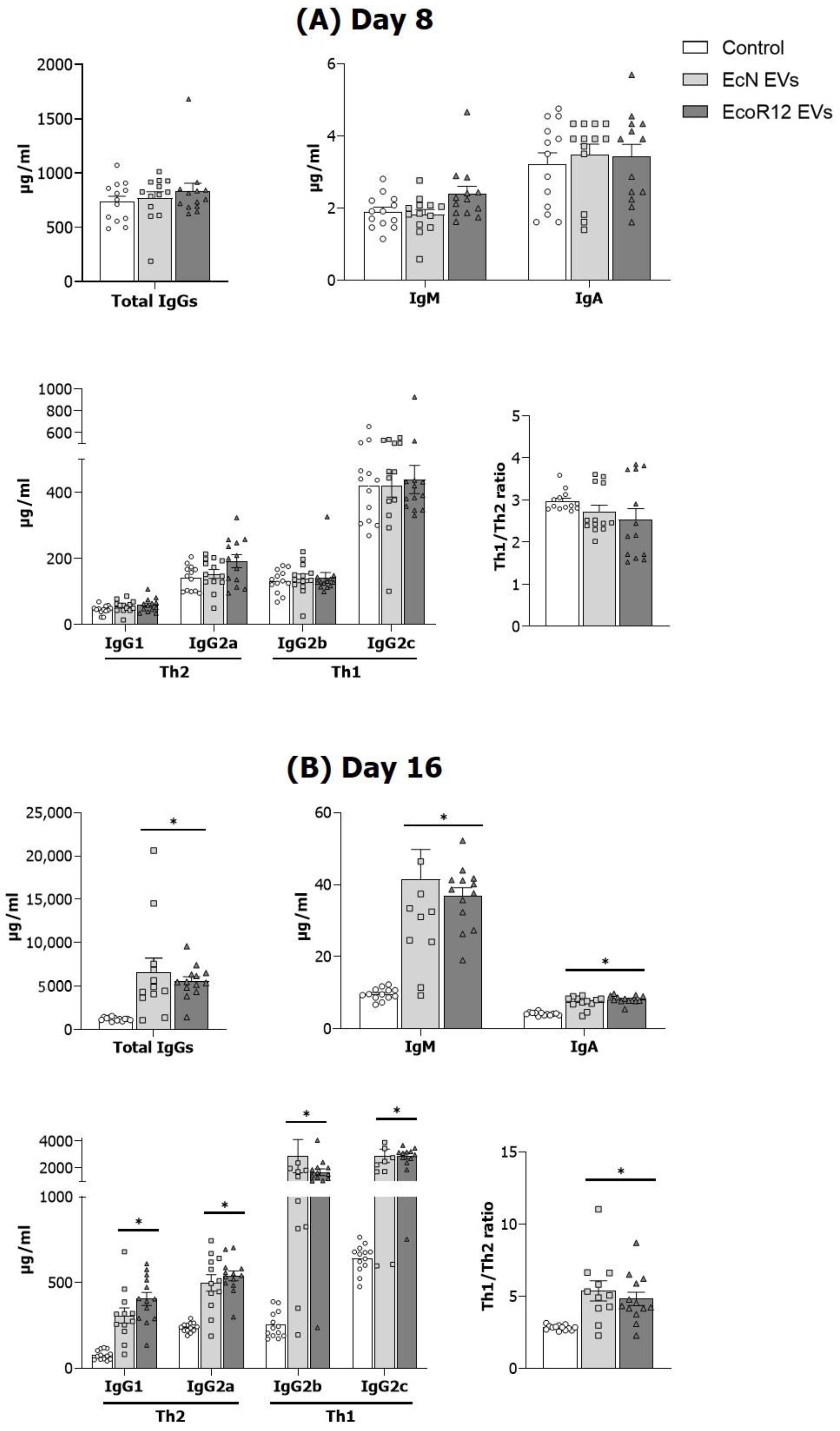
3.2. EVs from EcN and EcoR12 Stimulate Immunoglobulin Production in Neonatal Rats
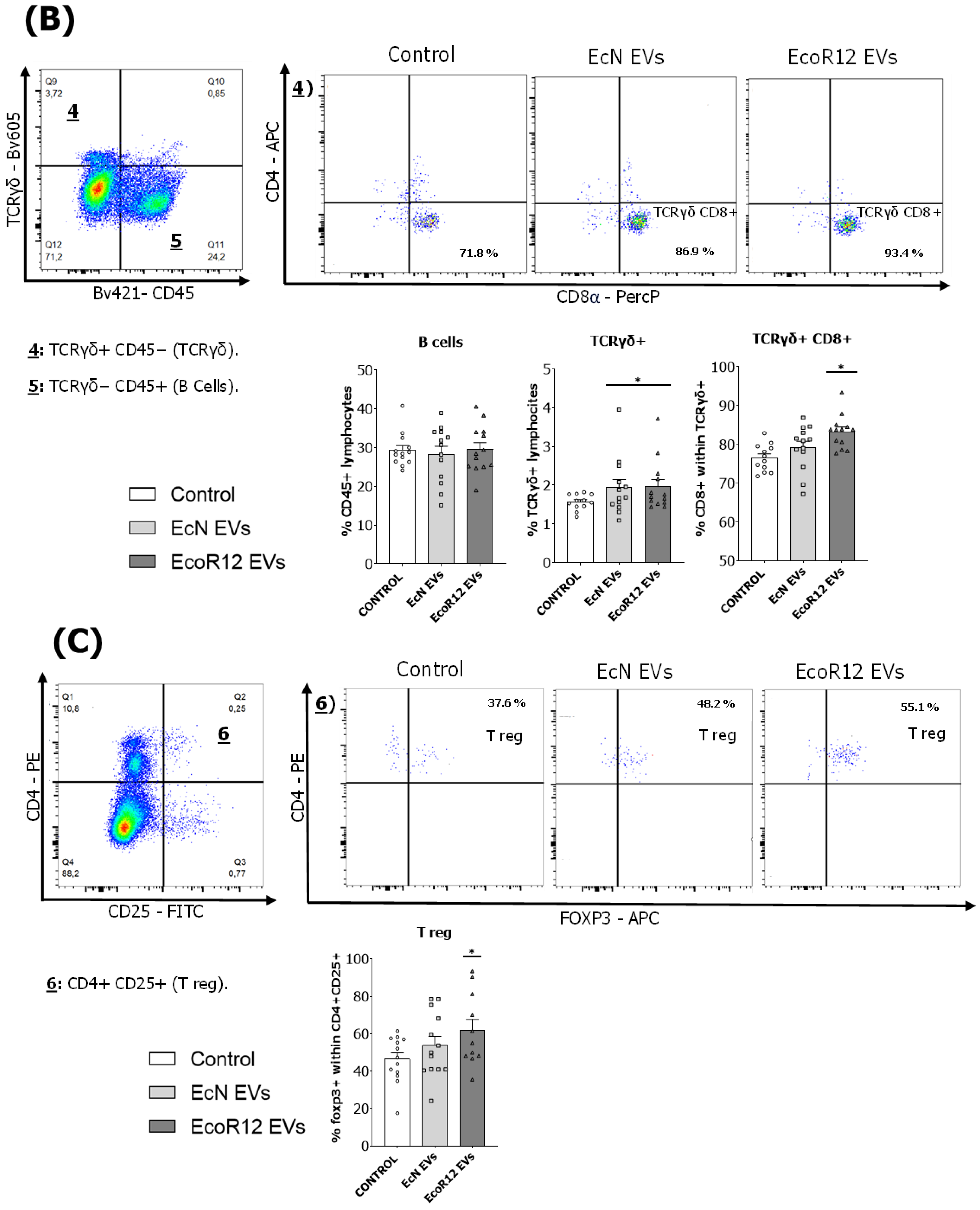
3.3. Analysis of the Spleen Lymphoid Subsets
3.4. Intestinal Gene Expression Analysis
3.5. Intestinal Histomorphometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caballero, S.; Pamer, E.G. Microbiota-Mediated Inflammation and Antimicrobial Defense in the Intestine. Annu. Rev. Immunol. 2015, 33, 227–256. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2015, 14, 20–32. [Google Scholar] [CrossRef]
- Tropini, C.; Earle, K.A.; Huang, K.C.; Sonnenburg, J.L. The Gut Microbiome: Connecting Spatial Organization to Function. Cell Host Microbe 2017, 21, 433–442. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Turner, J.R. Tight Junction Pore and Leak Pathways: A Dynamic Duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef]
- Gleeson, J.P.; Fein, K.C.; Chaudhary, N.; Doerfler, R.; Newby, A.N.; Whitehead, K.A. The enhanced intestinal permeability of infant mice enables oral protein and macromolecular absorption without delivery technology. Int. J. Pharm. 2021, 593, 120120. [Google Scholar] [CrossRef]
- Weström, B.; Sureda, E.A.; Pierzynowska, K.; Pierzynowski, S.G.; Pérez-Cano, F.-J. The Immature Gut Barrier and Its Importance in Establishing Immunity in Newborn Mammals. Front. Immunol. 2020, 11, 1153. [Google Scholar] [CrossRef]
- Sureda, E.A.; Weström, B.; Pierzynowski, S.G.; Prykhodko, O. Maturation of the Intestinal Epithelial Barrier in Neonatal Rats Coincides with Decreased FcRn Expression, Replacement of Vacuolated Enterocytes and Changed Blimp-1 Expression. PLoS ONE 2016, 11, e0164775. [Google Scholar] [CrossRef]
- Sánchez, B.; Urdaci, M.C.; Margolles, A. Extracellular proteins secreted by probiotic bacteria as mediators of effects that promote mucosa–bacteria interactions. Microbiology 2010, 156, 3232–3242. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Liu, H.; Zeng, X.; Zhang, G.; Qiao, S.; Wang, G.S.; et al. Bridging intestinal immunity and gut microbiota by metabolites. Cell. Mol. Life Sci. 2019, 76, 3917–3937. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ren, Y.; Fu, X. Inter-kingdom signaling between gut microbiota and their host. Cell. Mol. Life Sci. 2019, 76, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Stentz, R.; Carvalho, A.L.; Jones, E.J.; Carding, S.R. Fantastic voyage: The journey of intestinal microbiota-derived microvesicles through the body. Biochem. Soc. Trans. 2018, 46, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Garrido, N.; Badia, J.; Baldomà, L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J. Extracell. Vesicles 2021, 10, e12161. [Google Scholar] [CrossRef]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430. [Google Scholar] [CrossRef]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef]
- Doré, E.; Boilard, E. Bacterial extracellular vesicles and their interplay with the immune system. Pharmacol. Ther. 2023, 247, 108443. [Google Scholar] [CrossRef]
- O’Donoghue, E.J.; Krachler, A.M. Mechanisms of outer membrane vesicle entry into host cells. Cell. Microbiol. 2016, 18, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Cañas, M.-A.; Giménez, R.; Fábrega, M.-J.; Toloza, L.; Baldomà, L.; Badia, J. Outer Membrane Vesicles from the Probiotic Escherichia coli Nissle 1917 and the Commensal ECOR12 Enter Intestinal Epithelial Cells via Clathrin-Dependent Endocytosis and Elicit Differential Effects on DNA Damage. PLoS ONE 2016, 11, e0160374. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Defourny, K.A.Y.; Smid, E.J.; Abee, T. Gram-Positive Bacterial Extracellular Vesicles and Their Impact on Health and Disease. Front. Microbiol. 2018, 9, 1502. [Google Scholar] [CrossRef]
- Hu, R.; Lin, H.; Wang, M.; Zhao, Y.; Liu, H.; Min, Y.; Yang, X.; Gao, Y.; Yang, M. Lactobacillus reuteri-derived extracellular vesicles maintain intestinal immune homeostasis against lipopolysaccharide-induced inflammatory responses in broilers. J. Anim. Sci. Biotechnol. 2021, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Olivo-Martínez, Y.; Bosch, M.; Badia, J.; Baldomà, L. Modulation of the Intestinal Barrier Integrity and Repair by Microbiota Extracellular Vesicles through the Differential Regulation of Trefoil Factor 3 in LS174T Goblet Cells. Nutrients 2023, 15, 2437. [Google Scholar] [CrossRef]
- Diaz-Garrido, N.; Badia, J.; Baldomà, L. Modulation of Dendritic Cells by Microbiota Extracellular Vesicles Influences the Cytokine Profile and Exosome Cargo. Nutrients 2022, 14, 344. [Google Scholar] [CrossRef]
- Ashrafian, F.; Shahriary, A.; Behrouzi, A.; Moradi, H.R.; Raftar, S.K.A.; Lari, A.; Hadifar, S.; Yaghoubfar, R.; Badi, S.A.; Khatami, S.; et al. Akkermansia muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front. Microbiol. 2019, 10, 2155. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef]
- Choi, J.; Kim, Y.-K.; Han, P.-L. Extracellular Vesicles Derived from Lactobacillus plantarum Increase BDNF Expression in Cultured Hippocampal Neurons and Produce Antidepressant-like Effects in Mice. Exp. Neurobiol. 2019, 28, 158–171. [Google Scholar] [CrossRef]
- Yaghoubfar, R.; Behrouzi, A.; Ashrafian, F.; Shahryari, A.; Moradi, H.R.; Choopani, S.; Hadifar, S.; Vaziri, F.; Nojoumi, S.A.; Fateh, A.; et al. Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice. Sci. Rep. 2020, 10, 22119. [Google Scholar] [CrossRef]
- Díez-Sainz, E.; Milagro, F.I.; Riezu-Boj, J.I.; Lorente-Cebrián, S. Effects of gut microbiota–derived extracellular vesicles on obesity and diabetes and their potential modulation through diet. J. Physiol. Biochem. 2022, 78, 485–499. [Google Scholar] [CrossRef]
- Gul, L.; Modos, D.; Fonseca, S.; Madgwick, M.; Thomas, J.P.; Sudhakar, P.; Booth, C.; Stentz, R.; Carding, S.R.; Korcsmaros, T. Extracellular vesicles produced by the human commensal gut bacterium Bacteroides thetaiotaomicron affect host immune pathways in a cell-type specific manner that are altered in inflammatory bowel disease. J. Extracell. Vesicles 2022, 11, e12189. [Google Scholar] [CrossRef] [PubMed]
- Fábrega, M.-J.; Rodríguez-Nogales, A.; Garrido-Mesa, J.; Algieri, F.; Badía, J.; Giménez, R.; Gálvez, J.; Baldomà, L. Intestinal Anti-inflammatory Effects of Outer Membrane Vesicles from Escherichia coli Nissle 1917 in DSS-Experimental Colitis in Mice. Front. Microbiol. 2017, 8, 1274. [Google Scholar] [CrossRef] [PubMed]
- Ochman, H.; Selander, R.K. Standard Reference Strains of Escherichia coli from Natural Populations. J. Bacteriol. 1984, 157, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Frias, A.; Manresa, A.; de Oliveira, E.; López-Iglesias, C.; Mercade, E. Membrane Vesicles: A Common Feature in the Extracellular Matter of Cold-Adapted Antarctic Bacteria. Microb. Ecol. 2010, 59, 476–486. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Van’t Land, B.; Stahl, B.; Garssen, J.; Rodríguez-Lagunas, M.J.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Supplementation With 2′-FL and scGOS/lcFOS Ameliorates Rotavirus-Induced Diarrhea in Suckling Rats. Front. Cell. Infect. Microbiol. 2018, 8, 372. [Google Scholar] [CrossRef]
- Torres-Castro, P.; Grases-Pintó, B.; Abril-Gil, M.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J.; Franch, À. Modulation of the Systemic Immune Response in Suckling Rats by Breast Milk TGF-β2, EGF and FGF21 Supplementation. Nutrients 2020, 12, 1888. [Google Scholar] [CrossRef]
- Al-Asmakh, M.; Zadjali, F. Use of Germ-Free Animal Models in Microbiota-Related Research. J. Microbiol. Biotechnol. 2015, 25, 1583–1588. [Google Scholar] [CrossRef]
- Lu, J.; Lu, L.; Yu, Y.; Oliphant, K.; Drobyshevsky, A.; Claud, E.C. Early preterm infant microbiome impacts adult learning. Sci. Rep. 2022, 12, 3310. [Google Scholar] [CrossRef]
- Pérez-Cano, F.J.; Franch, À.; Castellote, C.; Castell, M. The Suckling Rat as a Model for Immunonutrition Studies in Early Life. J. Immunol. Res. 2012, 2012, 537310. [Google Scholar] [CrossRef] [PubMed]
- Pácha, J.; Allaire, J.M.; Morampudi, V.; Crowley, S.M.; Stahl, M.; Yu, H.; Bhullar, K.; Knodler, L.A.; Bressler, B.; Jacobson, K.; et al. Development of Intestinal Transport Function in Mammals. Physiol. Rev. 2000, 80, 1633–1667. [Google Scholar] [CrossRef] [PubMed]
- Bitto, N.J.; Kaparakis-Liaskos, M. The Therapeutic Benefit of Bacterial Membrane Vesicles. Int. J. Mol. Sci. 2017, 18, 1287. [Google Scholar] [CrossRef] [PubMed]
- Raeven, R.H.M.; van der Maas, L.; Tilstra, W.; Uittenbogaard, J.P.; Bindels, T.H.E.; Kuipers, B.; van der Ark, A.; Pennings, J.L.A.; van Riet, E.; Jiskoot, W.; et al. Immunoproteomic Profiling of Bordetella pertussis Outer Membrane Vesicle Vaccine Reveals Broad and Balanced Humoral Immunogenicity. J. Proteome Res. 2015, 14, 2929–2942. [Google Scholar] [CrossRef] [PubMed]
- Bottero, D.; Gaillard, M.; Zurita, E.; Moreno, G.; Martinez, D.S.; Bartel, E.; Bravo, S.; Carriquiriborde, F.; Errea, A.; Castuma, C.; et al. Characterization of the immune response induced by pertussis OMVs-based vaccine. Vaccine 2016, 34, 3303–3309. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Yang, C.; Wu, Y.; Zhou, X.; Liu, H.; Wang, Y. Bacterial outer membrane vesicles as a platform for biomedical applications: An update. J. Control. Release 2020, 323, 253–268. [Google Scholar] [CrossRef]
- Maddux, A.B.; Douglas, I.S. Is the developmentally immature immune response in paediatric sepsis a recapitulation of immune tolerance? Immunology 2015, 145, 1–10. [Google Scholar] [CrossRef]
- Dong, C. Cytokine Regulation and Function in T Cells. Annu. Rev. Immunol. 2021, 39, 51–76. [Google Scholar] [CrossRef]
- Jones, E.J.; Booth, C.; Fonseca, S.; Parker, A.; Cross, K.; Miquel-Clopés, A.; Hautefort, I.; Mayer, U.; Wileman, T.; Stentz, R.; et al. The Uptake, Trafficking, and Biodistribution of Bacteroides thetaiotaomicron Generated Outer Membrane Vesicles. Front. Microbiol. 2020, 11, 57. [Google Scholar] [CrossRef]
- Hendrix, A.; De Wever, O. Systemically circulating bacterial extracellular vesicles: Origin, fate, and function. Trends Microbiol. 2022, 30, 213–216. [Google Scholar] [CrossRef]
- Tulkens, J.; Vergauwen, G.; Van Deun, J.; Geeurickx, E.; Dhondt, B.; Lippens, L.; De Scheerder, M.-A.; Miinalainen, I.; Rappu, P.; De Geest, B.G.; et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut 2020, 69, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Chakaroun, R.M.; Massier, L.; Kovacs, P. Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders? Nutrients 2020, 12, 1082. [Google Scholar] [CrossRef] [PubMed]
- Kühl, A.A.; Erben, U.; Kredel, L.I.; Siegmund, B. Diversity of Intestinal Macrophages in Inflammatory Bowel Diseases. Front. Immunol. 2015, 6, 613. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 2010, 29, 781–788. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Underwood, M.A.; Umberger, E.; Patel, R.M. Safety and efficacy of probiotic administration to preterm infants: Ten common questions. Pediatr. Res. 2020, 88, 48–55. [Google Scholar] [CrossRef]
- Baldassarre, M.E.; Di Mauro, A.; Capozza, M.; Rizzo, V.; Schettini, F.; Panza, R.; Laforgia, N. Dysbiosis and Prematurity: Is There a Role for Probiotics? Nutrients 2019, 11, 1273. [Google Scholar] [CrossRef]
- Indrio, F.; Neu, J.; Pettoello-Mantovani, M.; Marchese, F.; Martini, S.; Salatto, A.; Aceti, A. Development of the Gastrointestinal Tract in Newborns as a Challenge for an Appropriate Nutrition: A Narrative Review. Nutrients 2022, 14, 1405. [Google Scholar] [CrossRef]
- Golubkova, A.; Hunter, C.J. Development of the Neonatal Intestinal Barrier, Microbiome, and Susceptibility to NEC. Microorganisms 2023, 11, 1247. [Google Scholar] [CrossRef]




| Day 8 | Control | EcN EVs | EcoR12 EVs |
| Animal length (cm) | 9.83 ± 0.15 | 9.64 ± 0.12 | 9.96 ± 0.07 |
| Body (cm) | 6.87 ± 0.11 | 6.86 ± 0.12 | 6.74 ± 0.11 |
| Tail (cm) | 3.05 ± 0.06 | 2.91 ± 0.05 | 3.15 ± 0.1 |
| Body mass index (g/cm2) | 0.24 ± 0.01 | 0.25 ± 0.01 | 0.27 ± 0.01 * |
| Lee index (g0.33/cm × 1000) | 331.15 ± 2.96 | 331.23 ± 2.47 | 343.78 ± 4.41 |
| Intestine weight (%) | 3.19 ± 0.06 | 3.38 ± 0.11 | 3.16 ± 0.05 |
| Intestine length (%) | 231.57 ± 7.39 | 226.00 ± 6.17 | 212.31 ± 4.49 |
| Cecum weight (%) | 0.19 ± 0.04 | 0.19 ± 0.03 | 0.24 ± 0.04 * |
| Spleen weight (%) | 0.48 ± 0.02 | 0.59 ± 0.03 * | 0.50 ± 0.03 |
| Liver weight (%) | 2.85 ± 0.02 | 3.03 ± 0.05 | 2.86± 0.06 |
| Thymus weight (%) | 0.29± 0.01 | 0.29 ± 0.01 | 0.30 ± 0.01 |
| Kidney weight (%) | 0.71 ± 0.01 | 0.69 ± 0.01 | 0.70 ± 0.01 |
| Heart weight (%) | 0.69 ± 0.02 | 0.77 ± 0.02 * | 0.72 ± 0.01 |
| Day 16 | Control | EcN EVs | EcoR12 EVs |
| Animal length (cm) | 14.42 ± 0.22 | 14.36 ± 0.17 | 14.61 ± 0.04 |
| Body (cm) | 9.32 ± 0.12 | 9.32 ± 0.08 | 9.51 ± 0.06 |
| Tail (cm) | 5.1 ± 0.09 | 5.03 ± 0.09 | 5.1 ± 0.05 |
| Body mass index (g/cm2) | 0.33 ± 0.01 | 0.32 ± 0.01 | 0.31 ± 0.01 |
| Lee index (g0.33/cm × 1000) | 328.6 ± 3.15 | 326.98 ± 2.26 | 321.77 ± 2.9 |
| Intestine weight (%) | 2.94 ± 0.04 | 2.97 ± 0.04 | 2.95 ± 0.04 |
| Intestine length (%) | 126.08 ± 5.51 | 132.18 ± 3.67 | 129.27 ± 3.53 |
| Cecum weight (%) | 0.21 ± 0.01 | 0.24 ± 0.01 | 0.25 ± 0.01 * |
| Spleen weight (%) | 0.44 ± 0.02 | 0.50 ± 0.01 * | 0.41 ± 0.01 |
| Liver weight (%) | 3.08 ± 0.03 | 3.24 ± 0.1 | 3.20 ± 0.07 |
| Thymus weight (%) | 0.52 ± 0.01 | 0.47 ± 0.01 | 0.51 ± 0.01 |
| Kidney weight (%) | 0.61 ± 0.01 | 0.63 ± 0.02 | 0.60 ± 0.01 |
| Heart weight (%) | 0.62 ± 0.02 | 0.65 ± 0.02 | 0.62 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Ruiz, S.; Sáez-Fuertes, L.; Casanova-Crespo, S.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J.; Badia, J.; Baldoma, L. Microbiota-Derived Extracellular Vesicles Promote Immunity and Intestinal Maturation in Suckling Rats. Nutrients 2023, 15, 4701. https://doi.org/10.3390/nu15214701
Martínez-Ruiz S, Sáez-Fuertes L, Casanova-Crespo S, Rodríguez-Lagunas MJ, Pérez-Cano FJ, Badia J, Baldoma L. Microbiota-Derived Extracellular Vesicles Promote Immunity and Intestinal Maturation in Suckling Rats. Nutrients. 2023; 15(21):4701. https://doi.org/10.3390/nu15214701
Chicago/Turabian StyleMartínez-Ruiz, Sergio, Laura Sáez-Fuertes, Sergi Casanova-Crespo, María J. Rodríguez-Lagunas, Francisco J. Pérez-Cano, Josefa Badia, and Laura Baldoma. 2023. "Microbiota-Derived Extracellular Vesicles Promote Immunity and Intestinal Maturation in Suckling Rats" Nutrients 15, no. 21: 4701. https://doi.org/10.3390/nu15214701
APA StyleMartínez-Ruiz, S., Sáez-Fuertes, L., Casanova-Crespo, S., Rodríguez-Lagunas, M. J., Pérez-Cano, F. J., Badia, J., & Baldoma, L. (2023). Microbiota-Derived Extracellular Vesicles Promote Immunity and Intestinal Maturation in Suckling Rats. Nutrients, 15(21), 4701. https://doi.org/10.3390/nu15214701







