Hydration for Adult Patients with Nephrolithiasis: Specificities and Current Recommendations
Abstract
:1. Introduction
2. Diet and Lithogenesis Mechanisms
- -
- Anatomical abnormalities, e.g., medullary sponge kidney, ureteropelvic junction obstruction, horseshoe kidney [12],
- -
- Rare inherited monogenic metabolic disorders [13], e.g., cystinuria characterized by a defective reabsorption of cystine in the renal proximal tubule [14], primary hyperoxaluria, a group of disorders of glyoxylate metabolism that cause the overproduction of endogenous oxalate [15], 2,8-dihydroxyadeninuria due to adenine phosphoribosyltransferase deficiency [16], autosomal dominant or recessive distal renal tubular acidosis type I associated with impaired acid excretion by intercalated cells in the renal collecting duct [17], Dent disease linked to proximal tubular defects [18], hereditary hypophosphatemic rickets with hypercalciuria characterized by renal phosphate wasting [19,20]),
- -
3. Fluid Intake Is the Main Determinant of Urine Volume (Figure 2)
3.1. Water Is the Main Constituent of the Human Body
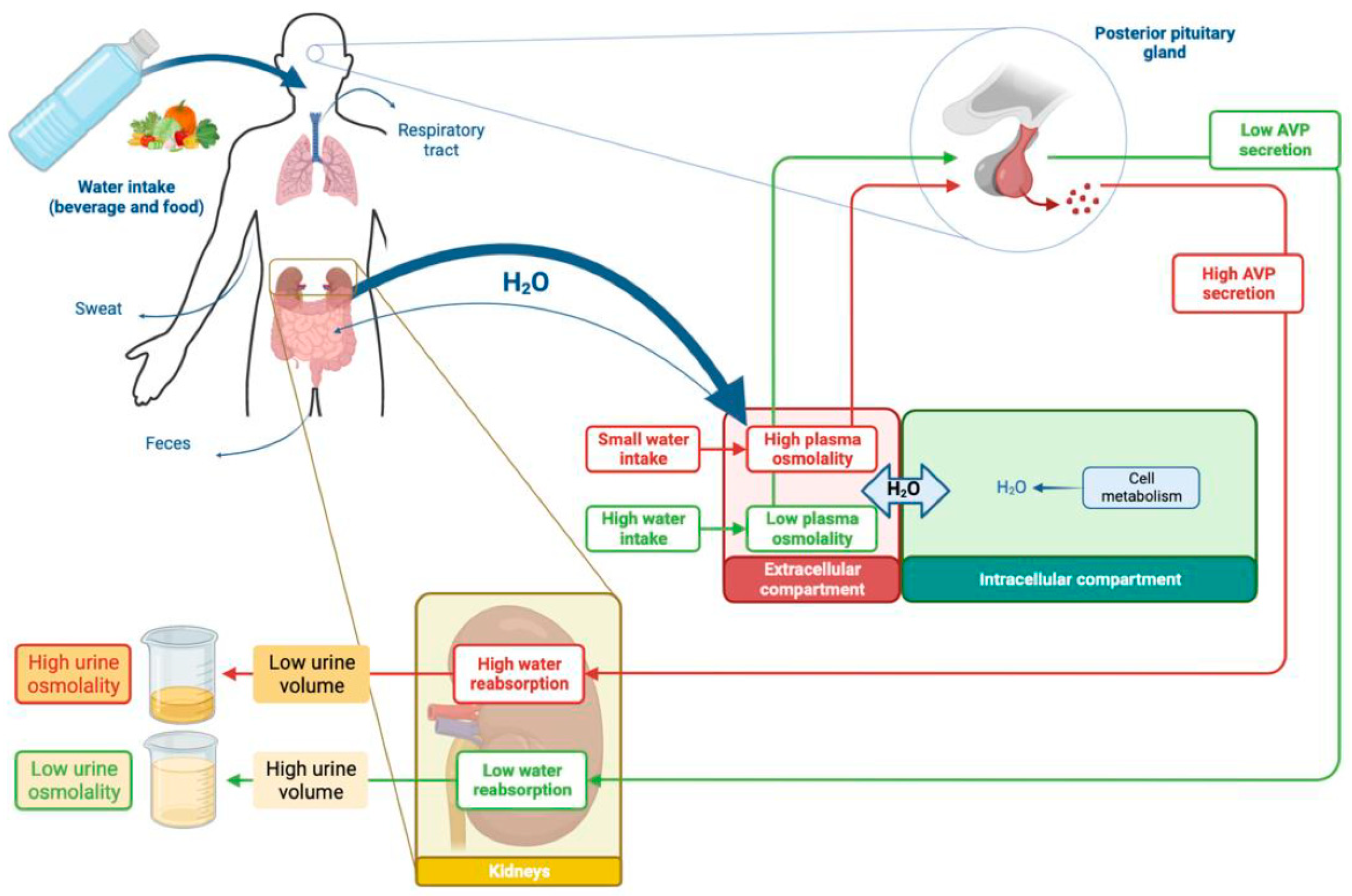
3.2. Water Homeostasis Depends on Inflows and Outflows
3.3. Kidneys Regulate Water Output and Urine Volume
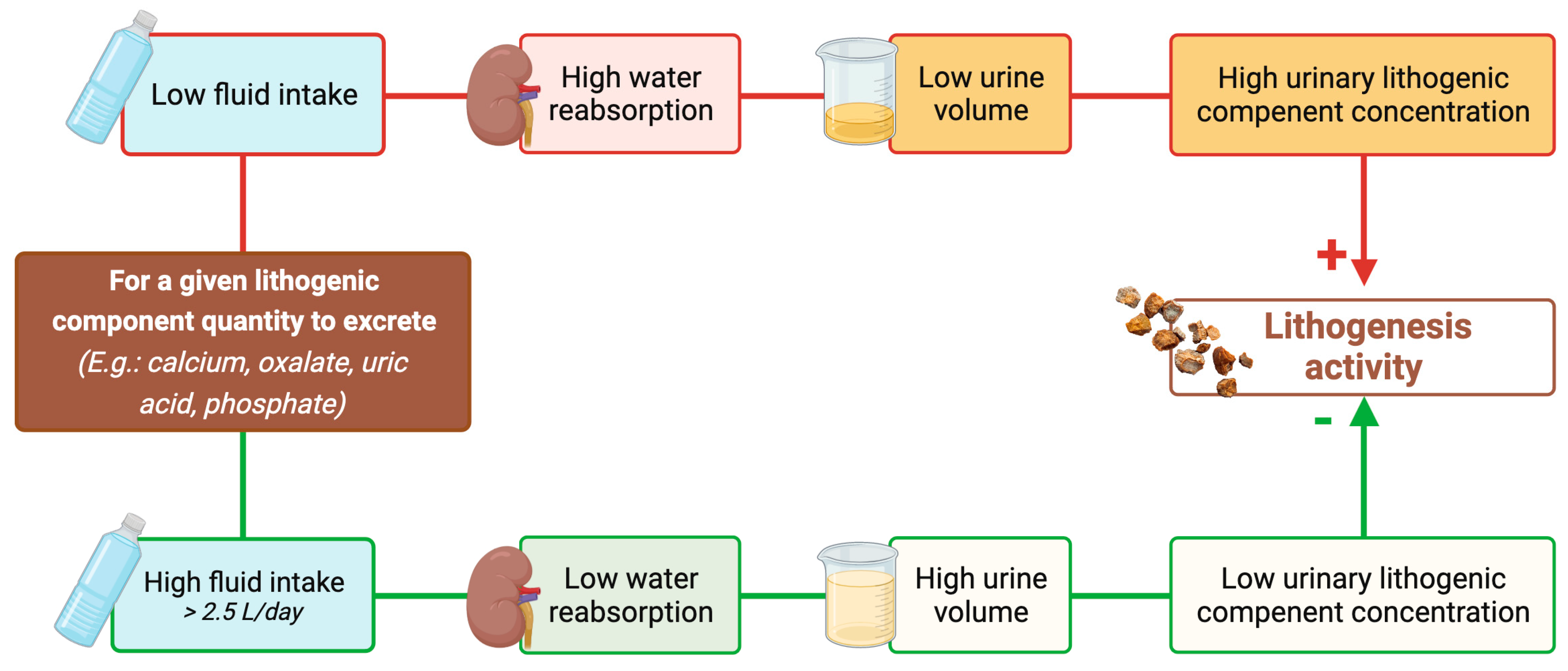
4. Reducing Urine Volume May Increase the Risk of Developing First Kidney Stones
5. Increasing Urine Volume in Stone Formers May Decrease the Risk of Stone Recurrence
6. Increasing Urine Volume in Cystinuric Patients May Decrease Stone Recurrence
7. Current Guidelines Recommend Increasing Fluid Intake to Prevent Stone Formation
8. How Can Physicians Help Their Patients to Increase Their Fluid Intake and Pay Attention to Its Composition?
8.1. Practical Ways to Increase Fluid Intake and Adherence to This Measure
8.2. Advice Regarding Types of Fluid Intake
9. Pharmacotherapy to Prevent Nephrolithiasis
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shoag, J.; Tasian, G.E.; Goldfarb, D.S.; Eisner, B.H. The new epidemiology of nephrolithiasis. Adv. Chronic Kidney Dis. 2015, 22, 273–278. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Krambeck, A.E.; Rule, A.D. Determining the true burden of kidney stone disease. Nat. Rev. Nephrol. 2020, 16, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Stamatelou, K.; Goldfarb, D.S. Epidemiology of Kidney Stones. Healthcare 2023, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Pearle, M.S.; Calhoun, E.A.; Curhan, G.C.; Urologic Diseases of America, P. Urologic diseases in America project: Urolithiasis. J. Urol. 2005, 173, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Enders, F.T.; Vaughan, L.E.; Bergstralh, E.J.; Knoedler, J.J.; Krambeck, A.E.; Lieske, J.C.; Rule, A.D. Stone Composition Among First-Time Symptomatic Kidney Stone Formers in the Community. Mayo. Clin. Proc. 2015, 90, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Rule, A.D.; Bergstralh, E.J.; Melton, L.J., 3rd; Li, X.; Weaver, A.L.; Lieske, J.C. Kidney stones and the risk for chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Gillen, D.L.; Worcester, E.M.; Coe, F.L. Decreased renal function among adults with a history of nephrolithiasis: A study of NHANES III. Kidney Int. 2005, 67, 685–690. [Google Scholar] [CrossRef] [PubMed]
- El-Zoghby, Z.M.; Lieske, J.C.; Foley, R.N.; Bergstralh, E.J.; Li, X.; Melton, L.J., 3rd; Krambeck, A.E.; Rule, A.D. Urolithiasis and the risk of ESRD. Clin. J. Am. Soc. Nephrol. 2012, 7, 1409–1415. [Google Scholar] [CrossRef]
- Lieske, J.C.; Rule, A.D.; Krambeck, A.E.; Williams, J.C.; Bergstralh, E.J.; Mehta, R.A.; Moyer, T.P. Stone composition as a function of age and sex. Clin. J. Am. Soc. Nephrol. 2014, 9, 2141–2146. [Google Scholar] [CrossRef]
- Daudon, M.; Traxer, O.; Lechevallier, E.; Saussine, C. Epidemiology of urolithiasis. Prog. Urol. 2008, 18, 802–814. [Google Scholar] [CrossRef]
- Corrales, M.; Doizi, S.; Barghouthy, Y.; Traxer, O.; Daudon, M. Classification of Stones According to Michel Daudon: A Narrative Review. Eur. Urol. Focus. 2021, 7, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Skolarikos, A.; Jung, H.; Neisius, A.; Petřík, A.; Somani, B.; Tailly, T.; Gambaro, G. EAU Guidelines on Urolithiasis. European Association of Urology. 2023. Available online: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Urolithiasis-2023.pdf (accessed on 15 August 2023).
- Singh, P.; Harris, P.C.; Sas, D.J.; Lieske, J.C. The genetics of kidney stone disease and nephrocalcinosis. Nat. Rev. Nephrol. 2022, 18, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Servais, A.; Thomas, K.; Dello Strologo, L.; Sayer, J.A.; Bekri, S.; Bertholet-Thomas, A.; Bultitude, M.; Capolongo, G.; Cerkauskiene, R.; Daudon, M.; et al. Cystinuria: Clinical practice recommendation. Kidney Int. 2021, 99, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Groothoff, J.W.; Metry, E.; Deesker, L.; Garrelfs, S.; Acquaviva, C.; Almardini, R.; Beck, B.B.; Boyer, O.; Cerkauskiene, R.; Ferraro, P.M.; et al. Clinical practice recommendations for primary hyperoxaluria: An expert consensus statement from ERKNet and OxalEurope. Nat. Rev. Nephrol. 2023, 19, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Bollee, G.; Harambat, J.; Bensman, A.; Knebelmann, B.; Daudon, M.; Ceballos-Picot, I. Adenine phosphoribosyltransferase deficiency. Clin. J. Am. Soc. Nephrol. 2012, 7, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.A.; Unwin, R.; Lopez-Garcia, S.C.; Kleta, R.; Bockenhauer, D.; Walsh, S. The pathophysiology of distal renal tubular acidosis. Nat. Rev. Nephrol. 2023, 19, 384–400. [Google Scholar] [CrossRef] [PubMed]
- Gianesello, L.; Del Prete, D.; Anglani, F.; Calo, L.A. Genetics and phenotypic heterogeneity of Dent disease: The dark side of the moon. Hum. Genet. 2021, 140, 401–421. [Google Scholar] [CrossRef]
- Dasgupta, D.; Wee, M.J.; Reyes, M.; Li, Y.; Simm, P.J.; Sharma, A.; Schlingmann, K.P.; Janner, M.; Biggin, A.; Lazier, J.; et al. Mutations in SLC34A3/NPT2c are associated with kidney stones and nephrocalcinosis. J. Am. Soc. Nephrol. 2014, 25, 2366–2375. [Google Scholar] [CrossRef]
- Wagner, C.A.; Rubio-Aliaga, I.; Hernando, N. Renal phosphate handling and inherited disorders of phosphate reabsorption: An update. Pediatr. Nephrol. 2019, 34, 549–559. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Khan, A.A.; Silverberg, S.J.; Fuleihan, G.E.; Marcocci, C.; Minisola, S.; Perrier, N.; Sitges-Serra, A.; Thakker, R.V.; Guyatt, G.; et al. Evaluation and Management of Primary Hyperparathyroidism: Summary Statement and Guidelines from the Fifth International Workshop. J. Bone Min. Res. 2022, 37, 2293–2314. [Google Scholar] [CrossRef]
- Calatroni, M.; Moroni, G.; Reggiani, F.; Ponticelli, C. Renal sarcoidosis. J. Nephrol. 2023, 36, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Nazzal, L.; Puri, S.; Goldfarb, D.S. Enteric hyperoxaluria: An important cause of end-stage kidney disease. Nephrol. Dial. Transpl. 2016, 31, 375–382. [Google Scholar] [CrossRef] [PubMed]
- West, B.; Luke, A.; Durazo-Arvizu, R.A.; Cao, G.; Shoham, D.; Kramer, H. Metabolic syndrome and self-reported history of kidney stones: The National Health and Nutrition Examination Survey (NHANES III) 1988-1994. Am. J. Kidney Dis. 2008, 51, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Stampfer, M.J.; Curhan, G.C. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005, 68, 1230–1235. [Google Scholar] [CrossRef]
- Daudon, M.; Jungers, P. Drug-induced renal calculi: Epidemiology, prevention and management. Drugs 2004, 64, 245–275. [Google Scholar] [CrossRef] [PubMed]
- Daudon, M.; Dessombz, A.; Frochot, V.; Letavernier, E.; Haymann, J.P.; Jungers, P.; Bazin, D. Comprehensive morphoconstitutional analysis of urinary stones improves etiological diagnosis and therapeutic strategy of nephrolithiasis. Comptes Rendus Chim. 2016, 19, 1470–1491. [Google Scholar] [CrossRef]
- Khan, S.R.; Pearle, M.S.; Robertson, W.G.; Gambaro, G.; Canales, B.K.; Doizi, S.; Traxer, O.; Tiselius, H.G. Kidney stones. Nat. Rev. Dis. Primers 2016, 2, 16008. [Google Scholar] [CrossRef]
- Robertson, G.L. Thirst and Vasopressin. In Seldin and Giebisch’s The Kidney, Physiology and Pathophysiology, 5th ed.; Alpern, R.J., Caplan, M.J., Moe, O.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, p. 1441. [Google Scholar]
- Jequier, E.; Constant, F. Water as an essential nutrient: The physiological basis of hydration. Eur. J. Clin. Nutr. 2010, 64, 115–123. [Google Scholar] [CrossRef]
- Rivard, C.J.; Wang, W.; Chan, L. Hypernatremic States. In Seldin and Giebisch’s The Kidney, Physiology and Pathophysiology, 5th ed.; Alpern, R.J., Caplan, M.J., Moe, O.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, p. 1541. [Google Scholar]
- Nielsen, S.; Kwon, T.H.; Dimke, H.; Skott, M.; Frøkiær, J. Aquaporin Water Channels in Mammalian Kidney. In Seldin and Giebisch’s The Kidney, Physiology and Pathophysiology, 5th ed.; Alpern, R.J., Caplan, M.J., Moe, O.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, p. 1405. [Google Scholar]
- Bichet, D.G. Genetics in endocrinology pathophysiology, diagnosis and treatment of familial nephrogenic diabetes insipidus. Eur. J. Endocrinol. 2020, 183, R29–R40. [Google Scholar] [CrossRef]
- Nielsen, S.; Frokiaer, J.; Marples, D.; Kwon, T.H.; Agre, P.; Knepper, M.A. Aquaporins in the kidney: From molecules to medicine. Physiol. Rev. 2002, 82, 205–244. [Google Scholar] [CrossRef]
- Sands, J.F.; Layton, H.E. The Urine Concentrating Mechanism and Urea Transporters. In Seldin and Giebisch’s The Kidney, Physiology and Pathophysiology, 5th ed.; Alpern, R.J., Caplan, M.J., Moe, O.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, p. 1463. [Google Scholar]
- Robertson, G.L. Differential diagnosis of polyuria. Annu. Rev. Med. 1988, 39, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Better, O.S.; Shabtai, M.; Kedar, S.; Melamud, A.; Berenheim, J.; Chaimovitz, C. Increased incidence of nephrolithiasis (N) in lifeguards (LG) in Israel. Adv. Exp. Med. Biol. 1980, 128, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Turner, L.R.; Hurst, C.; Mai, H.; Zhang, Y.; Tong, S. Exposure to ambient heat and urolithiasis among outdoor workers in Guangzhou, China. Sci. Total Environ. 2014, 472, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Lotan, Y.; Antonelli, J.; Jimenez, I.B.; Gharbi, H.; Herring, R.; Beaver, A.; Dennis, A.; Von Merveldt, D.; Carter, S.; Cohen, A.; et al. The kidney stone and increased water intake trial in steel workers: Results from a pilot study. Urolithiasis 2017, 45, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Siener, R.; Hesse, A. Fluid intake and epidemiology of urolithiasis. Eur. J. Clin. Nutr. 2003, 57 (Suppl. S2), S47–S51. [Google Scholar] [CrossRef] [PubMed]
- Atan, L.; Andreoni, C.; Ortiz, V.; Silva, E.K.; Pitta, R.; Atan, F.; Srougi, M. High kidney stone risk in men working in steel industry at hot temperatures. Urology 2005, 65, 858–861. [Google Scholar] [CrossRef]
- Borghi, L.; Meschi, T.; Amato, F.; Novarini, A.; Romanelli, A.; Cigala, F. Hot occupation and nephrolithiasis. J. Urol. 1993, 150, 1757–1760. [Google Scholar] [CrossRef]
- Pin, N.T.; Ling, N.Y.; Siang, L.H. Dehydration from outdoor work and urinary stones in a tropical environment. Occup. Med. 1992, 42, 30–32. [Google Scholar] [CrossRef]
- Masterson, J.H.; Jourdain, V.J.; Collard, D.A.; Choe, C.H.; Christman, M.S.; L’Esperance, J.O.; Auge, B.K. Changes in urine parameters after desert exposure: Assessment of stone risk in United States Marines transiently exposed to a desert environment. J. Urol. 2013, 189, 165–170. [Google Scholar] [CrossRef]
- Fakheri, R.J.; Goldfarb, D.S. Ambient temperature as a contributor to kidney stone formation: Implications of global warming. Kidney Int. 2011, 79, 1178–1185. [Google Scholar] [CrossRef]
- Malieckal, D.A.; Goldfarb, D.S. Occupational kidney stones. Curr. Opin. Nephrol. Hypertens 2020, 29, 232–236. [Google Scholar] [CrossRef]
- Frank, M.; De Vries, A. Prevention of urolithiasis. Education to adequate fluid intake in a new town situated in the Judean Desert Mountains. Arch. Environ. Health 1966, 13, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.J.; Rangel, L.J.; Krambeck, A.E. The effect of work location on urolithiasis in health care professionals. Urolithiasis 2013, 41, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Mass, A.Y.; Goldfarb, D.S.; Shah, O. Taxi cab syndrome: A review of the extensive genitourinary pathology experienced by taxi cab drivers and what we can do to help. Rev. Urol. 2014, 16, 99–104. [Google Scholar] [PubMed]
- Borghi, L.; Meschi, T.; Amato, F.; Briganti, A.; Novarini, A.; Giannini, A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: A 5-year randomized prospective study. J. Urol. 1996, 155, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N. Engl. J. Med. 1993, 328, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Stampfer, M.J.; Curhan, G.C. Dietary factors and the risk of incident kidney stones in men: New insights after 14 years of follow-up. J. Am. Soc. Nephrol. 2004, 15, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Curhan, G.C.; Willett, W.C.; Knight, E.L.; Stampfer, M.J. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch. Intern. Med. 2004, 164, 885–891. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Speizer, F.E.; Spiegelman, D.; Stampfer, M.J. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann. Intern. Med. 1997, 126, 497–504. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Speizer, F.E.; Stampfer, M.J. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001, 59, 2290–2298. [Google Scholar] [CrossRef]
- Littlejohns, T.J.; Neal, N.L.; Bradbury, K.E.; Heers, H.; Allen, N.E.; Turney, B.W. Fluid Intake and Dietary Factors and the Risk of Incident Kidney Stones in UK Biobank: A Population-based Prospective Cohort Study. Eur. Urol. Focus 2020, 6, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.Y.; Sakhaee, K.; Crowther, C.; Brinkley, L. Evidence justifying a high fluid intake in treatment of nephrolithiasis. Ann. Intern. Med. 1980, 93, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Borghi, L.; Meschi, T.; Schianchi, T.; Briganti, A.; Guerra, A.; Allegri, F.; Novarini, A. Urine volume: Stone risk factor and preventive measure. Nephron 1999, 81 (Suppl. S1), 31–37. [Google Scholar] [CrossRef] [PubMed]
- Hosking, D.H.; Erickson, S.B.; Van den Berg, C.J.; Wilson, D.M.; Smith, L.H. The stone clinic effect in patients with idiopathic calcium urolithiasis. J. Urol. 1983, 130, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Daudon, M.; Hennequin, C.; Boujelben, G.; Lacour, B.; Jungers, P. Serial crystalluria determination and the risk of recurrence in calcium stone formers. Kidney Int. 2005, 67, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Sarica, K.; Inal, Y.; Erturhan, S.; Yagci, F. The effect of calcium channel blockers on stone regrowth and recurrence after shock wave lithotripsy. Urol. Res. 2006, 34, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Rossetti, S.; Friend, K.; Erickson, S.B.; Lieske, J.C. Treatment effect, adherence, and safety of high fluid intake for the prevention of incident and recurrent kidney stones: A systematic review and meta-analysis. J. Nephrol. 2016, 29, 211–219. [Google Scholar] [CrossRef]
- Barbey, F.; Joly, D.; Rieu, P.; Mejean, A.; Daudon, M.; Jungers, P. Medical treatment of cystinuria: Critical reappraisal of long-term results. J. Urol. 2000, 163, 1419–1423. [Google Scholar] [CrossRef]
- Daudon, M.; Cohen-Solal, F.; Barbey, F.; Gagnadoux, M.F.; Knebelmann, B.; Jungers, P. Cystine crystal volume determination: A useful tool in the management of cystinuric patients. Urol. Res. 2003, 31, 207–211. [Google Scholar] [CrossRef]
- Prot-Bertoye, C.; Lebbah, S.; Daudon, M.; Tostivint, I.; Jais, J.P.; Lillo-Le Louët, A.; Pontoizeau, C.; Cochat, P.; Bataille, P.; Bridoux, F.; et al. Adverse events associated with currently used medical treatments for cystinuria and treatment goals: Results from a series of 442 patients in France. BJU Int. 2019, 124, 849–861. [Google Scholar] [CrossRef]
- Daudon, M.; Traxer, O.; Jungers, P. Lithiase Urinaire, 2nd ed.; Lavoisier Medecine-Sciences Publications: Paris, France, 2012; p. 402. [Google Scholar]
- Borghi, L.; Schianchi, T.; Meschi, T.; Guerra, A.; Allegri, F.; Maggiore, U.; Novarini, A. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N. Engl. J. Med. 2002, 346, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Stampfer, M.J.; Mount, D.B.; Curhan, G.C. DASH-style diet and 24-hour urine composition. Clin. J. Am. Soc. Nephrol. 2010, 5, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Bhojani, N.; Bjazevic, J.; Wallace, B.; Lee, L.; Kaler, K.S.; Dion, M.; Cowan, A.; Sultan, N.; Chew, B.H.; Razvi, H. UPDATE—Canadian Urological Association guideline: Evaluation and medical management of kidney stones. Can. Urol. Assoc. J. 2022, 16, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Pearle, M.S.; Goldfarb, D.S.; Assimos, D.G.; Curhan, G.; Denu-Ciocca, C.J.; Matlaga, B.R.; Monga, M.; Penniston, K.L.; Preminger, G.M.; Turk, T.M.; et al. Medical management of kidney stones: AUA guideline. J. Urol. 2014, 192, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Dallas, P.; Forciea, M.A.; Starkey, M.; Denberg, T.D. Clinical Guidelines Committee of the American College of, P. Dietary and pharmacologic management to prevent recurrent nephrolithiasis in adults: A clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 2014, 161, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C., Jr.; Gambaro, G.; Rodgers, A.; Asplin, J.; Bonny, O.; Costa-Bauza, A.; Ferraro, P.M.; Fogazzi, G.; Fuster, D.G.; Goldfarb, D.S.; et al. Urine and stone analysis for the investigation of the renal stone former: A consensus conference. Urolithiasis 2021, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Daudon, M. Crystalluria. Nephrol. Ther. 2015, 11, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Daudon, M.; Letavernier, E.; Frochot, V.; Haymann, J.P.; Bazin, D.; Jungers, P. Respective influence of calcium and oxalate urine concentration on the formation of calcium oxalate monohydrate or dihydrate crystals. Comptes Rendus Chim. 2016, 19, 1504–1513. [Google Scholar] [CrossRef]
- Lotan, Y.; Buendia Jimenez, I.; Lenoir-Wijnkoop, I.; Daudon, M.; Molinier, L.; Tack, I.; Nuijten, M.J. Increased water intake as a prevention strategy for recurrent urolithiasis: Major impact of compliance on cost-effectiveness. J. Urol. 2013, 189, 935–939. [Google Scholar] [CrossRef]
- Parks, J.H.; Coe, F.L. Evidence for durable kidney stone prevention over several decades. BJU Int. 2009, 103, 1238–1246. [Google Scholar] [CrossRef]
- McCauley, L.R.; Dyer, A.J.; Stern, K.; Hicks, T.; Nguyen, M.M. Factors influencing fluid intake behavior among kidney stone formers. J. Urol. 2012, 187, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Tarplin, S.; Monga, M.; Stern, K.L.; McCauley, L.R.; Sarkissian, C.; Nguyen, M.M. Predictors of Reporting Success With Increased Fluid Intake Among Kidney Stone Patients. Urology 2016, 88, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.X.; Prasad, N.S.; Rangan, G.K.; Allman-Farinelli, M.; Rangan, A.M. A systematic review to determine the most effective interventions to increase water intake. Nephrology 2016, 21, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Khorami, M.H.; Hashemi, R.; Bagherian-Sararoudi, R.; Sichani, M.M.; Tadayon, F.; Shahdoost, A.A.; Arezegar, S.H. The assessment of 24 24-h urine volume by measurement of urine specific gravity with dipstick in adults with nephrolithiasis. Adv. Biomed. Res. 2012, 1, 86. [Google Scholar] [CrossRef]
- Travers, S.; Prot-Bertoye, C.; Daudon, M.; Courbebaisse, M.; Baron, S. How to Monitor Hydration Status and Urine Dilution in Patients with Nephrolithiasis. Nutrients 2023, 15, 1642. [Google Scholar] [CrossRef]
- Conroy, D.E.; West, A.B.; Brunke-Reese, D.; Thomaz, E.; Streeper, N.M. Just-in-time adaptive intervention to promote fluid consumption in patients with kidney stones. Health Psychol. 2020, 39, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Borofsky, M.S.; Dauw, C.A.; York, N.; Terry, C.; Lingeman, J.E. Accuracy of daily fluid intake measurements using a “smart” water bottle. Urolithiasis 2018, 46, 343–348. [Google Scholar] [CrossRef]
- Cohen, R.; Fernie, G.; Roshan Fekr, A. Monitoring fluid intake by commercially available smart water bottles. Sci. Rep. 2022, 12, 4402. [Google Scholar] [CrossRef]
- Wright, H.C.; Alshara, L.; DiGennaro, H.; Kassis, Y.E.; Li, J.; Monga, M.; Calle, J.; Sivalingam, S. The impact of smart technology on adherence rates and fluid management in the prevention of kidney stones. Urolithiasis 2022, 50, 29–36. [Google Scholar] [CrossRef]
- Stout, T.E.; Lingeman, J.E.; Krambeck, A.E.; Humphreys, M.R.; Zisman, A.; Elfering, S.; Large, T.; Dahm, P.; Borofsky, M. A Randomized Trial Evaluating the Use of a Smart Water Bottle to Increase Fluid Intake in Stone Formers. J. Ren. Nutr. 2022, 32, 389–395. [Google Scholar] [CrossRef]
- Seay, N.W.; Lehrich, R.W.; Greenberg, A. Diagnosis and Management of Disorders of Body Tonicity-Hyponatremia and Hypernatremia: Core Curriculum 2020. Am. J. Kidney Dis. 2020, 75, 272–286. [Google Scholar] [CrossRef]
- Sterns, R.H.; Silver, S.M.; Hix, J.K. Hyponatremia. In Seldin and Giebisch’s The Kidney, Physiology and Pathophysiology, 5th ed.; Alpern, R.J., Caplan, M.J., Moe, O.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, p. 1511. [Google Scholar]
- Filippone, E.J.; Ruzieh, M.; Foy, A. Thiazide-Associated Hyponatremia: Clinical Manifestations and Pathophysiology. Am. J. Kidney Dis. 2020, 75, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Curhan, G.C. Fructose consumption and the risk of kidney stones. Kidney Int. 2008, 73, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A. Effect of cola consumption on urinary biochemical and physicochemical risk factors associated with calcium oxalate urolithiasis. Urol. Res. 1999, 27, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Taylor, E.N.; Gambaro, G.; Curhan, G.C. Soda and other beverages and the risk of kidney stones. Clin. J. Am. Soc. Nephrol. 2013, 8, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Shuster, J.; Jenkins, A.; Logan, C.; Barnett, T.; Riehle, R.; Zackson, D.; Wolfe, H.; Dale, R.; Daley, M.; Malik, I.; et al. Soft drink consumption and urinary stone recurrence: A randomized prevention trial. J. Clin. Epidemiol. 1992, 45, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Spiegelman, D.; Stampfer, M.J. Prospective study of beverage use and the risk of kidney stones. Am. J. Epidemiol. 1996, 143, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Taylor, E.N.; Gambaro, G.; Curhan, G.C. Caffeine intake and the risk of kidney stones. Am. J. Clin. Nutr. 2014, 100, 1596–1603. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Mao, Z.; He, X.; Zhang, Q.; Zhang, D. A meta-analysis of coffee intake and risk of urolithiasis. Urol. Int. 2014, 93, 220–228. [Google Scholar] [CrossRef]
- Siener, R. Nutrition and Kidney Stone Disease. Nutrients 2021, 13, 1917. [Google Scholar] [CrossRef]
- Large, T.; Williams, J., Jr.; Asplin, J.R.; Krambeck, A. Using Low-Calorie Orange Juice as a Dietary Alternative to Alkali Therapy. J. Endourol. 2020, 34, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, C.; Wang, X.L.; Liu, T.Z.; Zeng, X.T.; Li, S.; Duan, X.W. Self-Fluid Management in Prevention of Kidney Stones: A PRISMA-Compliant Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Medicine 2015, 94, e1042. [Google Scholar] [CrossRef] [PubMed]
- Stoots, S.J.M.; Kamphuis, G.M.; Geraghty, R.; Vogt, L.; Henderickx, M.; Hameed, B.M.Z.; Ibrahim, S.; Pietropaolo, A.; Jamnadass, E.; Aljumaiah, S.M.; et al. Global Variations in the Mineral Content of Bottled Still and Sparkling Water and a Description of the Possible Impact on Nephrological and Urological Diseases. J. Clin. Med. 2021, 10, 2807. [Google Scholar] [CrossRef] [PubMed]
- Stoots, S.J.M.; Geraghty, R.; Kamphuis, G.M.; Jamnadass, E.; Henderickx, M.; Ventimiglia, E.; Traxer, O.; Keller, E.X.; DeConinck, V.; Talso, M.; et al. Variations in the Mineral Content of Bottled “Still” Water Across Europe: Comparison of 182 Brands Across 10 Countries. J. Endourol. 2021, 35, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Stoots, S.J.M.; Geraghty, R.; Kamphuis, G.M.; Jamnadass, E.; Henderickx, M.; Ventimiglia, E.; Traxer, O.; Keller, E.X.; De Coninck, V.; Talso, M.; et al. Variations in the mineral content of bottled ‘carbonated or sparkling’ water across Europe: A comparison of 126 brands across 10 countries. Cent. Eur. J. Urol. 2021, 74, 71–75. [Google Scholar] [CrossRef]
- Michael, K.; Somani, B.K. Variation in Tap Water Mineral Content in the United Kingdom: Is It Relevant for Kidney Stone Disease? J. Clin. Med. 2022, 11, 5118. [Google Scholar] [CrossRef] [PubMed]
- Hubert, J.; Hubert, C.; Jungers, P.; Daudon, M.; Hartemann, P. Drinking water and urinary stones. Which drinking water and which modalities of diuresis? Prog. Urol. 2002, 12, 692–699. [Google Scholar]
- Siener, R. Can the manipulation of urinary pH by beverages assist with the prevention of stone recurrence? Urolithiasis 2016, 44, 51–56. [Google Scholar] [CrossRef]
- Kessler, T.; Hesse, A. Cross-over study of the influence of bicarbonate-rich mineral water on urinary composition in comparison with sodium potassium citrate in healthy male subjects. Br. J. Nutr. 2000, 84, 865–871. [Google Scholar] [CrossRef]
- Siener, R.; Jahnen, A.; Hesse, A. Influence of a mineral water rich in calcium, magnesium and bicarbonate on urine composition and the risk of calcium oxalate crystallization. Eur. J. Clin. Nutr. 2004, 58, 270–276. [Google Scholar] [CrossRef]
- Dai, J.C.; Pearle, M.S. Diet and Stone Disease in 2022. J. Clin. Med. 2022, 11, 4740. [Google Scholar] [CrossRef]
- Edvardsson, V.O.; Runolfsdottir, H.L.; Thorsteinsdottir, U.A.; Sch Agustsdottir, I.M.; Oddsdottir, G.S.; Eiriksson, F.; Goldfarb, D.S.; Thorsteinsdottir, M.; Palsson, R. Comparison of the effect of allopurinol and febuxostat on urinary 2,8-dihydroxyadenine excretion in patients with Adenine phosphoribosyltransferase deficiency (APRTd): A clinical trial. Eur. J. Intern. Med. 2018, 48, 75–79. [Google Scholar] [CrossRef]

| Fluid Amount | Diuresis | Urine Specific Gravity | Reference | |
|---|---|---|---|---|
| General preventive measures | ||||
| European Association of Urology | 2.5–3.0 L/day | 2.0–2.5 L/day | <1.010 | [12] |
| Canadian Urological Association | 2.5 L/day | [69] | ||
| American College of Physicians | At least 2.0 L/day | [71] | ||
| American Urological Association | At least 2.5 L /day | [70] | ||
| Cystinuria | ||||
| European Association of Urology | 3.5 L/day | >3 L/day | [12] | |
| Canadian urological Association | 3.5–4 L/day | >3 L/day | [69] | |
| European Reference Network for Rare Kidney Diseases | >3 L/day | ≤1.005 | [14] | |
| Primary Hyperoxaluria | ||||
| European Association of Urology | 3.5–4.0 L/day | [12] | ||
| European Reference Network for Rare Kidney Diseases | 3.5–4.0 L/day | [15] | ||
| 2,8-dihydroxyandenine stones and xanthine stones | ||||
| European Association of Urology | <1.010 | [12] | ||
| Urinary Parameters | Concentration | Reference |
|---|---|---|
| Calcium | <3.8 mmol/L | [60,74] |
| Oxalate | <0.31 mmol/L | [74] |
| Inorganic phosphate | <24 mmol/L at pH < 6.5 | [73] |
| Urate | <2.4 mmol/L at 5.3 ≤ pH <5.5 | [73] |
| <2.8 mmol/L at 5.5 ≤ pH < 6 | ||
| <3.5 mmol/L at pH ≥ 6 | ||
| Cystine | <1 mmol/L (250 mg/l) | [14] |
| Citrate | >1 mmol/L | [73] |
| Magnesium | >1.5 mmol/L | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Courbebaisse, M.; Travers, S.; Bouderlique, E.; Michon-Colin, A.; Daudon, M.; De Mul, A.; Poli, L.; Baron, S.; Prot-Bertoye, C. Hydration for Adult Patients with Nephrolithiasis: Specificities and Current Recommendations. Nutrients 2023, 15, 4885. https://doi.org/10.3390/nu15234885
Courbebaisse M, Travers S, Bouderlique E, Michon-Colin A, Daudon M, De Mul A, Poli L, Baron S, Prot-Bertoye C. Hydration for Adult Patients with Nephrolithiasis: Specificities and Current Recommendations. Nutrients. 2023; 15(23):4885. https://doi.org/10.3390/nu15234885
Chicago/Turabian StyleCourbebaisse, Marie, Simon Travers, Elise Bouderlique, Arthur Michon-Colin, Michel Daudon, Aurélie De Mul, Laura Poli, Stéphanie Baron, and Caroline Prot-Bertoye. 2023. "Hydration for Adult Patients with Nephrolithiasis: Specificities and Current Recommendations" Nutrients 15, no. 23: 4885. https://doi.org/10.3390/nu15234885
APA StyleCourbebaisse, M., Travers, S., Bouderlique, E., Michon-Colin, A., Daudon, M., De Mul, A., Poli, L., Baron, S., & Prot-Bertoye, C. (2023). Hydration for Adult Patients with Nephrolithiasis: Specificities and Current Recommendations. Nutrients, 15(23), 4885. https://doi.org/10.3390/nu15234885






