Histidine Triad Nucleotide-Binding Protein 1 Improves Critical Limb Ischemia by Regulating Mitochondrial Homeostasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Flow Cytometry
2.3. Cell Culture
2.4. Mitotracker ROS Staining
2.5. MitoSOX Fluorescence Staining
2.6. Measurements of Oxygen Consumption Rate
2.7. Western Blotting
2.8. Immunofluorescence
2.9. Tube Formation Assay
2.10. siRNA-Mediated Gene Knockdown
2.11. Statistics
3. Results
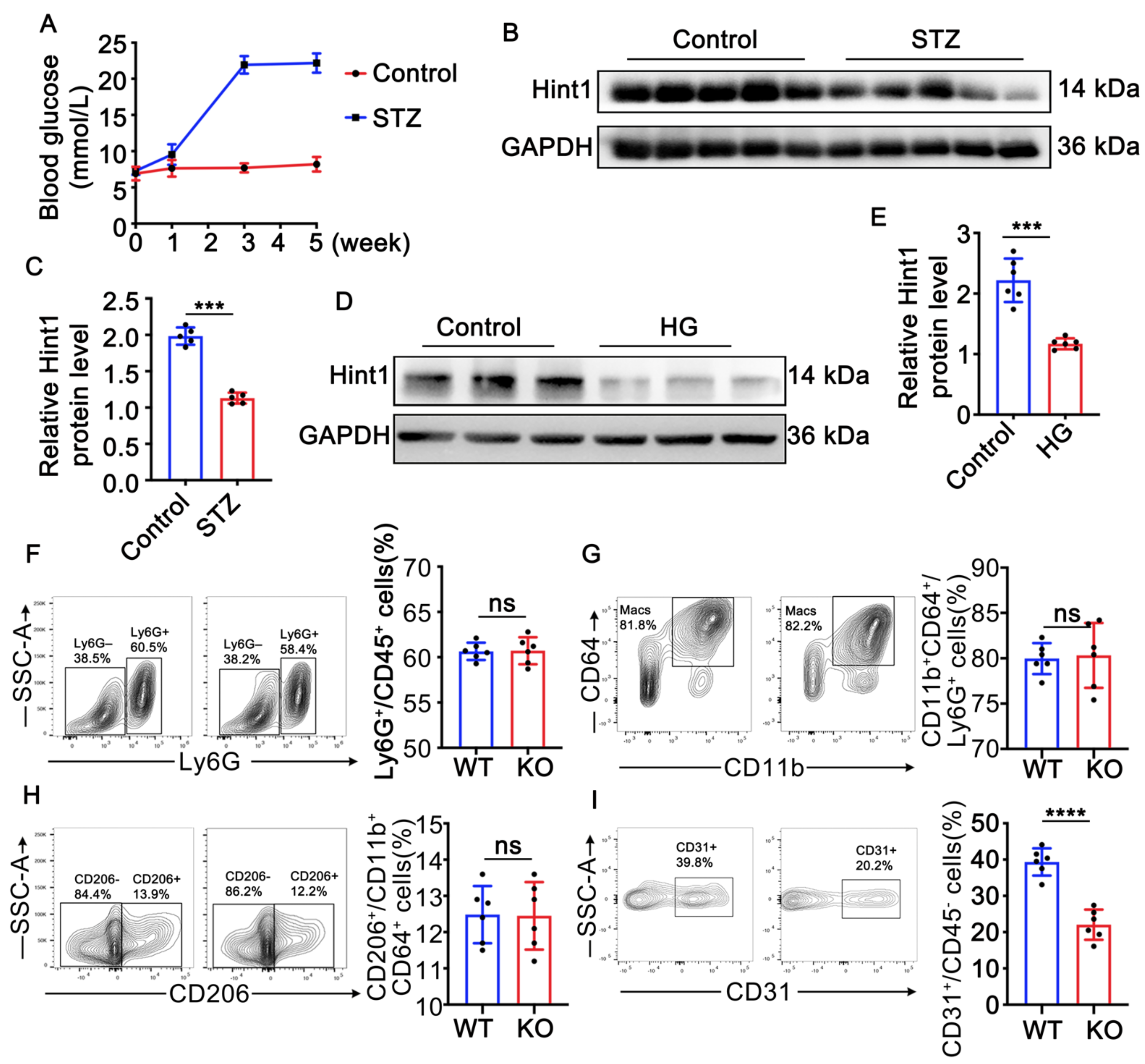
3.1. Hint1 Expression Is Reduced in STZ-Induced Mouse Muscle and High-Glucose (HG)-Treated Endothelial Cells
3.2. Hint1 Deficiency Barely Affects Inflammation in Ischemic Muscle in Diabetic Mice
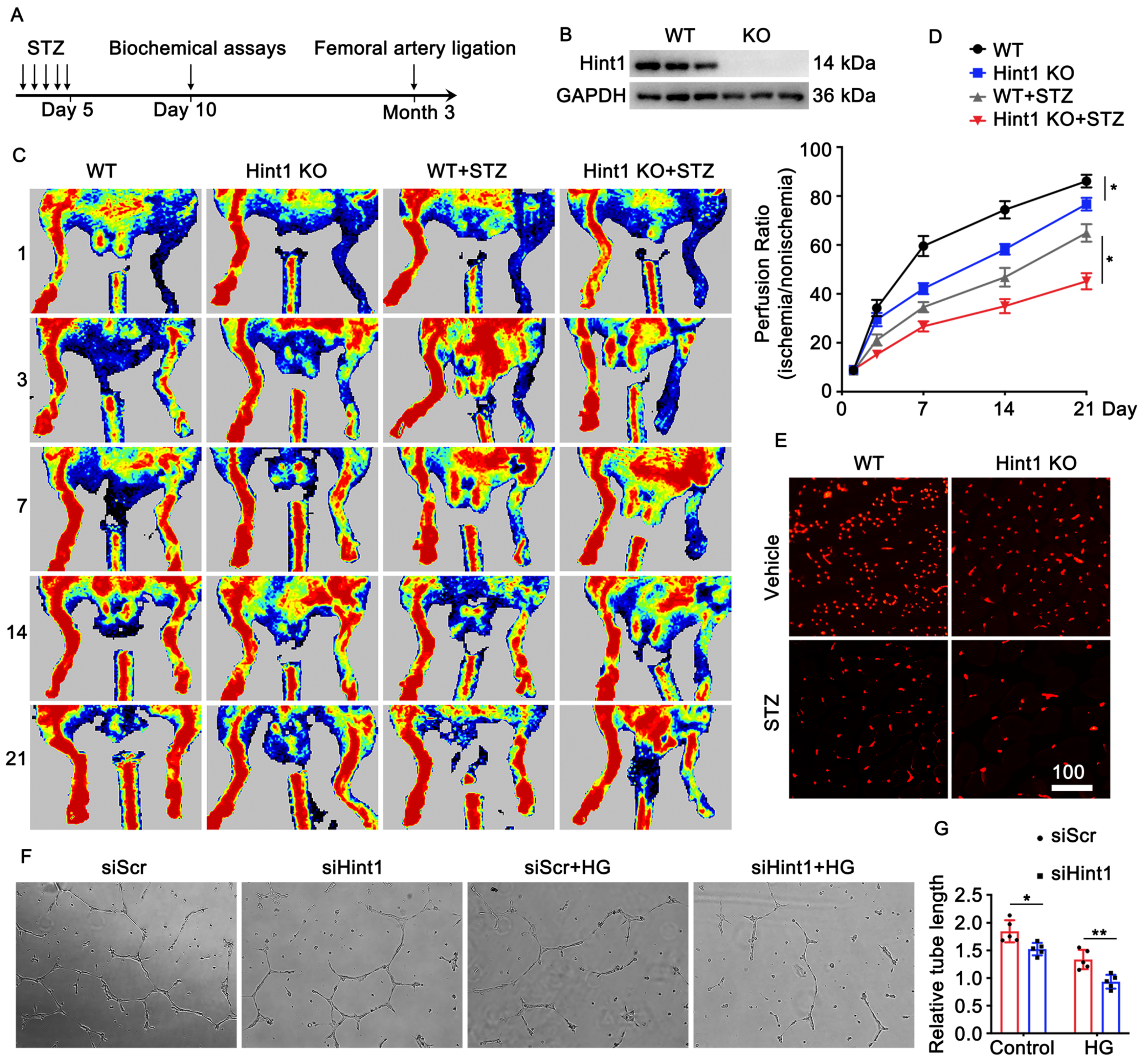
3.3. Hint1 Deficiency Impairs Blood Flow Recovery
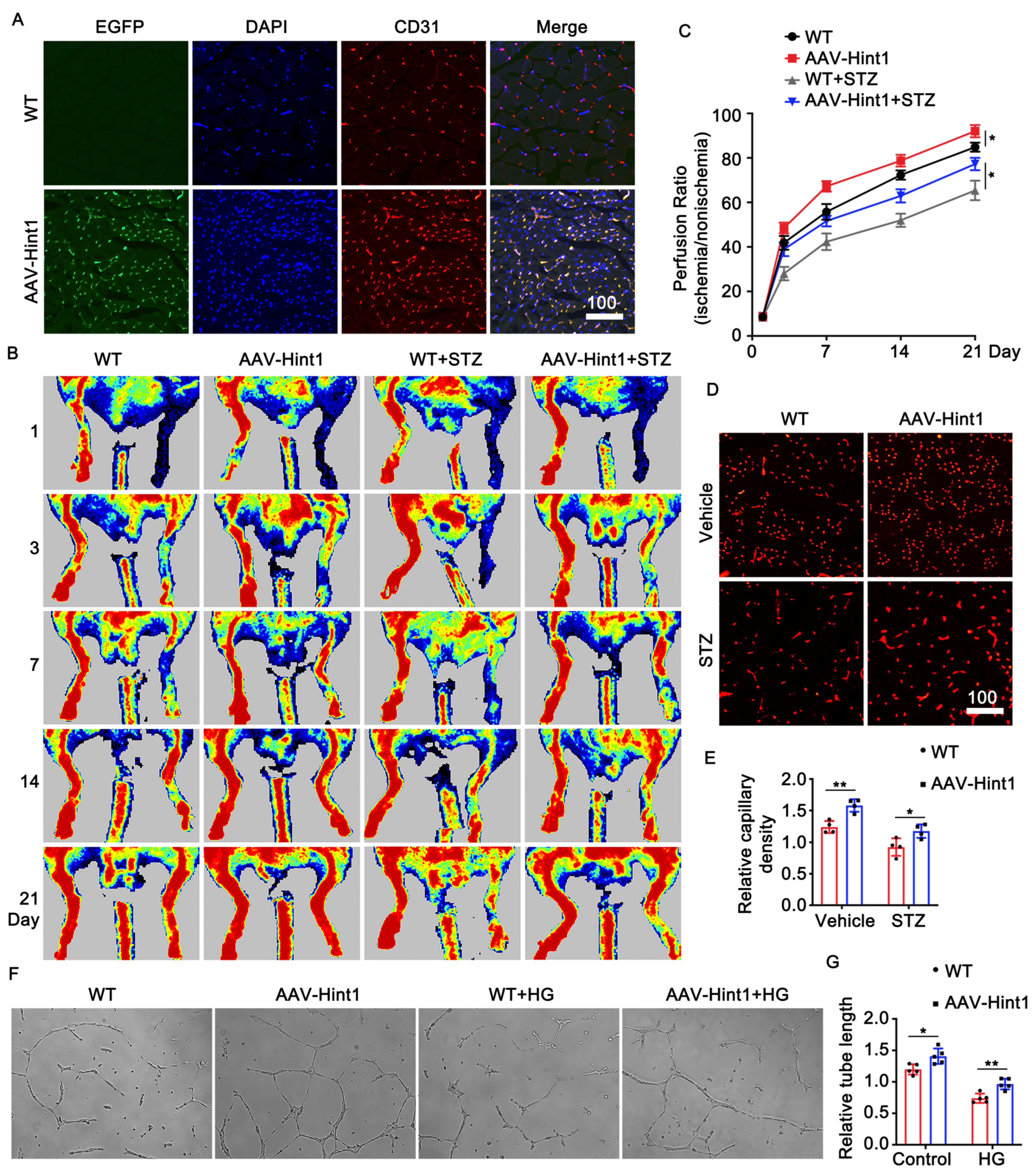
3.4. Endothelium-Specific Overexpression of Hint1 Improves Perfusion Recovery
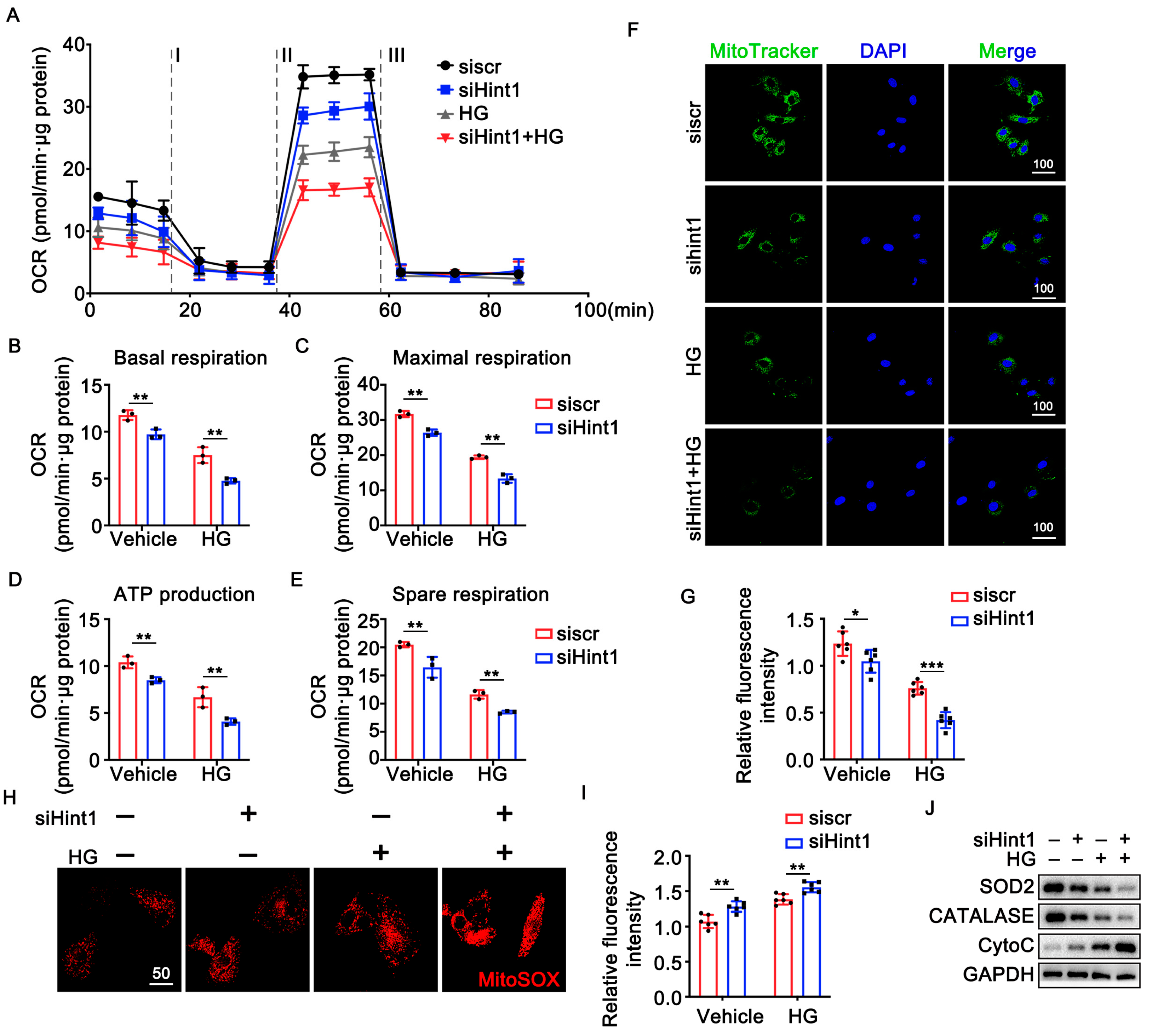
3.5. The Silencing of Hint1 Damages Mitochondrial Function in ECs
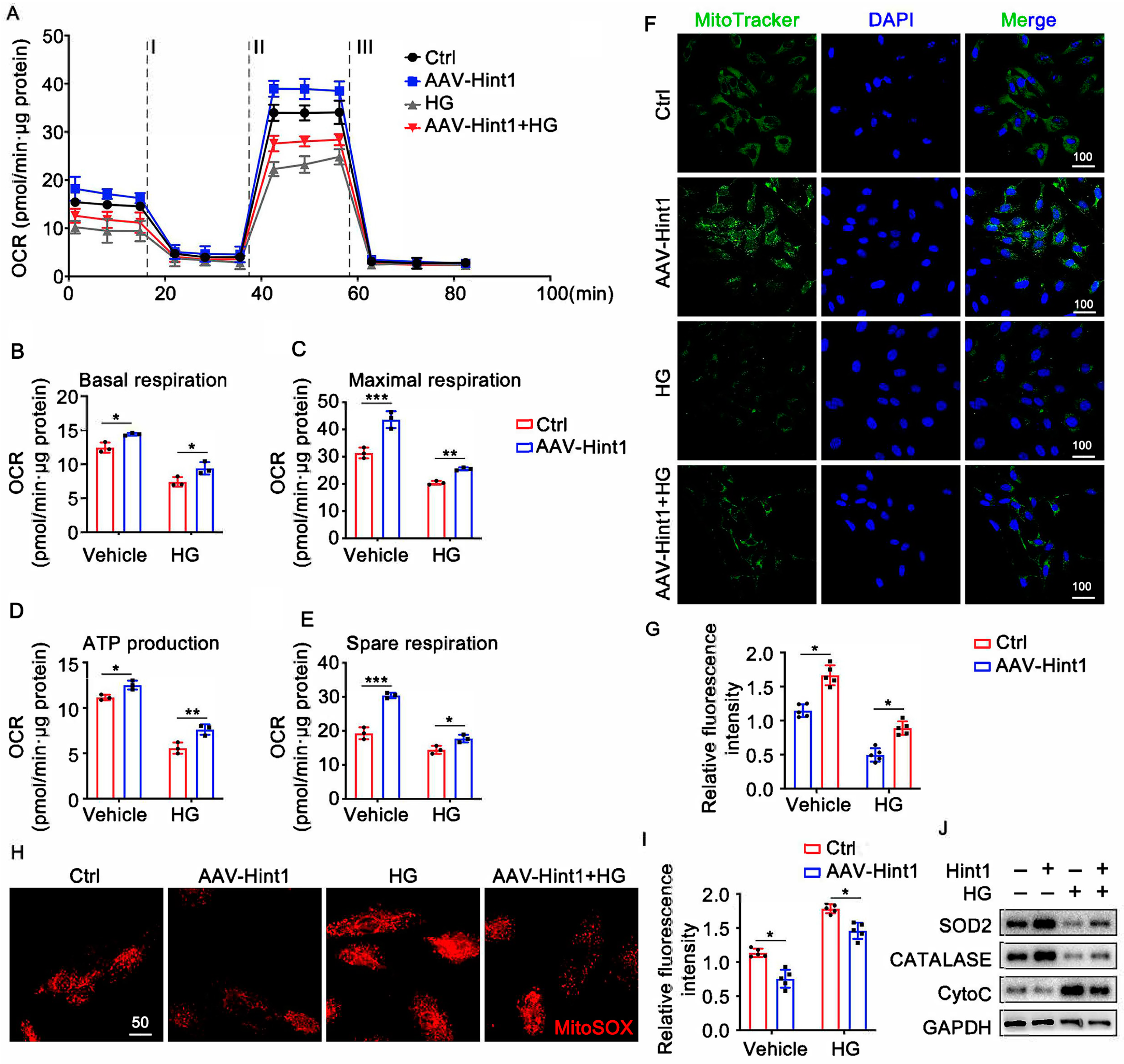
3.6. Hint1 Overexpression Ameliorates Mitochondrial Dysfunction in ECs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bussolino, F.; Mantovani, A.; Persico, G. Molecular mechanisms of blood vessel formation. Trends Biochem. Sci. 1997, 22, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Murabito, J.M.; Evans, J.C.; Nieto, K.; Larson, M.G.; Levy, D.; Wilson, P.W. Prevalence and clinical correlates of peripheral arterial disease in the Framingham Offspring Study. Am. Heart J. 2002, 143, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Schanzer, A.; Conte, M.S. Critical limb ischemia. Curr. Treat. Options Cardiovasc. Med. 2010, 12, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Faglia, E.; Clerici, G.; Clerissi, J.; Gabrielli, L.; Losa, S.; Mantero, M.; Caminiti, M.; Curci, V.; Lupattelli, T.; Morabito, A. Early and five-year amputation and survival rate of diabetic patients with critical limb ischemia: Data of a cohort study of 564 patients. Eur. J. Vasc. Endovasc. Surg. 2006, 32, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.-Y.; Wang, J.; Lee, S.-Y.; Chao, C.-T.; Hung, K.-Y.; Chien, K.-L. The Impact of Glucose-Lowering Strategy on the Risk of Increasing Frailty Severity among 49,519 Patients with Diabetes Mellitus: A Longitudinal Cohort Study. Aging Dis. 2023, 14, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Tongers, J.; Losordo, D.W. Human studies of angiogenic gene therapy. Circ. Res. 2009, 105, 724–736. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef]
- Brenner, C.; Garrison, P.; Gilmour, J.; Peisach, D.; Ringe, D.; Petsko, G.A.; Lowenstein, J.M. Crystal structures of HINT demonstrate that histidine triad proteins are GalT-related nucleotide-binding proteins. Nat. Struct. Biol. 1997, 4, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Zimoń, M.; Baets, J.; Almeida-Souza, L.; De Vriendt, E.; Nikodinovic, J.; Parman, Y.; Battaloǧlu, E.; Matur, Z.; Guergueltcheva, V.; Tournev, I.; et al. Loss-of-function mutations in HINT1 cause axonal neuropathy with neuromyotonia. Nat. Genet. 2012, 44, 1080–1083. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Z.; Wang, J.; Ma, X.; Dang, Y. HINT1 in Neuropsychiatric Diseases: A Potential Neuroplastic Mediator. Neural Plast. 2017, 2017, 5181925. [Google Scholar] [CrossRef]
- Razin, E.; Zhang, Z.C.; Nechushtan, H.; Frenkel, S.; Lee, Y.-N.; Arudchandran, R.; Rivera, J. Suppression of microphthalmia transcriptional activity by its association with protein kinase C-interacting protein 1 in mast cells. J. Biol. Chem. 1999, 274, 34272–34276. [Google Scholar] [CrossRef]
- Weiske, J.; Huber, O. The histidine triad protein Hint1 triggers apoptosis independent of its enzymatic activity. J. Biol. Chem. 2006, 281, 27356–27366. [Google Scholar] [CrossRef] [PubMed]
- Cen, B.; Li, H.; Weinstein, I.B. Histidine triad nucleotide-binding protein 1 up-regulates cellular levels of p27KIP1 by targeting ScfSKP2 ubiquitin ligase and Src. J. Biol. Chem. 2009, 284, 5265–5276. [Google Scholar] [CrossRef] [PubMed]
- Biscetti, F.; Straface, G.; De Cristofaro, R.; Lancellotti, S.; Rizzo, P.; Arena, V.; Stigliano, E.; Pecorini, G.; Egashira, K.; De Angelis, G.; et al. High-mobility group box-1 protein promotes angiogenesis after peripheral ischemia in diabetic mice through a VEGF-dependent mechanism. Diabetes 2010, 59, 1496–1505. [Google Scholar] [CrossRef]
- Caporali, A.; Meloni, M.; Miller, A.M.; Vierlinger, K.; Cardinali, A.; Spinetti, G.; Nailor, A.; Faglia, E.; Losa, S.; Gotti, A.; et al. Soluble ST2 is regulated by p75 neurotrophin receptor and predicts mortality in diabetic patients with critical limb ischemia. Arter. Thromb. Vasc. Biol. 2012, 32, e149–e160. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pan, L.; Hong, W.; Chen, S.; Bai, P.; Luo, W.; Sun, X.; He, F.; Jia, X.; Cai, J.; et al. GPR174 knockdown enhances blood flow recovery in hindlimb ischemia mice model by upregulating AREG expression. Nat. Commun. 2022, 13, 7519. [Google Scholar] [CrossRef]
- Ferro, A.; Queen, L.R.; Priest, R.M.; Xu, B.; Ritter, J.M.; Poston, L.; Ward, J.P.T. Activation of nitric oxide synthase by β2-adrenoceptors in human umbilical vein endothelium in vitro. Br. J. Pharmacol. 1999, 126, 1872–1880. [Google Scholar] [CrossRef]
- van der Windt, G.J.; Chang, C.; Pearce, E.L. Measuring Bioenergetics in T Cells Using a Seahorse Extracellular Flux Analyzer. Curr. Protoc. Immunol. 2016, 113, 3.16B.1–3.16B.14. [Google Scholar] [CrossRef] [PubMed]
- Miguel, V.; Ramos, R.; García-Bermejo, L.; Rodríguez-Puyol, D.; Lamas, S. The program of renal fibrogenesis is controlled by microRNAs regulating oxidative metabolism. Redox Biol. 2020, 40, 101851. [Google Scholar] [CrossRef]
- Liu, Z.; Han, Y.; Li, L.; Lu, H.; Meng, G.; Li, X.; Shirhan, M.; Peh, M.T.; Xie, L.; Zhou, S.; et al. The hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E−/−mice. Br. J. Pharmacol. 2013, 169, 1795–1809. [Google Scholar] [CrossRef]
- Arnaoutova, I.; Kleinman, H.K. In vitro angiogenesis: Endothelial cell tube formation on gelled basement membrane extract. Nat. Protoc. 2010, 5, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.-J.; Olsen, K.; Hamblin, M.; Zhang, J.; Schwendeman, S.P.; Chen, Y.E. Vascular endothelial cell-specific MicroRNA-15a inhibits angiogenesis in hindlimb ischemia. J. Biol. Chem. 2012, 287, 27055–27064. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Dong, Y.-Y.; Lei, G.; Zhou, Y.; Liu, P.; Dang, Y.-H. HINT1 Is Involved in the Chronic Mild Stress Elicited Oxidative Stress and Apoptosis through the PKC ε/ALDH-2/4HNE Pathway in Prefrontal Cortex of Rats. Front. Behav. Neurosci. 2021, 15, 690344. [Google Scholar] [CrossRef] [PubMed]
- Strom, A.; Tong, C.L.; Wagner, C.R. Histidine triad nucleotide-binding proteins HINT1 and HINT2 share similar substrate specificities and little affinity for the signaling dinucleotide Ap4A. FEBS Lett. 2020, 594, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Li, H.; Wu, H.-C.; Shen, J.; Wang, L.; Yu, M.-W.; Lee, P.-H.; Weinstein, I.B.; Santella, R.M. Silencing of Hint1, a novel tumor suppressor gene, by promoter hypermethylation in hepatocellular carcinoma. Cancer Lett. 2009, 275, 277–284. [Google Scholar] [CrossRef]
- Cen, B.; Deguchi, A.; Weinstein, I.B. Activation of protein kinase g Increases the expression of p21CIP1, p27KIP1, and histidine triad protein 1 through Sp1. Cancer Res. 2008, 68, 5355–5362. [Google Scholar] [CrossRef]
- Weiske, J.; Huber, O. The histidine triad protein Hint1 interacts with Pontin and Reptin and inhibits TCF–β-catenin-mediated transcription. J. Cell Sci. 2005, 118 Pt 14, 3117–3129. [Google Scholar] [CrossRef]
- Bieganowski, P.; Garrison, P.N.; Hodawadekar, S.C.; Faye, G.; Barnes, L.D.; Brenner, C. Adenosine monophosphoramidase activity of Hint and Hnt1 supports function of Kin28, Ccl1, and Tfb3. J. Biol. Chem. 2002, 277, 10852–10860. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis. Annu. Rev. Med. 2006, 57, 1–18. [Google Scholar] [CrossRef]
- Ren, S.-C.; Chen, X.; Gong, H.; Wang, H.; Wu, C.; Li, P.-H.; Chen, X.-F.; Qu, J.-H.; Tang, X. SIRT6 in Vascular Diseases, from Bench to Bedside. Aging Dis. 2022, 13, 1015–1029. [Google Scholar] [CrossRef]
- Risau, W.; Flamme, I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 1995, 11, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Marcu, R.; Zheng, Y.; Hawkins, B.J. Mitochondria and Angiogenesis. Adv. Exp. Med. Biol. 2017, 982, 371–406. [Google Scholar] [CrossRef] [PubMed]
- Dranka, B.P.; Hill, B.G.; Darley-Usmar, V.M. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. 2010, 48, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Krützfeldt, A.; Spahr, R.; Mertens, S.; Siegmund, B.; Piper, H. Metabolism of exogenous substrates by coronary endothelial cells in culture. J. Mol. Cell. Cardiol. 1990, 22, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Coutelle, O.; Hornig-Do, H.; Witt, A.; Andree, M.; Schiffmann, L.M.; Piekarek, M.; Brinkmann, K.; Seeger, J.M.; Liwschitz, M.; Miwa, S.; et al. Embelin inhibits endothelial mitochondrial respiration and impairs neoangiogenesis during tumor growth and wound healing. EMBO Mol. Med. 2014, 6, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Gohongi, T.; Kadambi, A.; Izumi, Y.; Ang, J.; Yun, C.-O.; Buerk, D.G.; Huang, P.L.; Jain, R.K. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. USA 2001, 98, 2604–2609. [Google Scholar] [CrossRef]
- Cleeter, M.W.; Cooper, J.M.; Darley-Usmar, V.M.; Moncada, S.; Schapira, A.H. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide: Implications for neurodegenerative diseases. FEBS Lett. 1994, 345, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Brenner, C.; Bieganowski PPace, H.C.; Huebner, K. The histidine triad superfamily of nucleotide-binding proteins. J. Cell Physiol. 1999, 181, 179–187. [Google Scholar] [CrossRef]
- Mootha, V.K.; Bunkenborg, J.; Olsen, J.V.; Hjerrild, M.; Wisniewski, J.R.; Stahl, E.; Bolouri, M.S.; Ray, H.N.; Sihag, S.; Kamal, M.; et al. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell 2003, 115, 629–640. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, T.; Cheng, S.; Lu, H.; Li, X.; Weng, X.; Ge, J. Histidine Triad Nucleotide-Binding Protein 1 Improves Critical Limb Ischemia by Regulating Mitochondrial Homeostasis. Nutrients 2023, 15, 4859. https://doi.org/10.3390/nu15234859
Gao T, Cheng S, Lu H, Li X, Weng X, Ge J. Histidine Triad Nucleotide-Binding Protein 1 Improves Critical Limb Ischemia by Regulating Mitochondrial Homeostasis. Nutrients. 2023; 15(23):4859. https://doi.org/10.3390/nu15234859
Chicago/Turabian StyleGao, Tingwen, Shuo Cheng, Hao Lu, Xiao Li, Xinyu Weng, and Junbo Ge. 2023. "Histidine Triad Nucleotide-Binding Protein 1 Improves Critical Limb Ischemia by Regulating Mitochondrial Homeostasis" Nutrients 15, no. 23: 4859. https://doi.org/10.3390/nu15234859





