What Is Food Noise? A Conceptual Model of Food Cue Reactivity
Abstract
:1. Introduction
2. The Anecdotal Evidence on “Food Noise”
3. Food Cue Reactivity: An Adaptive Characteristic That Can Lead to Maladaptive Eating Behaviors
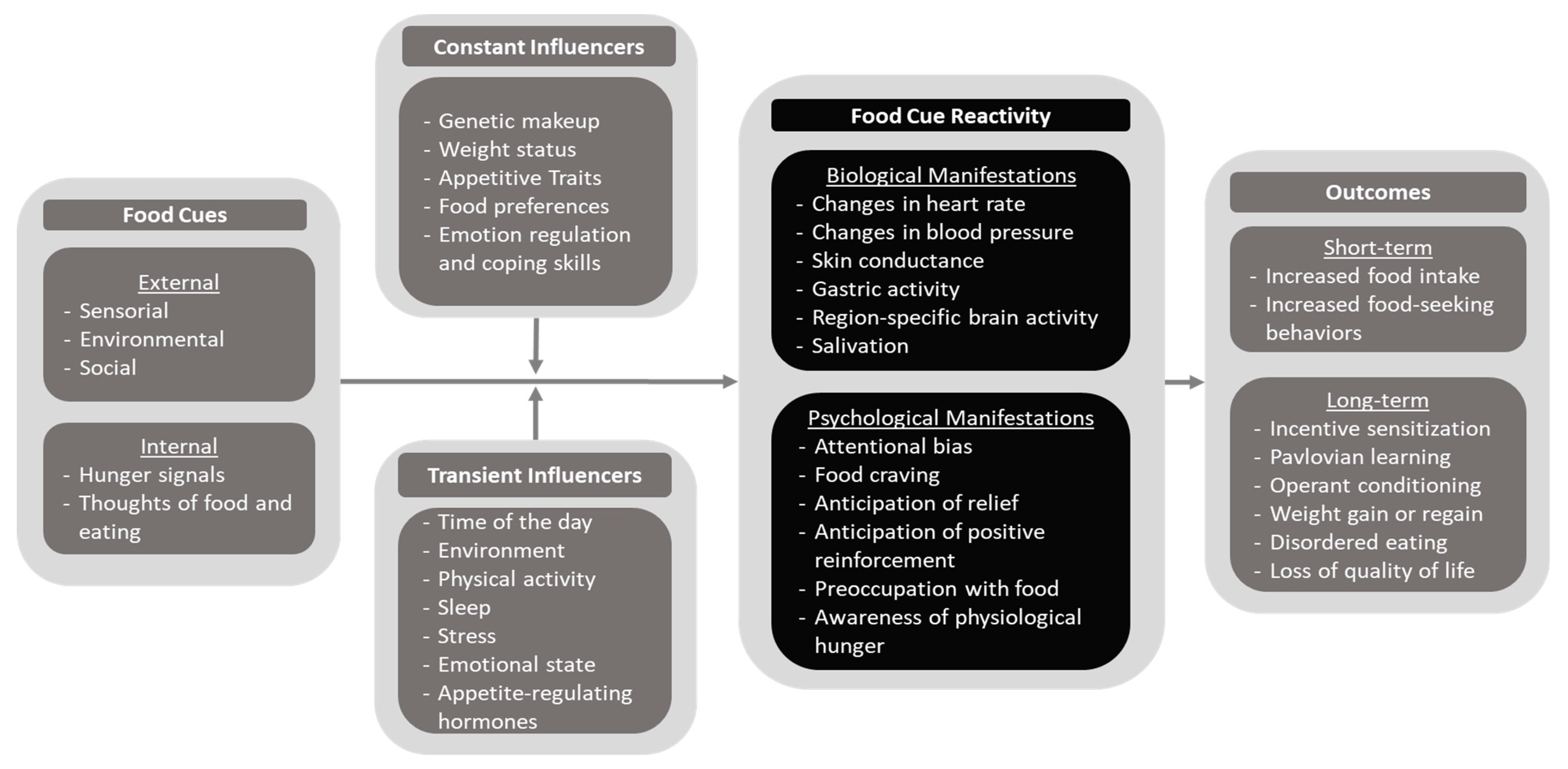
4. The Cue–Influencer–Reactivity–Outcome (CIRO) Model of Food Cue Reactivity
5. Methods for Assessing Food Cue Reactivity
6. Biological Manifestations of Food Cue Reactivity
7. Psychological Manifestations of Food Cue Reactivity
8. Outcomes of Increased Food Cue Reactivity
9. GLP-1RAs and Their Possible Role in Managing Behavioral Addictions and Substance Use Disorders: Additional Insights from the CIRO Model of Food Cue Reactivity
10. Research and Public Health Implications
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blüher, M.; Aras, M.; Aronne, L.J.; Batterham, R.L.; Giorgino, F.; Ji, L.; Pietiläinen, K.H.; Schnell, O.; Tonchevska, E.; Wilding, J.P.H. New insights into the treatment of obesity. Diabetes Obes. Metab. 2023, 25, 2058–2072. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, Q.W.; Yang, X.Y.; Yang, W.; Li, D.R.; Jin, J.Y.; Zhang, H.C.; Zhang, X.F. GLP-1 receptor agonists for the treatment of obesity: Role as a promising approach. Front. Endocrinol. 2023, 14, 1085799. [Google Scholar] [CrossRef]
- Rubino, D.M.; Greenway, F.L.; Khalid, U.; O’Neil, P.M.; Rosenstock, J.; Sørrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T. Effect of Weekly Subcutaneous Semaglutide vs. Daily Liraglutide on Body Weight in Adults with Overweight or Obesity without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA 2022, 327, 138–150. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Van Gaal, L.F.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wharton, S.; Yokote, K.; Zeuthen, N.; et al. Impact of Semaglutide on Body Composition in Adults with Overweight or Obesity: Exploratory Analysis of the STEP 1 Study. J. Endocr. Soc. 2021, 5 (Suppl. S1), A16–A17. [Google Scholar] [CrossRef]
- Nauck, M.A.; D’Alessio, D.A. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc. Diabetol. 2022, 21, 169. [Google Scholar] [CrossRef]
- de Mesquita, Y.L.L.; Pera Calvi, I.; Reis Marques, I.; Cruz, S.A.; Padrao, E.M.H.; de Paula Carvalho, P.E.; da Silva, C.H.A.; Cardoso, R.; Moura, F.A.; Rafalskiy, V.V. Efficacy and safety of the dual GIP and GLP-1 receptor agonist tirzepatide for weight loss: A meta-analysis of randomized controlled trials. Int. J. Obes. 2023, 47, 883–892. [Google Scholar] [CrossRef]
- Rosenstock, J.; Frias, J.; Jastreboff, A.M.; Du, Y.; Lou, J.; Gurbuz, S.; Thomas, M.K.; Hartman, M.L.; Haupt, A.; Milicevic, Z.; et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: A randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet 2023, 402, 529–544. [Google Scholar] [CrossRef]
- Abdi Beshir, S.; Ahmed Elnour, A.; Soorya, A.; Mohamed, A.P.; Goh, S.S.L.; Hussain, N.; Al Haddad, A.H.I.; Hussain, F.; Khidir, I.Y.; Abdelnassir, Z. A narrative review of approved and emerging anti-obesity medications. Saudi Pharm. J. 2023, 31, 101757. [Google Scholar] [CrossRef]
- Shaefer, C.F., Jr.; Kushner, P.; Aguilar, R. User’s guide to mechanism of action and clinical use of GLP-1 receptor agonists. Postgrad. Med. 2015, 127, 818–826. [Google Scholar] [CrossRef]
- Collins, L.; Costello, R.A. Glucagon-Like Peptide-1 Receptor Agonists; StatPearls Publishing: Tampa, FL, USA, 2023. [Google Scholar]
- Farr, O.M.; Sofopoulos, M.; Tsoukas, M.A.; Dincer, F.; Thakkar, B.; Sahin-Efe, A.; Filippaios, A.; Bowers, J.; Srnka, A.; Gavrieli, A.; et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: A crossover, randomised, placebo-controlled trial. Diabetologia 2016, 59, 954–965. [Google Scholar]
- López-Ferreras, L.; Richard, J.E.; Noble, E.E.; Eerola, K.; Anderberg, R.H.; Olandersson, K.; Taing, L.; Kanoski, S.E.; Hayes, M.R.; Skibicka, K.P. Lateral hypothalamic GLP-1 receptors are critical for the control of food reinforcement, ingestive behavior and body weight. Mol. Psychiatry 2018, 23, 1157–1168. [Google Scholar] [CrossRef]
- Gabery, S.; Salinas, C.G.; Paulsen, S.J.; Ahnfelt-Rønne, J.; Alanentalo, T.; Baquero, A.F.; Buckley, S.T.; Farkas, E.; Fekete, C.; Frederiksen, K.S.; et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020, 5, e133429. [Google Scholar] [CrossRef]
- Trapp, S.; Brierley, D.I. Brain GLP-1 and the regulation of food intake: GLP-1 action in the brain and its implications for GLP-1 receptor agonists in obesity treatment. Br. J. Pharmacol. 2022, 179, 557–570. [Google Scholar] [CrossRef]
- Dong, M.; Wen, S.; Zhou, L. The Relationship between the Blood-Brain-Barrier and the Central Effects of Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter-2 Inhibitors. Diabetes Metab. Syndr. Obes. 2022, 15, 2583–2597. [Google Scholar] [CrossRef]
- Chuong, V.; Farokhnia, M.; Khom, S.; Pince, C.L.; Elvig, S.K.; Vlkolinsky, R.; Marchette, R.C.; Koob, G.F.; Roberto, M.; Vendruscolo, L.F.; et al. The glucagon-like peptide-1 (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI Insight 2023, 8, e170671. [Google Scholar] [CrossRef]
- Schreiber, L.R.N.; Odlaug, B.L.; Grant, J.E. The overlap between binge eating disorder and substance use disorders: Diagnosis and neurobiology. J. Behav. Addict. 2013, 2, 191–198. [Google Scholar] [CrossRef]
- Giacomini, J.L.; Sadeghian, K.; Baldo, B.A. Eating driven by the gustatory insula: Contrasting regulation by infralimbic vs. prelimbic cortices. Neuropsychopharmacology 2022, 47, 1358–1366. [Google Scholar] [CrossRef]
- Douglass, A.M.; Kucukdereli, H.; Ponserre, M.; Markovic, M.; Gründemann, J.; Strobel, C.; Morales, P.L.A.; Conzelmann, K.-K.; Lüthi, A.; Klein, R. Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat. Neurosci. 2017, 20, 1384–1394. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.W.; Le Roux, C.W.; Ortiz, R.V.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- Ghusn, W.; De la Rosa, A.; Sacoto, D.; Cifuentes, L.; Campos, A.; Feris, F.; Hurtado, M.D.; Acosta, A. Weight Loss Outcomes Associated with Semaglutide Treatment for Patients with Overweight or Obesity. JAMA Netw. Open 2022, 5, e2231982. [Google Scholar] [CrossRef]
- Cassata, C. Drugs Like Ozempic and Wegovy Cut Cravings and Turn down “Food Noise”; Healthline Media: San Francisco, CA, USA, 2023; Available online: https://www.healthline.com/health-news/drugs-like-ozempic-and-wegovy-cut-cravings-and-turn-down-food-noise (accessed on 5 September 2023).
- Blum, D. People on Drugs Like Ozempic Say Their “Food Noise” Has Disappeared. The New York Times. 21 June 2023. Available online: https://www.nytimes.com/2023/06/21/well/eat/ozempic-food-noise.html (accessed on 5 September 2023).
- O’Neill, M. What Is “Food Noise”? How Drugs Like Ozempic and Wegovy Quiet Obsessive Thoughts about Food. Health. 3 July 2023. Available online: https://www.health.com/food-noise-ozempic-wegovy-7555112 (accessed on 5 September 2023).
- Hohman, M. Some Ozempic Patients Report Less “Food Noise”. Here’s What That Means. Yahoo News. 23 June 2023. Available online: https://news.yahoo.com/ozempic-brain-4-patients-share-190933574.html (accessed on 9 November 2023).
- Kuhn, C. Patients Say Drugs Like Ozempic Help with “Food Noise.” Here’s What That Means. PBS. 25 September 2023. Available online: https://www.pbs.org/newshour/health/patients-say-drugs-like-ozempic-help-with-food-noise-heres-what-that-means (accessed on 9 November 2023).
- Ain, H.U. Food Related Intrusive Thoughts: A Pilot Study; University of Richmond: Richmond, VA, USA, 2023; Available online: https://scholarship.richmond.edu/honors-theses/1676/ (accessed on 5 September 2023).
- Kornacka, M.; Czepczor-Bernat, K.; Napieralski, P.; Brytek-Matera, A. Rumination, mood, and maladaptive eating behaviors in overweight and healthy populations. Eat. Weight. Disord. 2021, 26, 273–285. [Google Scholar] [CrossRef]
- Morales, I.; Berridge, K.C. “Liking” and “wanting” in eating and food reward: Brain mechanisms and clinical implications. Physiol. Behav. 2020, 227, 113152. [Google Scholar] [CrossRef]
- Berridge, K.C. “Liking” and “wanting” food rewards: Brain substrates and roles in eating disorders. Physiol. Behav. 2009, 97, 537–550. [Google Scholar] [CrossRef]
- Reichenberger, J.; Richard, A.; Smyth, J.M.; Fischer, D.; Pollatos, O.; Blechert, J. It’s craving time: Time of day effects on momentary hunger and food craving in daily life. Nutrition 2018, 55–56, 15–20. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef]
- Meule, A.; Kübler, A.; Blechert, J. Time course of electrocortical food-cue responses during cognitive regulation of craving. Front. Psychol. 2013, 4, 669. [Google Scholar] [CrossRef]
- Belfort-DeAguiar, R.; Seo, D. Food Cues and Obesity: Overpowering Hormones and Energy Balance Regulation. Curr. Obes. Rep. 2018, 7, 122–129. [Google Scholar] [CrossRef]
- Smith, R.; Kelly, B.; Yeatman, H.; Boyland, E. Food Marketing Influences Children’s Attitudes, Preferences and Consumption: A Systematic Critical Review. Nutrients 2019, 11, 875. [Google Scholar] [CrossRef]
- Pollack, C.C.; Gilbert-Diamond, D.; Emond, J.A.; Eschholz, A.; Evans, R.K.; Boyland, E.J.; Masterson, T.D. Twitch user perceptions, attitudes and behaviours in relation to food and beverage marketing on Twitch compared with YouTube. J. Nutr. Sci. 2021, 10, e32. [Google Scholar] [CrossRef]
- Evans, R.; Christiansen, P.; Masterson, T.; Pollack, C.; Albadri, S.; Boyland, E. Recall of food marketing on videogame livestreaming platforms: Associations with adolescent diet-related behaviours and health. Appetite 2023, 186, 106584. [Google Scholar] [CrossRef]
- Tetley, A.; Brunstrom, J.; Griffiths, P. Individual differences in food-cue reactivity. The role of BMI and everyday portion-size selections. Appetite 2009, 52, 614–620. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Boutelle, K.N. Food cue reactivity: Neurobiological and behavioral underpinnings. Rev. Endocr. Metab. Disord. 2022, 23, 683–696. Available online: https://link.springer.com/article/10.1007/s11154-022-09724-x (accessed on 12 October 2023). [CrossRef]
- Nederkoorn, C.; Smulders, F.T.; Jansen, A. Cephalic phase responses, craving and food intake in normal subjects. Appetite 2000, 35, 45–55. [Google Scholar] [CrossRef]
- Wardle, J.; Guthrie, C.A.; Sanderson, S.; Rapoport, L. Development of the Children’s Eating Behaviour Questionnaire. J. Child. Psychol. Psychiatry 2001, 42, 963–970. [Google Scholar] [CrossRef]
- Hunot, C.; Fildes, A.; Croker, H.; Llewellyn, C.H.; Wardle, J.; Beeken, R.J. Appetitive traits and relationships with BMI in adults: Development of the Adult Eating Behaviour Questionnaire. Appetite 2016, 105, 356–363. [Google Scholar] [CrossRef]
- Rapuano, K.M.; Zieselman, A.L.; Kelley, W.M.; Sargent, J.D.; Heatherton, T.F.; Gilbert-Diamond, D. Genetic risk for obesity predicts nucleus accumbens size and responsivity to real-world food cues. Proc. Natl. Acad. Sci. USA 2017, 114, 160–165. [Google Scholar] [CrossRef]
- Boutelle, K.N.; Manzano, M.A.; Eichen, D.M. Appetitive traits as targets for weight loss: The role of food cue responsiveness and satiety responsiveness. Physiol. Behav. 2020, 224, 113018. [Google Scholar] [CrossRef]
- Paquet, C.; de Montigny, L.; Labban, A.; Buckeridge, D.; Ma, Y.; Arora, N.; Dubé, L. The moderating role of food cue sensitivity in the behavioral response of children to their neighborhood food environment: A cross-sectional study. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 86. [Google Scholar] [CrossRef]
- Serrano-Gonzalez, M.; Herting, M.M.; Lim, S.L.; Sullivan, N.J.; Kim, R.; Espinoza, J.; Koppin, C.M.; Javier, J.R.; Kim, M.S.; Luo, S. Developmental Changes in Food Perception and Preference. Front. Psychol. 2021, 12, 654200. [Google Scholar] [CrossRef]
- Pursey, K.M.; Stanwell, P.; Callister, R.J.; Brain, K.; Collins, C.E.; Burrows, T.L. Neural responses to visual food cues according to weight status: A systematic review of functional magnetic resonance imaging studies. Front. Nutr. 2014, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Poghosyan, V.; Ioannou, S.; Al-Amri, K.M.; Al-Mashhadi, S.A.; Al-Mohammed, F.; Al-Otaibi, T.; Al-Saeed, W. Spatiotemporal profile of altered neural reactivity to food images in obesity: Reward system is altered automatically and predicts efficacy of weight loss intervention. Front. Neurosci. 2023, 17, 948063. [Google Scholar] [CrossRef]
- Boswell, R.G.; Kober, H. Food cue reactivity and craving predict eating and weight gain: A meta-analytic review. Obes. Rev. 2016, 17, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Masterson, T.D.; Kirwan, C.B.; Davidson, L.E.; LeCheminant, J.D. Neural reactivity to visual food stimuli is reduced in some areas of the brain during evening hours compared to morning hours: An fMRI study in women. Brain Imaging Behav. 2016, 10, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Demos, K.E.; Sweet, L.H.; Hart, C.N.; McCaffery, J.M.; Williams, S.E.; Mailloux, K.A.; Trautvetter, J.; Owens, M.M.; Wing, R.R. The Effects of Experimental Manipulation of Sleep Duration on Neural Response to Food Cues. Sleep 2017, 40, zsx125. [Google Scholar] [CrossRef]
- Rauch, H.G.L.; Hume, D.J.; Howells, F.M.; Kroff, J.; Lambert, E.V. Food Cue Reactivity and the Brain-Heart Axis During Cognitive Stress Following Clinically Relevant Weight Loss. Front. Nutr. 2018, 5, 135. [Google Scholar] [CrossRef]
- Tryon, M.S.; Carter, C.S.; Decant, R.; Laugero, K.D. Chronic stress exposure may affect the brain’s response to high calorie food cues and predispose to obesogenic eating habits. Physiol. Behav. 2013, 120, 233–242. [Google Scholar] [CrossRef]
- Schneider-Worthington, C.R.; Smith, K.E.; Roemmich, J.N.; Salvy, S.J. External food cue responsiveness and emotional eating in adolescents: A multimethod study. Appetite 2022, 168, 105789. [Google Scholar] [CrossRef]
- Arend, A.K.; Schnepper, R.; Lutz, A.P.C.; Eichin, K.N.; Blechert, J. Prone to food in bad mood-Emotion-potentiated food-cue reactivity in patients with binge-eating disorder. Int. J. Eat. Disord. 2022, 55, 564–569. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Sinha, R.; Lacadie, C.; Small, D.M.; Sherwin, R.S.; Potenza, M.N. Neural correlates of stress- and food cue-induced food craving in obesity: Association with insulin levels. Diabetes Care 2013, 36, 394–402. [Google Scholar] [CrossRef]
- Luo, S.; O’Connor, S.G.; Belcher, B.R.; Page, K.A. Effects of Physical Activity and Sedentary Behavior on Brain Response to High-Calorie Food Cues in Young Adults. Obesity 2018, 26, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Flack, K.D.; Anderson, R.E., 3rd; McFee, K.F.; Kryscio, R.; Rush, C.R. Exercise increases attentional bias towards food cues in individuals classified as overweight to obese. Physiol. Behav. 2022, 247, 113711. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.; Salem, V.; Long, C.J.; Makwana, A.; Newbould, R.D.; Rabiner, E.A.; Ghatei, M.A.; Bloom, S.R.; Matthews, P.M.; Beaver, J.D.; et al. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011, 14, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Wever, M.C.M.; van Meer, F.; Charbonnier, L.; Crabtree, D.R.; Buosi, W.; Giannopoulou, A.; Androutsos, O.; Johnstone, A.M.; Manios, Y.; Meek, C.L.; et al. Associations between ghrelin and leptin and neural food cue reactivity in a fasted and sated state. Neuroimage 2021, 240, 118374. [Google Scholar] [CrossRef]
- Keesman, M.; Aarts, H.; Vermeent, S.; Häfner, M.; Papies, E.K. Consumption Simulations Induce Salivation to Food Cues. PLoS ONE 2016, 11, e0165449. [Google Scholar] [CrossRef]
- Zheng, L.; Miao, M.; Gan, Y. A systematic and meta-analytic review on the neural correlates of viewing high- and low-calorie foods among normal-weight adults. Neurosci. Biobehav. Rev. 2022, 138, 104721. [Google Scholar] [CrossRef]
- Ghobadi-Azbari, P.; Mahdavifar Khayati, R.; Ekhtiari, H. Habituation or sensitization of brain response to food cues: Temporal dynamic analysis in an functional magnetic resonance imaging study. Front. Hum. Neurosci. 2023, 17, 1076711. [Google Scholar] [CrossRef]
- Hermann, P.; Gál, V.; Kóbor, I.; Kirwan, C.B.; Kovács, P.; Kitka, T.; Lengyel, Z.; Bálint, E.; Varga, B.; Csekő, C.; et al. Efficacy of weight loss intervention can be predicted based on early alterations of fMRI food cue reactivity in the striatum. Neuroimage Clin. 2019, 23, 101803. [Google Scholar] [CrossRef]
- Cosme, D.; Zeithamova, D.; Stice, E.; Berkman, E.T. Multivariate neural signatures for health neuroscience: Assessing spontaneous regulation during food choice. Soc. Cogn. Affect. Neurosci. 2020, 15, 1120–1134. [Google Scholar] [CrossRef]
- Potthoff, J.; Face, A.L.; Schienle, A. The Color Nutrition Information Paradox: Effects of Suggested Sugar Content on Food Cue Reactivity in Healthy Young Women. Nutrients 2020, 12, 312. [Google Scholar] [CrossRef]
- Hagan, K.E.; Alasmar, A.; Exum, A.; Chinn, B.; Forbush, K.T. A systematic review and meta-analysis of attentional bias toward food in individuals with overweight and obesity. Appetite 2020, 151, 104710. [Google Scholar] [CrossRef]
- Cepeda-Benito, A.; Gleaves, D.H.; Williams, T.L.; Erath, S.A. The development and validation of the state and trait food-cravings questionnaires. Behav. Ther. 2000, 31, 151–173. [Google Scholar] [CrossRef]
- Kang Sim, D.E.; Eichen, D.M.; Strong, D.R.; Manzano, M.A.; Boutelle, K.N. Development and validation of the food cue responsivity scale. Physiol. Behav. 2023, 258, 114028. [Google Scholar] [CrossRef]
- Meule, A. Twenty Years of the Food Cravings Questionnaires: A Comprehensive Review. Curr. Addict. Rep. 2020, 7, 30–43. [Google Scholar] [CrossRef]
- Nijs, I.M.T.; Franken, I.H.A.; Muris, P. The modified Trait and State Food-Cravings Questionnaires: Development and validation of a general index of food craving. Appetite 2007, 49, 38–46. [Google Scholar] [CrossRef]
- Beechy, L.; Galpern, J.; Petrone, A.; Das, S.K. Assessment tools in obesity—Psychological measures, diet, activity, and body composition. Physiol. Behav. 2012, 107, 154–171. [Google Scholar] [CrossRef]
- Folkvord, F.; Anschütz, D.J.; Boyland, E.; Kelly, B.; Buijzen, M. Food advertising and eating behavior in children. Curr. Opin. Behav. Sci. 2016, 9, 26–31. [Google Scholar] [CrossRef]
- Folkvord, F.; Hermans, R.C.J. Food Marketing in an Obesogenic Environment: A Narrative Overview of the Potential of Healthy Food Promotion to Children and Adults. Curr. Addict. Rep. 2020, 7, 431–436. [Google Scholar] [CrossRef]
- Busetto, L.; Bettini, S.; Makaronidis, J.; Roberts, C.A.; Halford, J.C.G.; Batterham, R.L. Mechanisms of weight regain. Eur. J. Intern. Med. 2021, 93, 3–7. [Google Scholar] [CrossRef]
- Meule, A.; Küppers, C.; Harms, L.; Friederich, H.-C.; Schmidt, U.; Blechert, J.; Brockmeyer, T. Food cue-induced craving in individuals with bulimia nervosa and binge-eating disorder. PLoS ONE 2018, 13, e0204151. [Google Scholar] [CrossRef]
- Boutelle, K.N.; Knatz, S.; Carlson, J.; Bergmann, K.; Peterson, C.B. An Open Trial Targeting Food Cue Reactivity and Satiety Sensitivity in Overweight and Obese Binge Eaters. Cogn. Behav. Pract. 2017, 24, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Schyns, G.; van den Akker, K.; Roefs, A.; Houben, K.; Jansen, A. Exposure therapy vs lifestyle intervention to reduce food cue reactivity and binge eating in obesity: A pilot study. J. Behav. Ther. Exp. Psychiatry 2020, 67, 101453. [Google Scholar] [CrossRef]
- Li, N.; Mitchison, D.; Touyz, S.; Hay, P. Cross-sectional comparison of health-related quality of life and other features in people with and without objective and subjective binge eating using a general population sample. BMJ Open 2019, 9, e024227. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.E.; Potenza, M.N.; Weinstein, A.; Gorelick, D.A. Introduction to behavioral addictions. Am. J. Drug Alcohol. Abuse 2010, 36, 233–241. [Google Scholar] [CrossRef]
- Doucleff, M. Ozempic Seems to Curb Cravings for Alcohol. Here’s What Scientists Think Is Going on. NPR. 28 August 2023. Available online: https://www.npr.org/sections/health-shots/2023/08/28/1194526119/ozempic-wegovy-drinking-alcohol-cravings-semaglutide (accessed on 19 October 2023).
- Zhang, S. Did Scientists Accidentally Invent an Anti-addiction Drug? The Atlantic. 19 May 2023. Available online: https://www.theatlantic.com/health/archive/2023/05/ozempic-addictive-behavior-drinking-smoking/674098/ (accessed on 19 October 2023).
- Jerlhag, E. The therapeutic potential of glucagon-like peptide-1 for persons with addictions based on findings from preclinical and clinical studies. Front. Pharmacol. 2023, 14, 1063033. [Google Scholar] [CrossRef] [PubMed]
- Aranäs, C.; Blid Sköldheden, S.; Jerlhag, E. Antismoking agents do not contribute synergistically to semaglutide’s ability to reduce alcohol intake in rats. Front. Pharmacol. 2023, 14, 1180512. [Google Scholar] [CrossRef]
- Aranäs, C.; Edvardsson, C.E.; Shevchouk, O.T.; Zhang, Q.; Witley, S.; Sköldheden, S.B.; Zentveld, L.; Vallöf, D.; Tufvesson-Alm, M.; Jerlhag, E. Semaglutide reduces alcohol intake and relapse-like drinking in male and female rats. EBioMedicine 2023, 93, 104642. [Google Scholar] [CrossRef]
- Poisson, C.L.; Engel, L.; Saunders, B.T. Dopamine Circuit Mechanisms of Addiction-Like Behaviors. Front. Neural Circuits 2021, 15, 752420. [Google Scholar] [CrossRef]
- Büchel, C.; Peters, J.; Banaschewski, T.; Bokde, A.L.W.; Bromberg, U.; Conrod, P.J.; Flor, H.; Papadopoulos, D.; Garavan, H.; Gowland, P.; et al. Blunted ventral striatal responses to anticipated rewards foreshadow problematic drug use in novelty-seeking adolescents. Nat. Commun. 2017, 8, 14140. [Google Scholar] [CrossRef]
- Xue, J.; Qian, D.; Zhang, B.; Yang, J.; Li, W.; Bao, Y.; Qiu, S.; Fu, Y.; Wang, S.; Yuan, T.-F.; et al. Midbrain dopamine neurons arbiter OCD-like behavior. Proc. Natl. Acad. Sci. USA 2022, 119, e2207545119. [Google Scholar] [CrossRef]
- Powell, W.; Song, X.; Mohamed, Y.; Walsh, D.; Parks, E.J.; McMahon, T.M.; Khan, M.; Waitman, L.R. Medications and conditions associated with weight loss in patients prescribed semaglutide based on real-world data. Obesity 2023, 31, 2482–2492. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Cao, J.; Wei, J.; Geng, W. Case Report: Semaglutide-associated depression: A report of two cases. Front. Psychiatry 2023, 14, 1238353. [Google Scholar] [CrossRef]
- O’Neil, P.M.; Aroda, V.R.; Astrup, A.; Kushner, R.; Lau, D.C.W.; Wadden, T.A.; Brett, J.; Cancino, A.; Wilding, J.P.H.; on behalf of the Satiety and Clinical Adiposity—Liraglutide Evidence in individuals with and without diabetes (SCALE) study groups. Neuropsychiatric safety with liraglutide 3.0 mg for weight management: Results from randomized controlled phase 2 and 3a trials. Diabetes Obes. Metab. 2017, 19, 1529–1536. [Google Scholar] [CrossRef]
- Davies, M.; Færch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Bailey, T.S.; Billings, L.K.; Davies, M.; Frias, J.P.; Koroleva, A.; Lingvay, I.; O’Neil, P.M.; Rubino, D.M.; Skovgaard, D.; et al. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults with Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA 2021, 325, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults with Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021, 325, 1414–1425. [Google Scholar] [CrossRef]
- Garvey, W.T.; Batterham, R.L.; Bhatta, M.; Buscemi, S.; Christensen, L.N.; Frias, J.P.; Kandler, K.; Rigas, G.; Wadden, T.A.; Wharton, S.; et al. Two-year effects of semaglutide in adults with overweight or obesity: The STEP 5 trial. Nat. Med. 2022, 28, 2083–2091. [Google Scholar] [CrossRef]
- Weghuber, D.; Barrett, T.; Barrientos-Pérez, M.; Gies, I.; Hesse, D.; Jeppesen, O.K.; Kelly, A.S.; Mastrandrea, L.D.; Sørrig, R.; Arslanian, S. Once-Weekly Semaglutide in Adolescents with Obesity. N. Engl. J. Med. 2022, 387, 2245–2257. [Google Scholar] [CrossRef]
- Youmshajekian, L. EMA Statement on Ongoing Review of GLP-1 Receptor Agonists; European Medicines Agency: Amsterdam, The Netherlands, 2023; Available online: https://www.ema.europa.eu/en/news/ema-statement-ongoing-review-glp-1-receptor-agonists (accessed on 14 November 2023).
- Vo, L.; Albrecht, S.S.; Kershaw, K.N. Multilevel interventions to prevent and reduce obesity. Curr. Opin. Endocr. Metab. Res. 2019, 4, 62–69. [Google Scholar] [CrossRef]
- Kumanyika, S.K.; Obarzanek, E.; Stettler, N.; Bell, R.; Field, A.E.; Fortmann, S.P.; Franklin, B.A.; Gillman, M.W.; Lewis, C.E.; Poston, W.C., II; et al. Population-based prevention of obesity: The need for comprehensive promotion of healthful eating, physical activity, and energy balance: A scientific statement from American Heart Association Council on Epidemiology and Prevention, Interdisciplinary Committee for Prevention (formerly the expert panel on population and prevention science). Circulation 2008, 118, 428–464. [Google Scholar]
- Golden, S.D.; Earp, J.A.L. Social ecological approaches to individuals and their contexts: Twenty years of health education & behavior health promotion interventions. Health Educ. Behav. 2012, 39, 364–372. [Google Scholar]
- Boyland, E.; Maden, M.; Coates, A.E.; Masterson, T.D.; Alblas, M.C.; Bruce, A.S.; Roberts, C.A. Food and non-alcoholic beverage marketing in children and adults: A systematic review and activation likelihood estimation meta-analysis of functional magnetic resonance imaging studies. Obes Rev. 2023, e13643. [Google Scholar] [CrossRef] [PubMed]
- Javed, Z.; Valero-Elizondo, J.; Maqsood, M.H.; Mahajan, S.; Taha, M.B.; Patel, K.V.; Sharma, G.; Hagan, K.; Blaha, M.J.; Blankstein, R.; et al. Social determinants of health and obesity: Findings from a national study of US adults. Obesity 2022, 30, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, J.C., 3rd; Espinoza, J.; Holzhausen, E.A.; Goran, M.I.; Alderete, T.L. The impact of social determinants of health on obesity and diabetes disparities among Latino communities in Southern California. BMC Public. Health 2023, 23, 37. [Google Scholar] [CrossRef] [PubMed]
- Brewis, A.A.; Bruening, M. Weight Shame, Social Connection, and Depressive Symptoms in Late Adolescence. Int. J. Environ. Res. Public. Health 2018, 15, 891. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.L.; Wooldridge, J.S.; Herbert, M.S.; Tynan, M.; Dochat, C.; Afari, N. Associations Among Stress, Internalized Weight Stigma, Emotional Eating, and Body Composition in Active-Duty Service Members Enrolling in a Randomized Controlled Trial of a Weight Management Program. Int. J. Behav. Med. 2023. [Google Scholar] [CrossRef]
- Salvia, M.G.; Ritholz, M.D.; Craigen, K.L.E.; Quatromoni, P.A. Women’s perceptions of weight stigma and experiences of weight-neutral treatment for binge eating disorder: A qualitative study. EClinicalMedicine 2023, 56, 101811. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, A.B.; Crosby, R.D.; Cao, L.; Wonderlich, S.A.; Mitchell, J.E.; Engel, S.G.; Peterson, C.B. A preliminary study of momentary, naturalistic indicators of binge-eating episodes in adults with obesity. Int. J. Eat. Disord. 2018, 51, 87–91. [Google Scholar] [CrossRef]
- Epstein, L.H.; Carr, K.A.; Guth, C.; Shapiro, L.; Leone, L.A.; Temple, J.L. The enriched home environment and dietary intake are related to percent overBMI in children. Prev. Med. Rep. 2021, 23, 101440. [Google Scholar] [CrossRef]
- Díaz de León-Guerrero, S.; Salazar-León, J.; Meza-Sosa, K.F.; Valle-Garcia, D.; Aguilar-León, D.; Pedraza-Alva, G.; Pérez-Martínez, L. An enriched environment re-establishes metabolic homeostasis by reducing obesity-induced inflammation. Dis. Model. Mech. 2022, 15, dmm048936. [Google Scholar] [CrossRef]
- Harris, C.; Czaja, K. Can Circadian Eating Pattern Adjustments Reduce Risk or Prevent Development of T2D? Nutrients 2023, 15, 1762. [Google Scholar] [CrossRef] [PubMed]
- Eagleton, S.G.; Na, M.; Savage, J.S. Food insecurity is associated with higher food responsiveness in low-income children: The moderating role of parent stress and family functioning. Pediatr. Obes. 2022, 17, e12837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Kilpatrick, L.A.; Dong, T.S.; Gee, G.C.; Labus, J.S.; Osadchiy, V.; Beltran-Sanchez, H.; Wang, M.C.; Vaughan, A.; et al. Discrimination exposure impacts unhealthy processing of food cues: Crosstalk between the brain and gut. Nat. Ment. Health 2023, 1, 841–852. [Google Scholar] [CrossRef]
| Term | Definition |
|---|---|
| Food cue | External and internal conditioned stimuli that can elicit food-related responses, including sensorial (e.g., seeing and smelling food), environmental (e.g., walking by one’s favorite restaurant), and social (e.g., seeing people eating) cues, as well as internal hunger cues (e.g., one’s stomach growling). |
| Food cue reactivity | Conditioned responses to food cues, including physiological and psychological manifestations that favor food-seeking behaviors. |
| Food noise | Heightened and/or persistent manifestations of food cue reactivity, often leading to food-related intrusive thoughts and maladaptive eating behaviors. |
| Subscale Domain | Example Question |
|---|---|
| An intense desire to eat | I’m craving tasty food |
| Anticipation of relief from negative states | If I ate something, I wouldn’t feel so sluggish and lethargic |
| Craving as a physiological state | If I ate right now, my stomach wouldn’t feel as empty |
| Obsessive preoccupation with food | My desire to eat something tasty seems overpowering |
| Anticipation of positive reinforcement | If I were to eat what I’m desiring, I am sure my mood would improve |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, D.; Edwards, C.; Emond, J.A.; Gilbert-Diamond, D.; Butt, M.; Rigby, A.; Masterson, T.D. What Is Food Noise? A Conceptual Model of Food Cue Reactivity. Nutrients 2023, 15, 4809. https://doi.org/10.3390/nu15224809
Hayashi D, Edwards C, Emond JA, Gilbert-Diamond D, Butt M, Rigby A, Masterson TD. What Is Food Noise? A Conceptual Model of Food Cue Reactivity. Nutrients. 2023; 15(22):4809. https://doi.org/10.3390/nu15224809
Chicago/Turabian StyleHayashi, Daisuke, Caitlyn Edwards, Jennifer A. Emond, Diane Gilbert-Diamond, Melissa Butt, Andrea Rigby, and Travis D. Masterson. 2023. "What Is Food Noise? A Conceptual Model of Food Cue Reactivity" Nutrients 15, no. 22: 4809. https://doi.org/10.3390/nu15224809





