The Role of a Plant-Only (Vegan) Diet in Gastroesophageal Reflux Disease: Online Survey of the Italian General Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Assessments
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savarino, E.; Bredenoord, A.J.; Fox, M.; Pandolfino, J.E.; Roman, S.; Gyawali, C.P.; International Working Group for Disorders of Gastrointestinal Motility and Function. Advances in the Physiological Assessment and Diagnosis of GERD. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 323. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, C.P.; Azagury, D.E.; Chan, W.W.; Chandramohan, S.M.; Clarke, J.O.; de Bortoli, N.; Figueredo, E.; Fox, M.; Jodorkovsky, D.; Lazarescu, A.; et al. Nonerosive Reflux Disease: Clinical Concepts. Ann. N. Y. Acad. Sci. 2018, 1434, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Savarino, E.; Marabotto, E.; Bodini, G.; Pellegatta, G.; Coppo, C.; Giambruno, E.; Brunacci, M.; Zentilin, P.; Savarino, V. Epidemiology and Natural History of Gastroesophageal Reflux Disease. Minerva Gastroenterol. Dietol. 2017, 63, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Savarino, E.; de Bortoli, N.; De Cassan, C.; Della Coletta, M.; Bartolo, O.; Furnari, M.; Ottonello, A.; Marabotto, E.; Bodini, G.; Savarino, V. The Natural History of Gastro-Esophageal Reflux Disease: A Comprehensive Review. Dis. Esophagus 2017, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Navarro Silvera, S.A.; Mayne, S.T.; Gammon, M.D.; Vaughan, T.L.; Chow, W.-H.; Dubin, J.A.; Dubrow, R.; Stanford, J.L.; West, A.B.; Rotterdam, H.; et al. Diet and Lifestyle Factors and Risk of Subtypes of Esophageal and Gastric Cancers: Classification Tree Analysis. Ann. Epidemiol. 2014, 24, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, J.; Kuribayashi, S.; Baeg, M.K.; de Bortoli, N.; Valitova, E.; Savarino, E.V.; Kusano, M.; Triadafilopoulos, G. Current and Future Perspectives in the Management of Gastroesophageal Reflux Disease. Ann. N. Y. Acad. Sci. 2018, 1434, 70–83. [Google Scholar] [CrossRef]
- Tack, J.; Pandolfino, J.E. Pathophysiology of Gastroesophageal Reflux Disease. Gastroenterology 2018, 154, 277–288. [Google Scholar] [CrossRef]
- Lacy, B.E.; Weiser, K.; Chertoff, J.; Fass, R.; Pandolfino, J.E.; Richter, J.E.; Rothstein, R.I.; Spangler, C.; Vaezi, M.F. The Diagnosis of Gastroesophageal Reflux Disease. Am. J. Med. 2010, 123, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Martinucci, I.; Guidi, G.; Savarino, E.V.; Frazzoni, M.; Tolone, S.; Frazzoni, L.; Fuccio, L.; Bertani, L.; Bodini, G.; Ceccarelli, L.; et al. Vegetal and Animal Food Proteins Have a Different Impact in the First Postprandial Hour of Impedance-pH Analysis in Patients with Heartburn. Gastroenterol. Res. Pract. 2018, 2018, 7572430. [Google Scholar] [CrossRef] [PubMed]
- de Bortoli, N.; Guidi, G.; Martinucci, I.; Savarino, E.; Imam, H.; Bertani, L.; Russo, S.; Franchi, R.; Macchia, L.; Furnari, M.; et al. Voluntary and Controlled Weight Loss Can Reduce Symptoms and Proton Pump Inhibitor Use and Dosage in Patients with Gastroesophageal Reflux Disease: A Comparative Study. Dis. Esophagus 2016, 29, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.; Gyawali, C.P. Dietary Factors Involved in GERD Management. Best Pract. Res. Clin. Gastroenterol. 2023, 62–63, 101826. [Google Scholar] [CrossRef]
- Nilsson, M.; Johnsen, R.; Ye, W.; Hveem, K.; Lagergren, J. Lifestyle Related Risk Factors in the Aetiology of Gastro-Oesophageal Reflux. Gut 2004, 53, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Murao, T.; Sakurai, K.; Mihara, S.; Marubayashi, T.; Murakami, Y.; Sasaki, Y. Lifestyle Change Influences on GERD in Japan: A Study of Participants in a Health Examination Program. Dig. Dis. Sci. 2011, 56, 2857–2864. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, X.; Fan, W.; Yu, J.; Wang, P.; Liu, D.; Song, M.; Liu, S.; Zuo, X.; Zhang, R.; et al. Differences in Dietary and Lifestyle Triggers between Non-Erosive Reflux Disease and Reflux Esophagitis-A Multicenter Cross-Sectional Survey in China. Nutrients 2023, 15, 3400. [Google Scholar] [CrossRef]
- Herdiana, Y. Functional Food in Relation to Gastroesophageal Reflux Disease (GERD). Nutrients 2023, 15, 3583. [Google Scholar] [CrossRef]
- Martinucci, I.; de Bortoli, N.; Savarino, E.; Nacci, A.; Romeo, S.O.; Bellini, M.; Savarino, V.; Fattori, B.; Marchi, S. Optimal Treatment of Laryngopharyngeal Reflux Disease. Ther. Adv. Chronic Dis. 2013, 4, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Katz, P.O.; Dunbar, K.B.; Schnoll-Sussman, F.H.; Greer, K.B.; Yadlapati, R.; Spechler, S.J. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am. J. Gastroenterol. 2022, 117, 27–56. [Google Scholar] [CrossRef]
- Zheng, Z.; Nordenstedt, H.; Pedersen, N.L.; Lagergren, J.; Ye, W. Lifestyle Factors and Risk for Symptomatic Gastroesophageal Reflux in Monozygotic Twins. Gastroenterology 2007, 132, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-W.; Chang, C.-M.; Chang, C.-S.; Kao, A.-W.; Chou, M.-C. Comparison of Presentation and Impact on Quality of Life of Gastroesophageal Reflux Disease between Young and Old Adults in a Chinese Population. World J. Gastroenterol. 2011, 17, 4614–4618. [Google Scholar] [CrossRef] [PubMed]
- Wahlqvist, P.; Karlsson, M.; Johnson, D.; Carlsson, J.; Bolge, S.C.; Wallander, M.-A. Relationship between Symptom Load of Gastro-Oesophageal Reflux Disease and Health-Related Quality of Life, Work Productivity, Resource Utilization and Concomitant Diseases: Survey of a US Cohort. Aliment. Pharmacol. Ther. 2008, 27, 960–970. [Google Scholar] [CrossRef]
- Wiklund, I. Review of the Quality of Life and Burden of Illness in Gastroesophageal Reflux Disease. Dig. Dis. 2004, 22, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Dent, J.; Brun, J.; Fendrick, A.; Fennerty, M.; Janssens, J.; Kahrilas, P.; Lauritsen, K.; Reynolds, J.; Shaw, M.; Talley, N. An Evidence-Based Appraisal of Reflux Disease Management--the Genval Workshop Report. Gut 1999, 44 (Suppl. S2), S1–S16. [Google Scholar] [CrossRef]
- Vakil, N.; van Zanten, S.V.; Kahrilas, P.; Dent, J.; Jones, R.; Global Consensus Group. The Montreal Definition and Classification of Gastroesophageal Reflux Disease: A Global Evidence-Based Consensus. Am. J. Gastroenterol. 2006, 101, 1900–1920; quiz 1943. [Google Scholar] [CrossRef]
- Apolone, G.; Mosconi, P. The Italian SF-36 Health Survey: Translation, Validation and Norming. J. Clin. Epidemiol. 1998, 51, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Ministero Della Salute, Italia. Alcol, Zero o Il Meno Possibile. Available online: https://www.salute.gov.it/portale/alcol/dettaglioContenutiAlcol.jsp?lingua=italiano&id=5526&area=alcol&menu=vuoto (accessed on 14 September 2023).
- Istituto Superiore di Sanità. Indicatori Passi: Consumo Di Bevande Alcoliche. Available online: https://www.epicentro.iss.it/passi/indicatori/alcol (accessed on 14 September 2023).
- Nirwan, J.S.; Hasan, S.S.; Babar, Z.-U.-D.; Conway, B.R.; Ghori, M.U. Global Prevalence and Risk Factors of Gastro-Oesophageal Reflux Disease (GORD): Systematic Review with Meta-Analysis. Sci. Rep. 2020, 10, 5814. [Google Scholar] [CrossRef] [PubMed]
- Martinucci, I.; Natilli, M.; Lorenzoni, V.; Pappalardo, L.; Monreale, A.; Turchetti, G.; Pedreschi, D.; Marchi, S.; Barale, R.; de Bortoli, N. Gastroesophageal Reflux Symptoms among Italian University Students: Epidemiology and Dietary Correlates Using Automatically Recorded Transactions. BMC Gastroenterol. 2018, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Tosetti, C.; Savarino, E.; Benedetto, E.; De Bastiani, R.; Study Group for the Evaluation of GERD Triggering Foods. Elimination of Dietary Triggers Is Successful in Treating Symptoms of Gastroesophageal Reflux Disease. Dig. Dis. Sci. 2021, 66, 1565–1571. [Google Scholar] [CrossRef]
- Savarino, V.; Marabotto, E.; Zentilin, P.; Furnari, M.; Bodini, G.; De Maria, C.; Tolone, S.; De Bortoli, N.; Frazzoni, M.; Savarino, E. Pathophysiology, Diagnosis, and Pharmacological Treatment of Gastro-Esophageal Reflux Disease. Expert Rev. Clin. Pharmacol. 2020, 13, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, C.P.; Yadlapati, R.; Fass, R.; Katzka, D.; Pandolfino, J.; Savarino, E.; Sifrim, D.; Spechler, S.; Zerbib, F.; Fox, M.R.; et al. Updates to the Modern Diagnosis of GERD: Lyon Consensus 2.0. Gut 2023, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ledeboer, M.; Masclee, A.A.; Batstra, M.R.; Jansen, J.B.; Lamers, C.B. Effect of Cholecystokinin on Lower Oesophageal Sphincter Pressure and Transient Lower Oesophageal Sphincter Relaxations in Humans. Gut 1995, 36, 39–44. [Google Scholar] [CrossRef]
- Chirila, I.; Morariu, I.D.; Barboi, O.B.; Drug, V.L. The Role of Diet in the Overlap between Gastroesophageal Reflux Disease and Functional Dyspepsia. Turk. J. Gastroenterol. 2016, 27, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Zalvan, C.H.; Hu, S.; Greenberg, B.; Geliebter, J. A Comparison of Alkaline Water and Mediterranean Diet vs Proton Pump Inhibition for Treatment of Laryngopharyngeal Reflux. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.G.; Kang, H.W.; Hahn, S.J.; Kim, J.H.; Lee, J.K.; Lim, Y.J.; Koh, M.-S.; Lee, J.H. Vegetarianism as a Protective Factor for Reflux Esophagitis: A Retrospective, Cross-Sectional Study between Buddhist Priests and General Population. Dig. Dis. Sci. 2013, 58, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Temple, N.; Woodside, J.V. Vegetarian Diets, Low-Meat Diets and Health: A Review. Public Health Nutr. 2012, 15, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Beezhold, B.L.; Johnston, C.S.; Daigle, D.R. Vegetarian Diets Are Associated with Healthy Mood States: A Cross-Sectional Study in Seventh Day Adventist Adults. Nutr. J. 2010, 9, 26. [Google Scholar] [CrossRef]
- Okuyama, M.; Takaishi, O.; Nakahara, K.; Iwakura, N.; Hasegawa, T.; Oyama, M.; Inoue, A.; Ishizu, H.; Satoh, H.; Fujiwara, Y. Associations among Gastroesophageal Reflux Disease, Psychological Stress, and Sleep Disturbances in Japanese Adults. Scand. J. Gastroenterol. 2017, 52, 44–49. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, R.A.; Verbruggen, M.A.; Offutt, E.J. Anti-Heartburn Effects of a Fenugreek Fiber Product. Phytother. Res. 2011, 25, 88–91. [Google Scholar] [CrossRef]
- Rauma, A.L.; Mykkänen, H. Antioxidant Status in Vegetarians versus Omnivores. Nutrition 2000, 16, 111–119. [Google Scholar] [CrossRef]
- Olyaee, M.; Sontag, S.; Salman, W.; Schnell, T.; Mobarhan, S.; Eiznhamer, D.; Keshavarzian, A. Mucosal Reactive Oxygen Species Production in Oesophagitis and Barrett’s Oesophagus. Gut 1995, 37, 168–173. [Google Scholar] [CrossRef]
- Peng, D.; Zaika, A.; Que, J.; El-Rifai, W. The Antioxidant Response in Barrett’s Tumorigenesis: A Double-Edged Sword. Redox Biol. 2021, 41, 101894. [Google Scholar] [CrossRef]
- Veugelers, P.J.; Porter, G.A.; Guernsey, D.L.; Casson, A.G. Obesity and Lifestyle Risk Factors for Gastroesophageal Reflux Disease, Barrett Esophagus and Esophageal Adenocarcinoma. Dis. Esophagus 2006, 19, 321–328. [Google Scholar] [CrossRef]
- Jiao, L.; Kramer, J.R.; Rugge, M.; Parente, P.; Verstovsek, G.; Alsarraj, A.; El-Serag, H.B. Dietary Intake of Vegetables, Folate, and Antioxidants and the Risk of Barrett’s Esophagus. Cancer Causes Control 2013, 24, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Levin, T.R.; Block, G.; Rumore, G.J.; Quesenberry, C.P.; Buffler, P.; Corley, D.A. Dietary Antioxidants, Fruits, and Vegetables and the Risk of Barrett’s Esophagus. Am. J. Gastroenterol. 2008, 103, 1614–1623; quiz 1624. [Google Scholar] [CrossRef] [PubMed]
- Thompson, O.M.; Beresford, S.A.A.; Kirk, E.A.; Vaughan, T.L. Vegetable and Fruit Intakes and Risk of Barrett’s Esophagus in Men and Women. Am. J. Clin. Nutr. 2009, 89, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.A.; Watson, R.G.P.; Murphy, S.J.; Johnston, B.T.; Comber, H.; Mc Guigan, J.; Reynolds, J.V.; Murray, L.J. Risk Factors for Barrett’s Oesophagus and Oesophageal Adenocarcinoma: Results from the FINBAR Study. World J. Gastroenterol. 2007, 13, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.C.B.; Antonsson, A.; Rodriguez-Acevedo, A.J.; Liyanage, U.E.; Green, A.C.; van der Pols, J.C. Dark Green Leafy Vegetable Intake, MTHFR Genotype, and Risk of Cutaneous Squamous Cell Carcinoma. Dermatology 2022, 238, 657–661. [Google Scholar] [CrossRef]
- Kubo, A.; Corley, D.A.; Jensen, C.D.; Kaur, R. Dietary Factors and the Risks of Oesophageal Adenocarcinoma and Barrett’s Oesophagus. Nutr. Res. Rev. 2010, 23, 230–246. [Google Scholar] [CrossRef]
- Hajizadeh, B.; Jessri, M.; Moasheri, S.M.; Rad, A.H.; Rashidkhani, B. Fruits and Vegetables Consumption and Esophageal Squamous Cell Carcinoma: A Case-Control Study. Nutr. Cancer 2011, 63, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Otles, S.; Ozgoz, S. Health Effects of Dietary Fiber. Acta Sci. Pol. Technol. Aliment. 2014, 13, 191–202. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant Starch: Promise for Improving Human Health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Velanovich, V. Comparison of Generic (SF-36) vs. Disease-Specific (GERD-HRQL) Quality-of-Life Scales for Gastroesophageal Reflux Disease. J. Gastrointest. Surg. 1998, 2, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Eloubeidi, M.A.; Provenzale, D. Health-Related Quality of Life and Severity of Symptoms in Patients with Barrett’s Esophagus and Gastroesophageal Reflux Disease Patients without Barrett’s Esophagus. Am. J. Gastroenterol. 2000, 95, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Maleki, I.; Masoudzadeh, A.; Khalilian, A.; Daheshpour, E. Quality of Life in Patients with Gastroesophageal Reflux Disease in an Iranian Population. Gastroenterol. Hepatol. Bed Bench 2013, 6, 96–100. [Google Scholar] [PubMed]
- Cheung, T.K.; Lam, P.K.Y.; Wei, W.I.; Wong, W.M.; Ng, M.L.; Gu, Q.; Hung, I.F.; Wong, B.C.Y. Quality of Life in Patients with Laryngopharyngeal Reflux. Digestion 2009, 79, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Dimenäs, E.; Glise, H.; Hallerbäck, B.; Hernqvist, H.; Svedlund, J.; Wiklund, I. Quality of Life in Patients with Upper Gastrointestinal Symptoms. An Improved Evaluation of Treatment Regimens? Scand. J. Gastroenterol. 1993, 28, 681–687. [Google Scholar] [CrossRef]
- Revicki, D.A.; Wood, M.; Maton, P.N.; Sorensen, S. The Impact of Gastroesophageal Reflux Disease on Health-Related Quality of Life. Am. J. Med. 1998, 104, 252–258. [Google Scholar] [CrossRef]
- Wiklund, I.; Bardhan, K.D.; Müller-Lissner, S.; Bigard, M.A.; Bianchi Porro, G.; Ponce, J.; Hosie, J.; Scott, M.; Weir, D.; Fulton, C.; et al. Quality of Life during Acute and Intermittent Treatment of Gastro-Oesophageal Reflux Disease with Omeprazole Compared with Ranitidine. Results from a Multicentre Clinical Trial. The European Study Group. Ital. J. Gastroenterol. Hepatol. 1998, 30, 19–27. [Google Scholar]
- Mathias, S.D.; Colwell, H.H.; Miller, D.P.; Pasta, D.J.; Henning, J.M.; Ofman, J.J. Health-Related Quality-of-Life and Quality-Days Incrementally Gained in Symptomatic Nonerosive GERD Patients Treated with Lansoprazole or Ranitidine. Dig. Dis. Sci. 2001, 46, 2416–2423. [Google Scholar] [CrossRef] [PubMed]
- Ofman, J.J. The Economic and Quality-of-Life Impact of Symptomatic Gastroesophageal Reflux Disease. Am. J. Gastroenterol. 2003, 98, S8–S14. [Google Scholar] [CrossRef]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A Randomised Controlled Trial of Dietary Improvement for Adults with Major Depression (the “SMILES” Trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef]
- Yin, W.; Löf, M.; Chen, R.; Hultman, C.M.; Fang, F.; Sandin, S. Mediterranean Diet and Depression: A Population-Based Cohort Study. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Pasco, J.A.; Mykletun, A.; Williams, L.J.; Hodge, A.M.; O’Reilly, S.L.; Nicholson, G.C.; Kotowicz, M.A.; Berk, M. Association of Western and Traditional Diets with Depression and Anxiety in Women. Am. J. Psychiatry 2010, 167, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Matison, A.P.; Mather, K.A.; Flood, V.M.; Reppermund, S. Associations between Nutrition and the Incidence of Depression in Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Prospective Observational Population-Based Studies. Ageing Res. Rev. 2021, 70, 101403. [Google Scholar] [CrossRef] [PubMed]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-Style Dietary Intervention Supplemented with Fish Oil Improves Diet Quality and Mental Health in People with Depression: A Randomized Controlled Trial (HELFIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef]
- Francis, H.M.; Stevenson, R.J.; Chambers, J.R.; Gupta, D.; Newey, B.; Lim, C.K. A Brief Diet Intervention Can Reduce Symptoms of Depression in Young Adults—A Randomised Controlled Trial. PLoS ONE 2019, 14, e0222768. [Google Scholar] [CrossRef] [PubMed]
- Istituto Superiore di Sanità. Sorveglianza PASSI 2021–2022. Available online: https://www.epicentro.iss.it/passi/dati/socio (accessed on 28 August 2023).
- Eurispes. Rapporto Italia 2023. Available online: https://eurispes.eu/news/risultati-del-rapporto-italia-2023/ (accessed on 28 August 2023).
- Gyawali, C.P.; Kahrilas, P.J.; Savarino, E.; Zerbib, F.; Mion, F.; Smout, A.J.P.M.; Vaezi, M.; Sifrim, D.; Fox, M.R.; Vela, M.F.; et al. Modern Diagnosis of GERD: The Lyon Consensus. Gut 2018, 67, 1351–1362. [Google Scholar] [CrossRef] [PubMed]

| a. Socio-Demographic Characteristics | Overall Sample n = 1077 | GERD− n = 982 (91.2%) | GERD+ n = 95 (8.8%) | p-Value |
|---|---|---|---|---|
| Gender, n (%) | 0.672 Fisher | |||
| Male | 75 (7.0%) | 70 (7.1%) | 5 (5.3%) | |
| Female | 1002 (93.0%) | 912 (92.9%) | 90 (94.7%) | |
| Age, mean (SD) | 37.1 (12.0) | 37.0 (11.9) | 37.7 (12.9) | 0.583 t test |
| BMI, mean (SD) | 22.2 (3.8) | 22.0 (3.5) | 24.1 (5.4) | <0.001 t test |
| Marital status, n (%) | 0.508 Fisher | |||
| Married | 664 (61.7%) | 602 (61.3%) | 62 (65.3%) | |
| Not married | 413 (38.3%) | 380 (38.7%) | 33 (34.7%) | |
| Education, n (%) | 0.027 Fisher | |||
| Professional qualification/Diploma | 362 (33.6%) | 321 (32.7%) | 41 (43.2%) | |
| Degree/Post-degree | 715 (66.4%) | 661 (67.3%) | 54 (56.8%) | |
| Occupation, n (%) | 0.097 Fisher | |||
| Employed | 765 (71.0%) | 705 (71.8%) | 60 (63.2%) | |
| Not employed | 312 (29.0%) | 277 (28.2%) | 35 (36.8%) | |
| b. Life habits | Overall Sample n = 1077 | GERD− n = 982 (91.2%) | GERD+ n = 95 (8.8%) | p-value |
| Dietary pattern, n (%) | 0.005 Fisher | |||
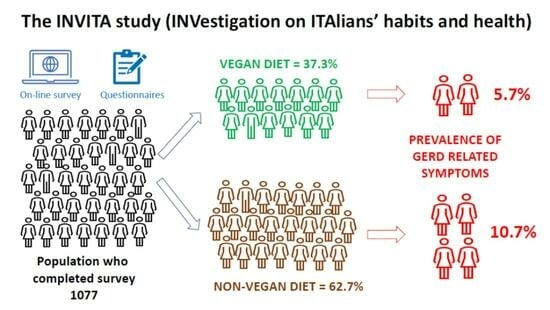
| Vegan | 402 (37.3%) | 379 (38.6%) | 23 (24.2%) | |
| Non-vegan | 675 (62.7%) | 603 (61.4%) | 72 (75.8%) | |
| Monthly alcohol consumption, n (%) | 32 missing | 30 missing | 2 missing | 0.864 Chi-square |
| No consumption | 224 (21.4%) | 206 (21.6%) | 18 (19.4%) | |
| Low/Moderate 1 | 770 (73.7%) | 700 (73.5%) | 70 (75.3%) | |
| At risk 2 | 51 (4.9%) | 46 (4.8%) | 5 (5.4%) | |
| Currently smoking, n (%) | 5 missing | 4 missing | 1 missing | 0.022 Fisher |
| No | 975 (91.0%) | 896 (91.6%) | 79 (84.0%) | |
| Yes | 97 (9.0%) | 82 (8.4%) | 15 (16.0%) | |
| c. Health-related quality of life (SF-36), mean (SD) | Overall Sample n = 1077 | GERD− n = 982 (91.2%) | GERD+ n = 95 (8.8%) | p-value t test |
| General health | 68.4 (17.2) | 69.7 (16.0) | 54.3 (22.5) | <0.001 |
| Physical functioning | 94.6 (9.9) | 95.2 (8.7) | 88.3 (17.4) | <0.001 |
| Role limitations due to emotional problems | 58.9 (40.9) | 60.0 (40.7) | 48.2 (41.5) | 0.007 |
| Bodily pain | 82.7 (20.3) | 84.0 (19.4) | 68.4 (23.4) | <0.001 |
| Emotional well-being | 65.8 (17.1) | 66.6 (16.7) | 57.9 (18.7) | <0.001 |
| Role limitations due to physical health | 84.6 (28.8) | 86.0 (27.2) | 70.0 (39.4) | <0.001 |
| Energy/fatigue | 53.8 (18.4) | 54.8 (17.9) | 43.9 (20.8) | <0.001 |
| Social functioning | 74.3 (22.7) | 75.4 (22.0) | 63.0 (25.8) | <0.001 |
| Independent Variable | OR (Unadjusted) | 95% CI | p-Value |
|---|---|---|---|
| Gender | |||
| Male | Ref. | - | - |
| Female | 1.38 | 0.54–3.51 | 0.497 |
| Age | 1.01 | 0.99–1.02 | 0.345 |
| BMI | 1.12 | 1.07–1.17 | <0.001 |
| Marital status | |||
| Married | Ref. | - | - |
| Not married | 0.84 | 0.54–1.31 | 0.449 |
| Education | |||
| Professional qualification/Diploma | Ref. | - | - |
| Degree/Post-degree | 0.64 | 0.42–0.98 | 0.040 |
| Occupation | |||
| Employed | Ref. | - | - |
| Not employed | 1.48 | 0.96–2.30 | 0.078 |
| Dietary pattern | |||
| Non-vegan | Ref. | - | - |
| Vegan | 0.51 | 0.31–0.83 | 0.006 |
| Monthly alcohol consumption | |||
| No consumption | Ref. | - | - |
| Low/Moderate 1 | 1.14 | 0.67–1.96 | 0.625 |
| At risk 2 | 1.24 | 0.44–3.52 | 0.681 |
| Currently smoking | |||
| No | Ref. | - | - |
| Yes | 2.07 | 1.14–3.77 | 0.016 |
| General health | 0.96 | 0.95–0.97 | <0.001 |
| Physical functioning | 0.98 | 0.98–0.99 | <0.001 |
| Role limitations due to emotional problems | 0.99 | 0.98–0.99 | 0.008 |
| Bodily pain | 0.97 | 0.96–0.98 | <0.001 |
| Emotional well-being | 0.97 | 0.96–0.98 | <0.001 |
| Role limitations due to physical health | 0.96 | 0.94–0.97 | <0.001 |
| Energy/fatigue | 0.97 | 0.96–0.98 | <0.001 |
| Social functioning | 0.98 | 0.97–0.99 | <0.001 |
| Independent Variable | OR (Adjusted) | 95% CI | p-Value |
|---|---|---|---|
| BMI | 1.07 | 1.02–1.13 | 0.007 |
| Education | |||
| Professional qualification/Diploma | Ref. | - | - |
| Degree/Post-degree | 0.74 | 0.46–1.19 | 0.219 |
| Dietary pattern | |||
| Non-vegan | Ref. | - | - |
| Vegan | 0.47 | 0.28–0.81 | 0.006 |
| Currently smoking | |||
| No | Ref. | - | - |
| Yes | 1.97 | 1.03–3.74 | 0.039 |
| General health | 0.97 | 0.96–0.99 | 0.001 |
| Physical functioning | 1.00 | 0.99–1.01 | 0.721 |
| Role limitations due to emotional problems | 1.00 | 0.99–1.01 | 0.317 |
| Bodily pain | 0.98 | 0.97–0.99 | 0.005 |
| Emotional well-being | 0.99 | 0.97–1.01 | 0.544 |
| Role limitations due to physical health | 1.00 | 0.98–1.02 | 0.921 |
| Energy/fatigue | 0.99 | 0.97–1.01 | 0.547 |
| Social functioning | 1.00 | 0.98–1.01 | 0.583 |
| Number of observations | 1077 | ||
| LR test, p-value | Chi2(12) = 94.45, p < 0.001 | ||
| Hosmer—Lemeshow goodness-of-fit (10 groups) | |||
| Chi2(df), p-value | Chi2(8) = 7.55, p = 0.479 | ||
| Pearson goodness-of-fit | |||
| Number of covariate patterns | 1072 | ||
| Chi2(df), p-value | Chi2(1059) = 1060.64, p = 0.480 | ||
| Area under ROC curve | 0.78 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, G.; Baroni, L.; Bonetto, C.; Visaggi, P.; Orazzini, M.; Solinas, I.; Guidi, G.; Pugliese, J.; Scaramuzza, G.; Ovidi, F.; et al. The Role of a Plant-Only (Vegan) Diet in Gastroesophageal Reflux Disease: Online Survey of the Italian General Population. Nutrients 2023, 15, 4725. https://doi.org/10.3390/nu15224725
Rizzo G, Baroni L, Bonetto C, Visaggi P, Orazzini M, Solinas I, Guidi G, Pugliese J, Scaramuzza G, Ovidi F, et al. The Role of a Plant-Only (Vegan) Diet in Gastroesophageal Reflux Disease: Online Survey of the Italian General Population. Nutrients. 2023; 15(22):4725. https://doi.org/10.3390/nu15224725
Chicago/Turabian StyleRizzo, Gianluca, Luciana Baroni, Chiara Bonetto, Pierfrancesco Visaggi, Mattia Orazzini, Irene Solinas, Giada Guidi, Jessica Pugliese, Giulia Scaramuzza, Filippo Ovidi, and et al. 2023. "The Role of a Plant-Only (Vegan) Diet in Gastroesophageal Reflux Disease: Online Survey of the Italian General Population" Nutrients 15, no. 22: 4725. https://doi.org/10.3390/nu15224725
APA StyleRizzo, G., Baroni, L., Bonetto, C., Visaggi, P., Orazzini, M., Solinas, I., Guidi, G., Pugliese, J., Scaramuzza, G., Ovidi, F., Buselli, I., Bellini, M., Savarino, E. V., & de Bortoli, N. (2023). The Role of a Plant-Only (Vegan) Diet in Gastroesophageal Reflux Disease: Online Survey of the Italian General Population. Nutrients, 15(22), 4725. https://doi.org/10.3390/nu15224725