An Update on Drug–Nutrient Interactions and Dental Decay in Older Adults
Highlights
- Long-term use of medications in the elderly leads to deficiencies in key vitamins and minerals (e.g., vitamin B12, magnesium, calcium, and iron).
- Nutrient deficiencies linked to medications may cause systemic health issues, including anemia, osteoporosis, and increased risk of dental caries and other oral health problems.
- Healthcare professionals need to monitor the nutritional status of elderly patients on long-term medications.
- Tailored dietary interventions or supplements should be considered to prevent nutrient deficiencies.
- Proper management can improve overall health outcomes and reduce the risk of systemic and oral health issues such as dental decay in the elderly.
Abstract
:1. Introduction
2. Importance of Micronutrients in the Older Adults
3. Older Adults and Polymedication
4. Drug–Nutrient Interactions
5. Host–Drug–Oral Microbiota–Nutrient Interactions
6. Drugs Used for Basic Elderly Pathologies vs. Frequency of Dental Caries
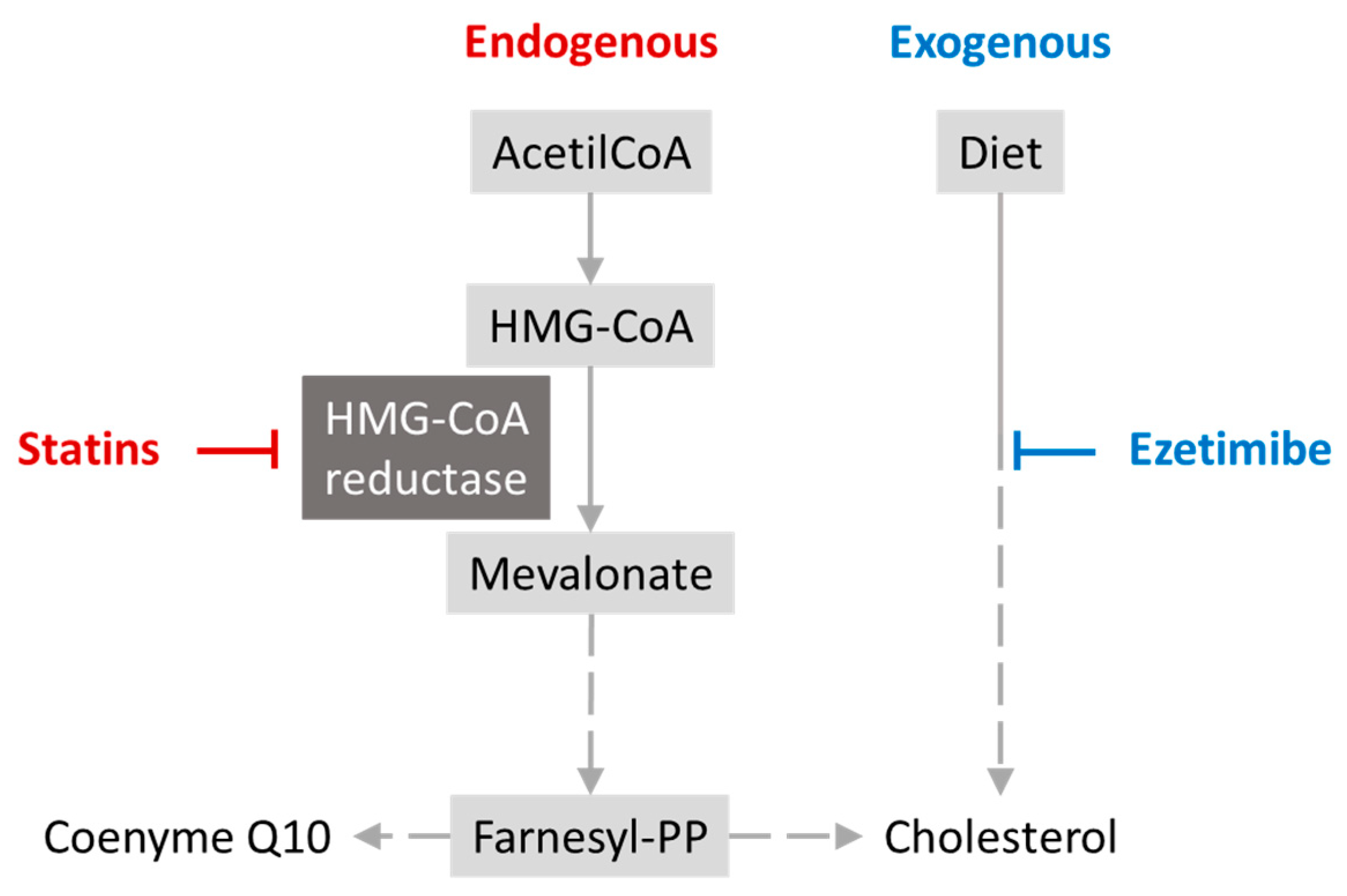
6.1. Antidyslipidemics–Statins
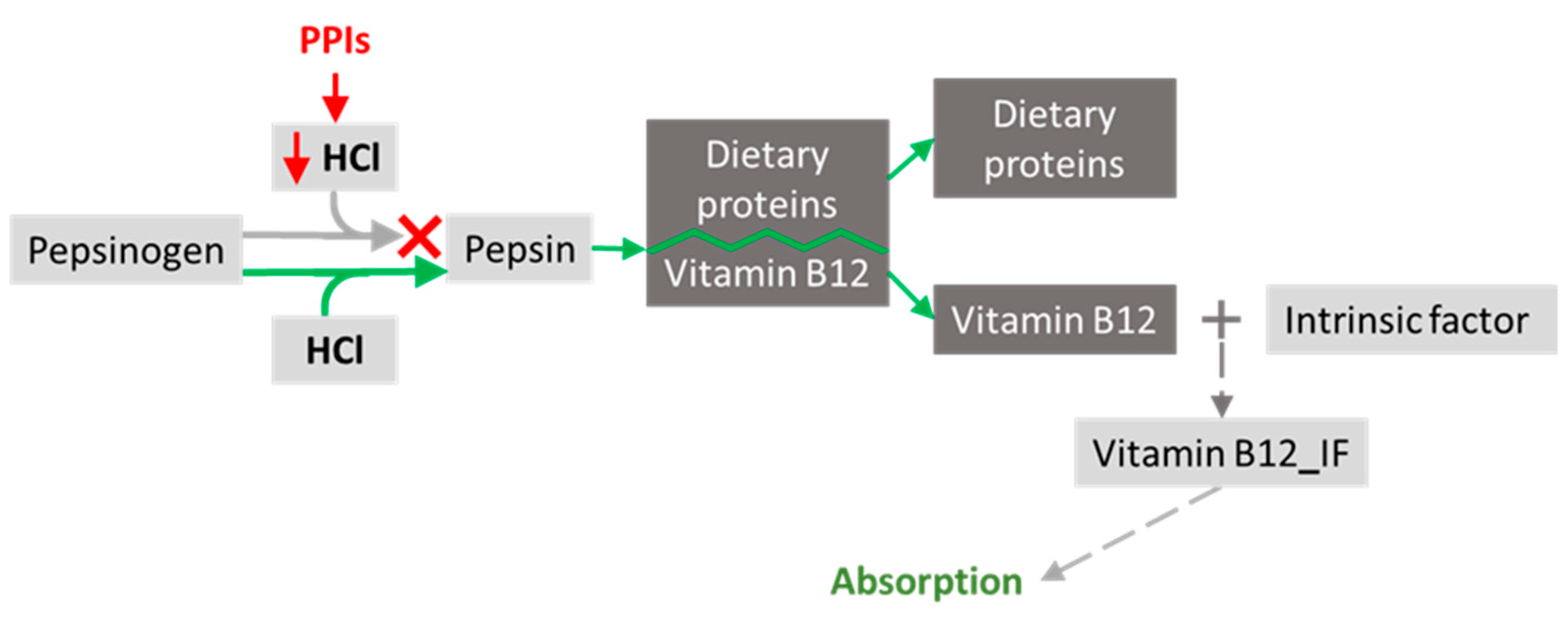
6.2. Gastric Secretion Modifiers–Proton Pump Inhibitors
6.3. Oral Hypoglycaemic Agent–Metformin
6.4. Central Nervous System (CNS) Medications–Antidepressants
6.5. Anti-Hypertensives
6.5.1. Angiotensin Converting Enzyme Inhibitors (ACE)
6.5.2. Calcium Channel Blockers (CCB)
6.5.3. Loop and Thiazide Diuretics
6.5.4. Potassium Sparing Diuretics (Triamterene) and Hydrochlorothiazide
6.6. Corticosteroids
7. Overview
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barbaccia, V.; Bravi, L.; Murmura, F.; Savelli, E.; Viganò, E. Mature and Older Adults’ Perception of Active Ageing and the Need for Supporting Services: Insights from a Qualitative Study. Int. J. Environ. Res. Public Health 2022, 19, 7660. [Google Scholar] [CrossRef] [PubMed]
- Bushra, R.; Aslam, N.; Khan, A.Y. Food-Drug Interactions. Oman Med. J. 2011, 26, 77. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R.; Tapsell, L.C. Food, Not Nutrients, Is the Fundamental Unit in Nutrition. Nutr. Rev. 2007, 65, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Ötles, S.; Senturk, A. Food and Drug Interactions: A General Review. Acta Sci. Pol. Technol. Aliment. 2014, 13, 89–102. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Benedetti, A.; Di Paolo, A.; Giannese, D.; Cupisti, A. Interactions between Food and Drugs, and Nutritional Status in Renal Patients: A Narrative Review. Nutrients 2022, 14, 212. [Google Scholar] [CrossRef]
- Bell, V.; Barros, A.B.; Fernandes, T.H. Food Fortification in Sub Saharan Africa: Science or Business? In Food and Nutrition Security in Africa; Fernandes, T.H., Ferrão, J., Facknath, S., Eds.; Alcance Ed.: Maputo, Mozambique, 2020; ISBN 978-989-8934-05-5. [Google Scholar]
- Cruz-Lopes, L.; Macena, M.; Guiné, R.P.F. Application of Nanotechnologies along the Food Supply Chain. Open Agric. 2021, 6, 749–760. [Google Scholar] [CrossRef]
- Khavinson, V.; Popovich, I.; Mikhailova, O. Towards Realization of Longer Life. Acta Biomed. Atenei Parm. 2020, 91, e2020054. [Google Scholar] [CrossRef]
- Lunenfeld, B.; Stratton, P. The Clinical Consequences of an Ageing World and Preventive Strategies. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 643–659. [Google Scholar] [CrossRef]
- Choubisa, D. Nutrition and Geriatric: An Overview. Dent. J. Adv. Stud. 2022, 10, 115–127. [Google Scholar] [CrossRef]
- Saunders, J.; Smith, T. Malnutrition: Causes and Consequences. Clin. Med. 2010, 10, 624–627. [Google Scholar] [CrossRef]
- Antoniadou, M.; Varzakas, T. Breaking the Vicious Circle of Diet, Malnutrition and Oral Health for the Independent Elderly. Crit. Rev. Food Sci. Nutr. 2021, 61, 3233–3255. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiao, X.; Zhang, X. Hydration Status in Older Adults: Current Knowledge and Future Challenges. Nutrients 2023, 15, 2609. [Google Scholar] [CrossRef] [PubMed]
- Ragonnaud, E.; Biragyn, A. Gut Microbiota as the Key Controllers of “Healthy” Aging of Elderly People. Immun. Ageing 2021, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Lee, W.F.; Salamanca, E.; Yao, W.L.; Su, J.N.; Wang, S.Y.; Hu, C.J.; Chang, W.J. Oral Microbiota Changes in Elderly Patients, an Indicator of Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2021, 18, 4211. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Park, J.C.; Park, Y.K. Nutrient Intakes and Medication Use in Elderly Individuals with and without Dry Mouths. Nutr. Res. Pract. 2020, 14, 143–151. [Google Scholar] [CrossRef]
- Gerdin, E.W.; Einarson, S.; Jonsson, M.; Aronsson, K.; Johansson, I. Impact of Dry Mouth Conditions on Oral Health-Related Quality of Life in Older People. Gerodontology 2005, 22, 219–226. [Google Scholar] [CrossRef]
- Plemons, J.M.; Al-Hashimi, I.; Marek, C.L. Managing Xerostomia and Salivary Gland Hypofunction: Executive Summary of a Report from the American Dental Association Council on Scientific Affairs. J. Am. Dent. Assoc. 2014, 145, 867–873. [Google Scholar] [CrossRef]
- Rusthen, S.; Young, A.; Herlofson, B.B.; Aqrawi, L.A.; Rykke, M.; Hove, L.H.; Palm, Ø.; Jensen, J.L.; Singh, P.B. Oral Disorders, Saliva Secretion, and Oral Health-Related Quality of Life in Patients with Primary Sjögren’s Syndrome. Eur. J. Oral Sci. 2017, 125, 265–271. [Google Scholar] [CrossRef]
- Pajukoski, H.; Meurman, J.H.; Odont, D.; Halonen, P.; Sulkava, R. Prevalence of Subjective Dry Mouth and Burning Mouth in Hospitalized Elderly Patients and Outpatients in Relation to Saliva, Medication, and Systemic Diseases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 92, 641–649. [Google Scholar] [CrossRef]
- Fejerskov, O.; Kidd, E.A.M. (Eds.) Dental Caries: The Disease and Its Clinical Management; Blackwell: Oxford, UK; Malden, MA, USA, 2003; ISBN 978-1-4051-0718-1. [Google Scholar]
- Saunders, R.H.; Meyerowitz, C. Dental Caries in Older Adults. Dent. Clin. N. Am. 2005, 49, 293–308. [Google Scholar] [CrossRef]
- Chan, A.K.Y.; Tamrakar, M.; Jiang, C.M.; Lo, E.C.M.; Leung, K.C.M.; Chu, C.H. A Systematic Review on Caries Status of Older Adults. Int. J. Environ. Res. Public Health 2021, 18, 10662. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Untreated Caries: A Systematic Review and Metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Gavriilidou, N.N.; Belibasakis, G.N. Root Caries: The Intersection between Periodontal Disease and Dental Caries in the Course of Ageing. Br. Dent. J. 2019, 227, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- López, R.; Smith, P.C.; Göstemeyer, G.; Schwendicke, F. Ageing, Dental Caries and Periodontal Diseases. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S145–S152. [Google Scholar] [CrossRef]
- Chu, C.H.; Mei, M.L.; Lo, E.C.M. Use of Fluorides in Dental Caries Management. Gen. Dent. 2010, 58, 37–43; quiz 44–45, 79–80. [Google Scholar]
- Marinho, V.C.C.; Worthington, H.V.; Walsh, T.; Chong, L.Y. Fluoride Gels for Preventing Dental Caries in Children and Adolescents. Cochrane Database Syst. Rev. 2015, 2015, CD002280. [Google Scholar] [CrossRef]
- Gao, S.S.; Zhang, S.; Mei, M.L.; Lo, E.C.-M.; Chu, C.-H. Caries Remineralisation and Arresting Effect in Children by Professionally Applied Fluoride Treatment—A Systematic Review. BMC Oral Health 2016, 16, 12. [Google Scholar] [CrossRef]
- Chan, A.K.Y.; Tamrakar, M.; Jiang, C.M.; Tsang, Y.C.; Leung, K.C.M.; Chu, C.H. Clinical Evidence for Professionally Applied Fluoride Therapy to Prevent and Arrest Dental Caries in Older Adults: A Systematic Review. J. Dent. 2022, 125, 104273. [Google Scholar] [CrossRef]
- Amargianitakis, M.; Antoniadou, M.; Rahiotis, C.; Varzakas, T. Probiotics, Prebiotics, Synbiotics and Dental Caries. New Perspectives, Suggestions, and Patient Coaching Approach for a Cavity-Free Mouth. Appl. Sci. 2021, 11, 5472. [Google Scholar] [CrossRef]
- Salazar, N.; Valdés-Varela, L.; González, S.; Gueimonde, M.; de los Reyes-Gavilán, C.G. Nutrition and the Gut Microbiome in the Elderly. Gut Microbes 2017, 8, 82–97. [Google Scholar] [CrossRef]
- Agence Française de Sécurité Sanitaire des Aliments. Opinion of the French Food Safety Agency on the Assessment of Nutritional Needs for Frail Elderly People and Those Suffering from Certain Pathologies, in Order to Establish Nutritional References and Provide Appropriate Nutritional Support; Agence Française de Sécurité Sanitaire des Aliments: Maisons-Alfort, France, 2009; Available online: https://www.anses.fr/en/content/opinion-french-food-safety-agency-assessment-nutritional-needs-frail-elderly-people-and (accessed on 20 October 2023).
- Zhang, Y.; Chen, R.; Zhang, D.D.; Qi, S.; Liu, Y. Metabolite Interactions between Host and Microbiota during Health and Disease: Which Feeds the Other? Biomed. Pharmacother. 2023, 160, 114295. [Google Scholar] [CrossRef] [PubMed]
- Genser, D. Food and Drug Interaction: Consequences for the Nutrition/Health Status. Ann. Nutr. Metab. 2008, 52, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Liu, I.T.; Liang, F.W.; Li, C.C.; Sun, Z.J.; Chang, Y.F.; Chao, T.H.; Wu, C.H. Effects of Age and Gender on Body Composition Indices as Predictors of Mortality in Middle-Aged and Old People. Sci. Rep. 2022, 12, 7912. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). Council on Family Health. In Drug Interactions: What You Should Know; FDA: Rockville, MD, USA, 2004. [Google Scholar]
- Savarino, G.; Corsello, A.; Corsello, G. Macronutrient Balance and Micronutrient Amounts through Growth and Development. Ital. J. Pediatr. 2021, 47, 109. [Google Scholar] [CrossRef] [PubMed]
- Wierzejska, R.E. Dietary Supplements—For Whom? The Current State of Knowledge about the Health Effects of Selected Supplement Use. Int. J. Environ. Res. Public Health 2021, 18, 8897. [Google Scholar] [CrossRef] [PubMed]
- Pognan, F.; Beilmann, M.; Boonen, H.C.M.; Czich, A.; Dear, G.; Hewitt, P.; Mow, T.; Oinonen, T.; Roth, A.; Steger-Hartmann, T.; et al. The Evolving Role of Investigative Toxicology in the Pharmaceutical Industry. Nat. Rev. Drug Discov. 2023, 22, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.D.; Drake, V.J.; Stevens, J.F. Medications and Micronutrients: Identifying Clinically Relevant Interactions and Addressing Nutritional Needs. J. Pharm. Technol. 2018, 34, 216–230. [Google Scholar] [CrossRef]
- Rechel, B.; Jagger, C.; McKee, M.; Cylus, J.; Normand, C.; Figueras, J.; North, J.; White, C. Living Longer, but in Better or Worse Health? European Observatory on Health Systems and Policies: Brussels, Belgium, 2020. [Google Scholar]
- Inui, T.; Hanley, B.; Tee, E.S.; Nishihira, J.; Tontisirin, K.; Van Dael, P.; Eggersdorfer, M. The Role of Micronutrients in Ageing Asia: What Can Be Implemented with the Existing Insights. Nutrients 2021, 13, 2222. [Google Scholar] [CrossRef]
- Farag, M.A.; Hamouda, S.; Gomaa, S.; Agboluaje, A.A.; Hariri, M.L.M.; Yousof, S.M. Dietary Micronutrients from Zygote to Senility: Updated Review of Minerals’ Role and Orchestration in Human Nutrition throughout Life Cycle with Sex Differences. Nutrients 2021, 13, 3740. [Google Scholar] [CrossRef]
- Brancaccio, M.; Mennitti, C.; Cesaro, A.; Fimiani, F.; Vano, M.; Gargiulo, B.; Caiazza, M.; Amodio, F.; Coto, I.; D’alicandro, G.; et al. The Biological Role of Vitamins in Athletes’ Muscle, Heart and Microbiota. Int. J. Environ. Res. Public Health 2022, 19, 1249. [Google Scholar] [CrossRef]
- Berger, M.M.; Pantet, O.; Schneider, A.; Ben-Hamouda, N. Micronutrient Deficiencies in Medical and Surgical Inpatients. J. Clin. Med. 2019, 8, 931. [Google Scholar] [CrossRef] [PubMed]
- van Steenwijk, H.P.; Bast, A.; de Boer, A. Immunomodulating Effects of Fungal Β-Glucans: From Traditional Use to Medicine. Nutrients 2021, 13, 1333. [Google Scholar] [CrossRef] [PubMed]
- Vlassopoulou, M.; Yannakoulia, M.; Pletsa, V.; Zervakis, G.I.; Kyriacou, A. Effects of Fungal Β-Glucans on Health—A Systematic Review of Randomized Controlled Trials. Food Funct. 2021, 12, 3366–3380. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Martelli, A.; Flori, L.; Colletti, A.; Cicero, A.F.G. Coenzyme Q10: Clinical Applications beyond Cardiovascular Diseases. Nutrients 2021, 13, 1697. [Google Scholar] [CrossRef]
- Manthena, S.; Rao, M.V.R.; Penubolu, L.P.; Putcha, M.; Harsha, A.V.N.S. Effectiveness of CoQ10 Oral Supplements as an Adjunct to Scaling and Root Planing in Improving Periodontal Health. J. Clin. Diagn. Res. JCDR 2015, 9, ZC26–ZC28. [Google Scholar] [CrossRef]
- Ho, M.J.; Li, E.C.; Wright, J.M. Blood Pressure Lowering Efficacy of Coenzyme Q10 for Primary Hypertension. Cochrane Database Syst. Rev. 2016, 3, CD007435. [Google Scholar] [CrossRef]
- Prakash, S.; Sunitha, J.; Hans, M. Role of Coenzyme Q(10) as an Antioxidant and Bioenergizer in Periodontal Diseases. Indian J. Pharmacol. 2010, 42, 334–337. [Google Scholar] [CrossRef]
- Schaeffer, L. The Role of Functional Groups in Drug–Receptor Interactions. In The Practice of Medicinal Chemistry: Fourth Edition; Academic Press: Cambridge, MA, USA, 2008; pp. 359–378. ISBN 9780124172050. [Google Scholar]
- Hathcock, J. Metabolic Mechanisms of Drug-Nutrient Interactions. Fed. Proc. 1985, 44, 124–129. [Google Scholar]
- Pozzi, C.; Santucci, M.; Marverti, G.; D’arca, D.; Tagliazucchi, L.; Ferrari, S.; Gozzi, G.; Losi, L.; Tassone, G.; Mangani, S.; et al. Structural Bases for the Synergistic Inhibition of Human Thymidylate Synthase and Ovarian Cancer Cell Growth by Drug Combinations. Cancers 2021, 13, 2061. [Google Scholar] [CrossRef]
- WHO Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 20 October 2023).
- Ismail, Z.; Ahmad, W.I.W.; Hamjah, S.H.; Astina, I.K. The Impact of Population Ageing: A Review. Iran. J. Public Health 2021, 50, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults—Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Chang, A.Y.; Tan, A.X.; Nadeau, K.C.; Odden, M.C. Aging Hearts in a Hotter, More Turbulent World: The Impacts of Climate Change on the Cardiovascular Health of Older Adults. Curr. Cardiol. Rep. 2022, 24, 749–760. [Google Scholar] [CrossRef]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What Is Polypharmacy? A Systematic Review of Definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Pazan, F.; Wehling, M. Polypharmacy in Older Adults: A Narrative Review of Definitions, Epidemiology and Consequences. Eur. Geriatr. Med. 2021, 12, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Alongi, M.; Anese, M. Re-Thinking Functional Food Development through a Holistic Approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Akamine, D.; Filho, M.K.; Peres, C.M. Drug-Nutrient Interactions in Elderly People. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 304–310. [Google Scholar] [CrossRef]
- Dhurjad, P.; Dhavaliker, C.; Gupta, K.; Sonti, R. Exploring Drug Metabolism by the Gut Microbiota: Modes of Metabolism and Experimental Approaches. Drug Metab. Dispos. 2022, 50, 224–234. [Google Scholar] [CrossRef]
- Fischbach, M.A. Microbiome: Focus on Causation and Mechanism. Cell 2018, 174, 785–790. [Google Scholar] [CrossRef]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between Drugs and the Gut Microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Vich Vila, A.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.R.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of Commonly Used Drugs on the Composition and Metabolic Function of the Gut Microbiota. Nat. Commun. 2020, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human Oral Microbiota and Its Modulation for Oral Health. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Corrêa, J.D.; Silva, T.A. The Oral Microbiota Is Modified by Systemic Diseases. J. Dent. Res. 2019, 98, 148–156. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral Microbiota in Human Systematic Diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Nagakubo, D.; Kaibori, Y. Oral Microbiota: The Influences and Interactions of Saliva, IgA, and Dietary Factors in Health and Disease. Microorganisms 2023, 11, 2307. [Google Scholar] [CrossRef] [PubMed]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.-M.; Dequenne, I.; de Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2019, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; Cheng, L.P.; Zhou, L.M.; Cheung, Y.T.; Zuo, Z. Herb-Drug Interactions between the Medicinal Mushrooms Lingzhi and Yunzhi and Cytotoxic Anticancer Drugs: A Systematic Review. Chin. Med. UK 2020, 15, 75. [Google Scholar] [CrossRef]
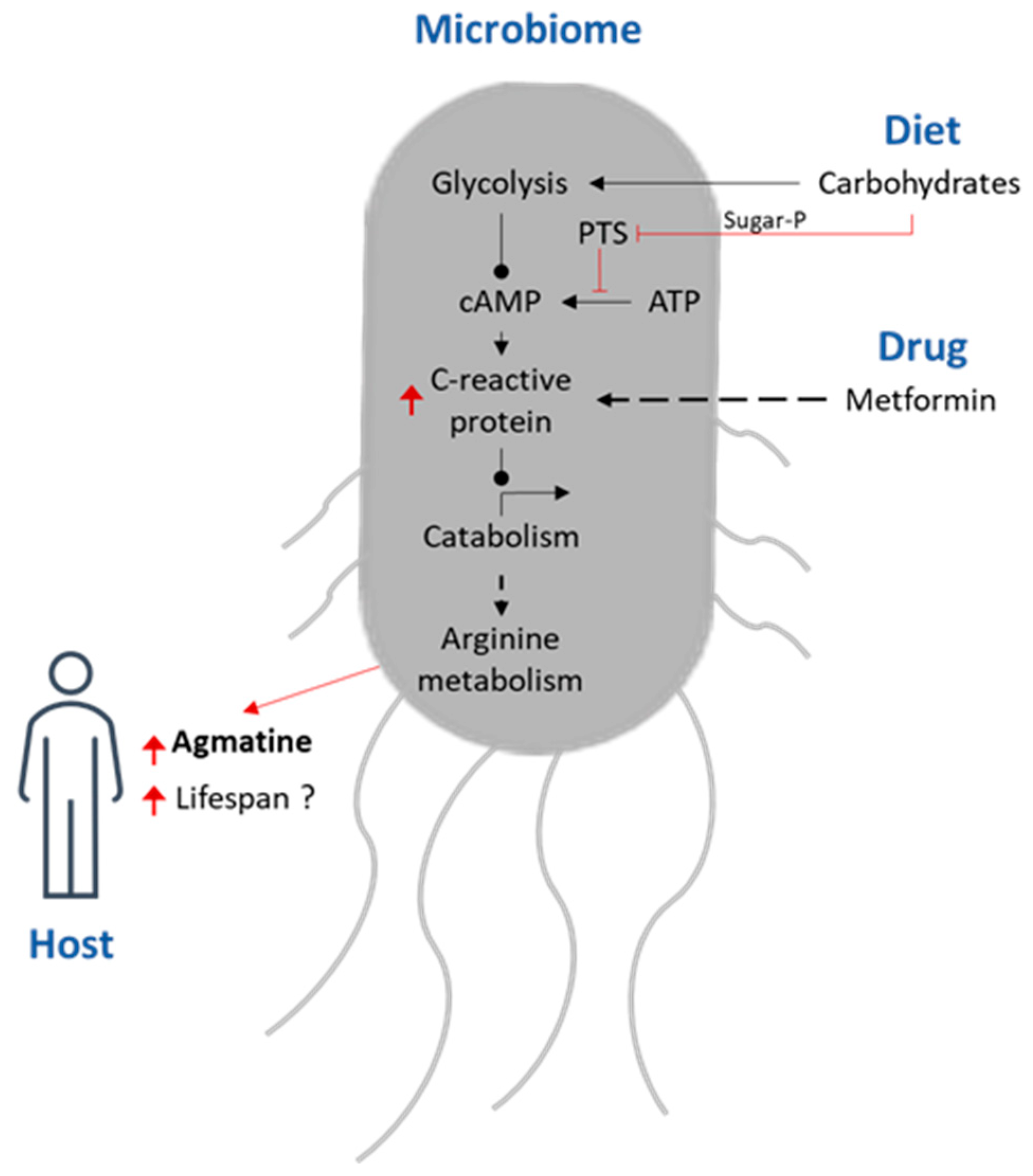
- Yang, F.; Xu, J.; Zhu, Y.; Wang, Y.; Xu, M.; Rao, Z. High-Level Production of the Agmatine in Engineered Corynebacterium Crenatum with the Inhibition-Releasing Arginine Decarboxylase. Microb. Cell Factories 2022, 21, 16. [Google Scholar] [CrossRef]
- Pryor, R.; Norvaisas, P.; Marinos, G.; Best, L.; Thingholm, L.B.; Quintaneiro, L.M.; De Haes, W.; Esser, D.; Waschina, S.; Lujan, C.; et al. Host-Microbe-Drug-Nutrient Screen Identifies Bacterial Effectors of Metformin Therapy. Cell 2019, 178, 1299–1312.e29. [Google Scholar] [CrossRef]
- Song, M.; Liu, T.; Liu, H.; Zhang, Q.; Zhang, Q.; Wang, Y.; Ma, X.; Cao, L.; Shi, H. Association between Metabolic Syndrome, C-Reactive Protein, and the Risk of Primary Liver Cancer: A Large Prospective Study. BMC Cancer 2022, 22, 853. [Google Scholar] [CrossRef] [PubMed]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-Tumor Activity. Cell 2016, 167, 829–842.e13. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.B.; Cassagnol, M. HMG-CoA Reductase Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Pop, G.; Farcaș, A.; Butucă, A.; Morgovan, C.; Arseniu, A.M.; Pumnea, M.; Teodoru, M.; Gligor, F.G. Post-Marketing Surveillance of Statins—A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance. Pharmaceuticals 2022, 15, 1536. [Google Scholar] [CrossRef] [PubMed]
- Horodinschi, R.N.; Stanescu, A.M.A.; Bratu, O.G.; Stoian, A.P.; Radavoi, D.G.; Diaconu, C.C. Treatment with Statins in Elderly Patients. Medicina 2019, 55, 721. [Google Scholar] [CrossRef] [PubMed]
- Zaleski, A.L.; Taylor, B.A.; Thompson, P.D. Coenzyme Q10 as Treatment for Statin-Associated Muscle Symptoms—A Good Idea, But…. Adv. Nutr. 2018, 9, 519S. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Walls, A. Chapter 12: Nutrient Deficiencies and Oral Health. Monogr. Oral Sci. 2020, 28, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Gossweiler, A.; Martinez-Mier, E.A. Chapter 6: Vitamins and Oral Health. Monogr. Oral Sci. 2020, 28, 59–67. [Google Scholar] [CrossRef]
- Turunen, M.; Olsson, J.; Dallner, G. Metabolism and Function of Coenzyme Q. Biochim. Biophys. Acta BBA-Biomembr. 2004, 1660, 171–199. [Google Scholar] [CrossRef]
- Hussain, A.; Kaler, J.; Ray, S.D.; Hussain, A.; Kaler, J.; Ray, S. The Benefits Outweigh the Risks of Treating Hypercholesterolemia: The Statin Dilemma. Cureus 2023, 15, e33648. [Google Scholar] [CrossRef]
- Schonewille, M.; De Boer, J.F.; Mele, L.; Wolters, H.; Bloks, V.W.; Wolters, J.C.; Kuivenhoven, J.A.; Tietge, U.J.F.; Brufau, G.; Groen, A.K. Statins Increase Hepatic Cholesterol Synthesis and Stimulate Fecal Cholesterol Elimination in Mice. J. Lipid Res. 2016, 57, 1455–1464. [Google Scholar] [CrossRef]
- Pérez-Castrillón, J.L.; Abad Manteca, L.; Vega, G.; Del Pino Montes, J.; De Luis, D.; Dueňas Laita, A. Vitamin D Levels and Lipid Response to Atorvastatin. Int. J. Endocrinol. 2010, 2010, 320721. [Google Scholar] [CrossRef] [PubMed]
- Peponis, M.; Antoniadou, M.; Pappa, E.; Rahiotis, C.; Varzakas, T. Vitamin D and Vitamin D Receptor Polymorphisms Relationship to Risk Level of Dental Caries. Appl. Sci. 2023, 13, 6014. [Google Scholar] [CrossRef]
- Rasheed, K.; Sethi, P.; Bixby, E. Severe Vitamin d Deficiency Induced Myopathy Associated with Rhabydomyolysis. N. Am. J. Med. Sci. 2013, 5, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Wakeman, M. A Literature Review of the Potential Impact of Medication on Vitamin D Status. Risk Manag. Healthc. Policy 2021, 14, 3357–3381. [Google Scholar] [CrossRef] [PubMed]
- Al-Jubori, S.H.; Al-Murad, M.A.; Al-Mashhadane, F.A. Effect of Oral Vitamin D3 on Dental Caries: An In-Vivo and In-Vitro Study. Cureus 2022, 14, e25360. [Google Scholar] [CrossRef]
- Kim, I.-J.; Lee, H.-S.; Ju, H.-J.; Na, J.-Y.; Oh, H.-W. A Cross-Sectional Study on the Association between Vitamin D Levels and Caries in the Permanent Dentition of Korean Children. BMC Oral Health 2018, 18, 43. [Google Scholar] [CrossRef]
- Boucher, B.J. The problems of vitamin d insufficiency in older people. Aging Dis. 2012, 3, 313–329. [Google Scholar]
- Uwitonze, A.M.; Rahman, S.; Ojeh, N.; Grant, W.B.; Kaur, H.; Haq, A.; Razzaque, M.S. Oral Manifestations of Magnesium and Vitamin D Inadequacy. J. Steroid Biochem. Mol. Biol. 2020, 200, 105636. [Google Scholar] [CrossRef]
- Brito, S.A.; de Almeida, C.L.F.; de Santana, T.I.; da Silva Oliveira, A.R.; do Nascimento Figueiredo, J.C.B.; Souza, I.T.; de Almeida, L.L.; da Silva, M.V.; Borges, A.S.; de Medeiros, J.W.; et al. Antiulcer Activity and Potential Mechanism of Action of the Leaves of Spondias mombin L. Oxid. Med. Cell. Longev. 2018, 2018, 1731459. [Google Scholar] [CrossRef]
- Torres-Bondia, F.; de Batlle, J.; Galván, L.; Buti, M.; Barbé, F.; Piñol-Ripoll, G. Evolution of the Consumption Trend of Proton Pump Inhibitors in the Lleida Health Region between 2002 and 2015. BMC Public Health 2022, 22, 818. [Google Scholar] [CrossRef]
- Heidelbaugh, J.J. Proton Pump Inhibitors and Risk of Vitamin and Mineral Deficiency: Evidence and Clinical Implications. Ther. Adv. Drug Saf. 2013, 4, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Weusten, B.L.; van de Wiel, A. Aphthous Ulcers and Vitamin B12 Deficiency. Neth. J. Med. 1998, 53, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.-L.; Brito, A.; Guéant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B12 Deficiency. Nat. Rev. Dis. Primer 2017, 3, 17040. [Google Scholar] [CrossRef] [PubMed]
- M Hugar, S.; S Dhariwal, N.; Majeed, A.; Badakar, C.; Gokhale, N.; Mistry, L. Assessment of Vitamin B12 and Its Correlation with Dental Caries and Gingival Diseases in 10- to 14-Year-Old Children: A Cross-Sectional Study. Int. J. Clin. Pediatr. Dent. 2017, 10, 142–146. [Google Scholar] [CrossRef]
- Stover, P.J. Vitamin B12 and Older Adults. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 24–27. [Google Scholar] [CrossRef]
- Prosapio, J.G.; Sankar, P.; Jialal, I. Physiology, Gastrin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Swarnakari, K.M.; Bai, M.; Manoharan, M.P.; Raja, R.; Jamil, A.; Csendes, D.; Gutlapalli, S.D.; Prakash, K.; Desai, D.M.; Desai, A.; et al. The Effects of Proton Pump Inhibitors in Acid Hypersecretion-Induced Vitamin B12 Deficiency: A Systematic Review (2022). Cureus 2022, 14, e31672. [Google Scholar] [CrossRef]
- Toh, J.W.T.; Ong, E.; Wilson, R. Hypomagnesaemia Associated with Long-Term Use of Proton Pump Inhibitors. Gastroenterol. Rep. 2015, 3, 243–253. [Google Scholar] [CrossRef]
- Ahmed, F.; Mohammed, A. Magnesium: The Forgotten Electrolyte—A Review on Hypomagnesemia. Med. Sci. 2019, 7, 56. [Google Scholar] [CrossRef]
- Srinutta, T.; Chewcharat, A.; Takkavatakarn, K.; Praditpornsilpa, K.; Eiam-Ong, S.; Jaber, B.L.; Susantitaphong, P. Proton Pump Inhibitors and Hypomagnesemia: A Meta-Analysis of Observational Studies. Medicine 2019, 98, e17788. [Google Scholar] [CrossRef]
- Losurdo, G.; Caccavo, N.L.B.; Indellicati, G.; Celiberto, F.; Ierardi, E.; Barone, M.; Di Leo, A. Effect of Long-Term Proton Pump Inhibitor Use on Blood Vitamins and Minerals: A Primary Care Setting Study. J. Clin. Med. 2023, 12, 2910. [Google Scholar] [CrossRef]
- Hansen, K.E.; Jones, A.N.; Lindstrom, M.J.; Davis, L.A.; Ziegler, T.E.; Penniston, K.L.; Alvig, A.L.; Shafer, M.M. Do Proton Pump Inhibitors Decrease Calcium Absorption? J. Bone Miner. Res. 2010, 25, 2786–2795. [Google Scholar] [CrossRef]
- Baj, J.; Flieger, W.; Teresiński, G.; Buszewicz, G.; Sitarz, R.; Forma, A.; Karakuła, K.; Maciejewski, R. Magnesium, Calcium, Potassium, Sodium, Phosphorus, Selenium, Zinc, and Chromium Levels in Alcohol Use Disorder: A Review. J. Clin. Med. 2020, 9, 1901. [Google Scholar] [CrossRef] [PubMed]
- Piskin, E.; Cianciosi, D.; Gulec, S.; Tomas, M.; Capanoglu, E. Iron Absorption: Factors, Limitations, and Improvement Methods. ACS Omega 2022, 7, 20441–20456. [Google Scholar] [CrossRef] [PubMed]
- Yiannikourides, A.; Latunde-Dada, G.O. A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders. Medicines 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Hamano, H.; Niimura, T.; Horinouchi, Y.; Zamami, Y.; Takechi, K.; Goda, M.; Imanishi, M.; Chuma, M.; Izawa-Ishizawa, Y.; Miyamoto, L.; et al. Proton Pump Inhibitors Block Iron Absorption through Direct Regulation of Hepcidin via the Aryl Hydrocarbon Receptor-Mediated Pathway. Toxicol. Lett. 2020, 318, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Bahdila, D.; Markowitz, K.; Pawar, S.; Chavan, K.; Fine, D.H.; Velliyagounder, K. The Effect of Iron Deficiency Anemia on Experimental Dental Caries in Mice. Arch. Oral Biol. 2019, 105, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.-Q.; Han, R.; Huang, P.-P.; Wang, S.-Y.; Lin, H.; Ma, L. Iron Deficiency and Early Childhood Caries: A Systematic Review and Meta-Analysis. Chin. Med. J. 2021, 134, 2832–2837. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Jain, A.; Jearth, V.; Mahajan, R.; Panigrahi, M.K.; Sharma, V.; Goenka, M.K.; Kochhar, R.; Makharia, G.; Reddy, D.N.; et al. Guidelines on Optimizing the Use of Proton Pump Inhibitors: PPI Stewardship. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol. 2023, 42, 601–628. [Google Scholar] [CrossRef]
- Nasri, H.; Rafieian-Kopaei, M. Metformin: Current Knowledge. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 658. [Google Scholar]
- Campbell, R.K.; White, J.R.; Saulie, B.A. Metformin: A New Oral Biguanide. Clin. Ther. 1996, 18, 360–371. [Google Scholar] [CrossRef]
- CPMP/4082/00; Summary Information on a Referral Opinion following an Arbitration. EMA: Amsterdam, The Netherlands, 2000.
- Infante, M.; Leoni, M.; Caprio, M.; Fabbri, A. Long-Term Metformin Therapy and Vitamin B12 Deficiency: An Association to Bear in Mind. World J. Diabetes 2021, 12, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Sayedali, E.; Yalin, A.E.; Yalin, S. Association between Metformin and Vitamin B12 Deficiency in Patients with Type 2 Diabetes. World J. Diabetes 2023, 14, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Y.; Tang, Z.-Q.; Bao, Z.-X. Vitamin B12 deficiency may play an etiological role in atrophic glossitis and its grading: A clinical case-control study. BMC Oral Health 2022, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, F.; Samman, S. Vitamin B12 in Health and Disease. Nutrients 2010, 2, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Pratama, S.; Lauren, B.C.; Wisnu, W. The Efficacy of Vitamin B12 Supplementation for Treating Vitamin B12 Deficiency and Peripheral Neuropathy in Metformin-Treated Type 2 Diabetes Mellitus Patients: A Systematic Review. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102634. [Google Scholar] [CrossRef] [PubMed]
- Sheffler, Z.M.; Patel, P.; Abdijadid, S. Antidepressants. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Martins, D.A.R. Análise da Evolução Do Consumo de Ansiolíticos e Antidepressivos em Portugal Continental Entre 2010 e 2020; Universidade Fernando Pessoa: Porto, Portugal, 2021. [Google Scholar]
- Mercurio, M.; de Filippis, R.; Spina, G.; De Fazio, P.; Segura-Garcia, C.; Galasso, O.; Gasparini, G. The Use of Antidepressants Is Linked to Bone Loss: A Systematic Review and Metanalysis. Orthop. Rev. 2022, 14, 38564. [Google Scholar] [CrossRef] [PubMed]
- Zerofsky, M.; Ryder, M.; Bhatia, S.; Stephensen, C.B.; King, J.; Fung, E.B. Effects of Early Vitamin D Deficiency Rickets on Bone and Dental Health, Growth and Immunity. Matern. Child. Nutr. 2016, 12, 898–907. [Google Scholar] [CrossRef]
- Chu, A.; Wadhwa, R. Selective Serotonin Reuptake Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023; ISBN 9781119226253. [Google Scholar]
- Warden, S.J.; Robling, A.G.; Haney, E.M.; Turner, C.H.; Bliziotes, M.M. The Emerging Role of Serotonin (5-Hydroxytryptamine) in the Skeleton and Its Mediation of the Skeletal Effects of Low-Density Lipoprotein Receptor-Related Protein 5 (LRP5). Bone 2010, 46, 4–12. [Google Scholar] [CrossRef]
- Tsapakis, E.M.; Gamie, Z.; Tran, G.T.; Adshead, S.; Lampard, A.; Mantalaris, A.; Tsiridis, E. The Adverse Skeletal Effects of Selective Serotonin Reuptake Inhibitors. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2012, 27, 156–169. [Google Scholar] [CrossRef]
- Yadav, V.K.; Oury, F.; Suda, N.; Liu, Z.-W.; Gao, X.-B.; Confavreux, C.; Klemenhagen, K.C.; Tanaka, K.F.; Gingrich, J.A.; Guo, X.E.; et al. A Serotonin-Dependent Mechanism Explains the Leptin Regulation of Bone Mass, Appetite, and Energy Expenditure. Cell 2009, 138, 976–989. [Google Scholar] [CrossRef]
- Yadav, V.K.; Balaji, S.; Suresh, P.S.; Liu, X.S.; Lu, X.; Li, Z.; Guo, X.E.; Mann, J.J.; Balapure, A.K.; Gershon, M.D.; et al. Pharmacological Inhibition of Gut-Derived Serotonin Synthesis Is a Potential Bone Anabolic Treatment for Osteoporosis. Nat. Med. 2010, 16, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Wee, A.K.H.; Sultana, R. Determinants of Vitamin B12 Deficiency in Patients with Type-2 Diabetes Mellitus—A Primary-Care Retrospective Cohort Study. BMC Prim. Care 2023, 24, 102. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.M.; Wang, Y.; Berk, M.; Collier, F.M.; Fernandes, T.J.; Constable, M.J.; Pasco, J.A.; Dodd, S.; Nicholson, G.C.; Kennedy, R.L.; et al. Selective Serotonin Reuptake Inhibitors Inhibit Human Osteoclast and Osteoblast Formation and Function. Biol. Psychiatry 2013, 74, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Fang, L.; Chen, Y.; Zhong, J.; Wang, H.; Xie, P. Effect of Selective Serotonin Reuptake Inhibitors on Bone Mineral Density: A Systematic Review and Meta-Analysis. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2018, 29, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Battaglino, R.; Fu, J.; Späte, U.; Ersoy, U.; Joe, M.; Sedaghat, L.; Stashenko, P. Serotonin Regulates Osteoclast Differentiation through Its Transporter. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2004, 19, 1420–1431. [Google Scholar] [CrossRef]
- Warden, S.J.; Haney, E.M. Skeletal Effects of Serotonin (5-Hydroxytryptamine) Transporter Inhibition: Evidence from in Vitro and Animal-Based Studies. J. Musculoskelet. Neuronal Interact. 2008, 8, 121. [Google Scholar]
- Wu, X.; Al-Abedalla, K.; Rastikerdar, E.; Abi Nader, S.; Daniel, N.G.; Nicolau, B.; Tamimi, F. Selective Serotonin Reuptake Inhibitors and the Risk of Osseointegrated Implant Failure: A Cohort Study. J. Dent. Res. 2014, 93, 1054–1061. [Google Scholar] [CrossRef]
- Yıldırım, G.; Eralp, F.E. Effect of Antidepressants and Its Orthodontic Implications. Essent. Dent. 2021, 1, 12–16. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. Is the Intake of Selective Serotonin Reuptake Inhibitors Associated with an Increased Risk of Dental Implant Failure? Int. J. Oral Maxillofac. Surg. 2017, 46, 782–788. [Google Scholar] [CrossRef]
- Altay, M.A.; Sindel, A.; Özalp, Ö.; Yildirimyan, N.; Kader, D.; Bilge, U.; Baur, D.A. Does the Intake of Selective Serotonin Reuptake Inhibitors Negatively Affect Dental Implant Osseointegration? A Retrospective Study. J. Oral Implantol. 2018, 44, 260–265. [Google Scholar] [CrossRef]
- Vila, G. The Effects of Different Types of Antidepressants on Dental Implant Failure: A Retrospective and In-Vitro. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2018. [Google Scholar]
- Branco-de-Almeida, L.S.; Franco, G.C.; Castro, M.L.; Dos Santos, J.G.; Anbinder, A.L.; Cortelli, S.C.; Kajiya, M.; Kawai, T.; Rosalen, P.L. Fluoxetine Inhibits Inflammatory Response and Bone Loss in a Rat Model of Ligature-Induced Periodontitis. J. Periodontol. 2012, 83, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.; Sharma, R.K.; Tewari, S.; Khurana, H.; Narula, S.C. Effect of Fluoxetine on Periodontal Status in Patients With Depression: A Cross-Sectional Observational Study. J. Periodontol. 2015, 86, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Spinler, S.A. Hyponatremia Associated with Selective Serotonin-Reuptake Inhibitors in Older Adults. Ann. Pharmacother. 2006, 40, 1618–1622. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Mechanic, O.J. Syndrome of Inappropriate Antidiuretic Hormone Secretion. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023; pp. 273–279. ISBN 9780128041130. [Google Scholar]
- Cuzzo, B.; Padala, S.A.; Lappin, S.L. Physiology, Vasopressin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Tomar, L.; Patra, P.; Nigam, A. A Study to Understand the Pattern of Hyponatremia in Patients Using Selective Serotonin Reuptake Inhibitors and Serotonin Dopamine Antagonists. Ind. Psychiatry J. 2021, 30, 113. [Google Scholar] [CrossRef] [PubMed]
- Schorah, C.J.; Sobala, G.M.; Sanderson, M.; Collis, N.; Primrose, J.N. Gastric Juice Ascorbic Acid: Effects of Disease and Implications for Gastric Carcinogenesis. Am. J. Clin. Nutr. 1991, 53, 287S–293S. [Google Scholar] [CrossRef]
- Dewhirst, R.A.; Fry, S.C. The Oxidation of Dehydroascorbic Acid and 2,3-Diketogulonate by Distinct Reactive Oxygen Species. Biochem. J. 2018, 475, 3451–3470. [Google Scholar] [CrossRef]
- Henry, E.B.; Carswell, A.; Wirz, A.; Fyffe, V.; McColl, K.E.L. Proton Pump Inhibitors Reduce the Bioavailability of Dietary Vitamin C. Aliment. Pharmacol. Ther. 2005, 22, 539–545. [Google Scholar] [CrossRef]
- Maxfield, L.; Crane, J.S. Vitamin C Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ems, T.; Lucia, K.S.; Huecker, M.R. Biochemistry, Iron Absorption. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Chen, H.Q.; Gong, J.Y.; Xing, K.; Liu, M.Z.; Ren, H.; Luo, J.Q. Pharmacomicrobiomics: Exploiting the Drug-Microbiota Interactions in Antihypertensive Treatment. Front. Med. 2022, 8, 742394. [Google Scholar] [CrossRef]
- Morgan, T.O.; Anderson, A.I.E.; MacInnis, R.J. Ace Inhibitors, Β-Blockers, Calcium Blockers, and Diuretics for the Control of Systolic Hypertension. Am. J. Hypertens. 2001, 14, 241–247. [Google Scholar] [CrossRef]
- Lalvay Armijos, D.A.; Castañeda Espin, A.O.; Cobos Carrera, D.F. Medicación Antihipertensiva y Sus Reacciones Adversas En La Cavidad Oral. Una Revisión Integrativa. Res. Soc. Dev. 2022, 11, e202111032624. [Google Scholar] [CrossRef]
- Streckfus, C.F.; Strahl, R.C.; Welsh, S. Anti-Hypertension Medications: An Epidemiological Factor in the Prevalence of Root Decay among Geriatric Patients Suffering from Hypertension. Clin. Prev. Dent. 1990, 12, 26–29. [Google Scholar] [PubMed]
- Sica, D.A. Interaction of Grapefruit Juice and Calcium Channel Blockers. Am. J. Hypertens. 2006, 19, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Ajeigbe, O.F.; Ademosun, A.O.; Oboh, G. Relieving the Tension in Hypertension: Food–Drug Interactions and Anti-Hypertensive Mechanisms of Food Bioactive Compounds. J. Food Biochem. 2021, 45, e13317. [Google Scholar] [CrossRef]
- Mohn, E.S.; Kern, H.J.; Saltzman, E.; Mitmesser, S.H.; McKay, D.L. Evidence of Drug–Nutrient Interactions with Chronic Use of Commonly Prescribed Medications: An Update. Pharmaceutics 2018, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Huang, D.; Jiang, J.; Li, Y.; Li, H.; Ke, Y. Captopril Alleviates Hypertension-Induced Renal Damage, Inflammation, and NF-ΚB Activation. Braz. J. Med. Biol. Res. 2018, 51, e7338. [Google Scholar] [CrossRef]
- Mozaffar, B.; Ardavani, A.; Muzafar, H.; Idris, I. The Effectiveness of Zinc Supplementation in Taste Disorder Treatment: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Nutr. Metab. 2023, 2023, 6711071. [Google Scholar] [CrossRef]
- Hernández-Camacho, J.D.; Vicente-García, C.; Parsons, D.S.; Navas-Enamorado, I. Zinc at the Crossroads of Exercise and Proteostasis. Redox Biol. 2020, 35, 101529. [Google Scholar] [CrossRef]
- Fang, M.M.; Lei, K.Y.; Kilgore, L.T. Effects of Zinc Deficiency on Dental Caries in Rats. J. Nutr. 1980, 110, 1032–1036. [Google Scholar] [CrossRef]
- Atasoy, H.B.; Ulusoy, Z.I.A. The Relationship between Zinc Deficiency and Children’s Oral Health. Pediatr. Dent. 2012, 34, 383–386. [Google Scholar]
- Kifor, I.; Moore, T.J.; Fallo, F.; Sperling, E.; Chiou, C.Y.; Menachery, A.; Williams, G.H. Potassium-Stimulated Angiotensin Release from Superfused Adrenal Capsules and Enzymatically Dispersed Cells of the Zona Glomerulosa. Endocrinology 1991, 129, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Cusick, A.S.; Thielemier, B. ACE Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Babu, N.V.; Roy, A. Comparative Analysis of the Status of Dental Caries and Selected Salivary Electrolytes in Children with Autism. Int. J. Clin. Pediatr. Dent. 2022, 15, S242–S246. [Google Scholar] [CrossRef] [PubMed]
- Ambikathanaya, U.; Hegde, U.; Tippeswamy; Ayas, M. Role of Salivary Electrolytes in Prevalence of Dental Caries among Diabetic and Non-Diabetic Adults. J. Clin. Diagn. Res. 2018, 12, ZC05–ZC08. [Google Scholar] [CrossRef]
- McKeever, R.G.; Hamilton, R.J. Calcium Channel Blockers. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022; pp. 689–695. ISBN 9780128122006. [Google Scholar]
- Basile, J. The Role of Existing and Newer Calcium Channel Blockers in the Treatment of Hypertension. J. Clin. Hypertens. 2007, 6, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Karadima, V.; Kraniotou, C.; Bellos, G.; Tsangaris, G.T. Drug-Micronutrient Interactions: Food for Thought and Thought for Action. EPMA J. 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Jurge, S.; Kuffer, R.; Scully, C.; Porter, S.R. Mucosal Disease Series. Number VI. Recurrent Aphthous Stomatitis. Oral Dis. 2006, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Tonsekar, P.; Tonsekar, V. Calcium-Channel-Blocker-Influenced Gingival Enlargement: A Conundrum Demystified. Oral 2021, 1, 236–249. [Google Scholar] [CrossRef]
- Nyska, A.; Shemesh, M.; Tal, H.; Dayan, D. Gingival Hyperplasia Induced by Calcium Channel Blockers: Mode of Action. Med. Hypotheses 1994, 43, 115–118. [Google Scholar] [CrossRef]
- Sabarudin, M.A.; Taib, H.; Mohamad, W.M.W.; Sabarudin, M.A.; Taib, H.; Mohamad, W.M.W. Refining the Mechanism of Drug-Influenced Gingival Enlargement and Its Management. Cureus 2022, 14, e25009. [Google Scholar] [CrossRef]
- Albu, C.-C.; Bencze, M.-A.; Dragomirescu, A.-O.; Suciu, I.; Tănase, M.; Albu, Ş.-D.; Russu, E.-A.; Ionescu, E. Folic Acid and Its Role in Oral Health: A Narrative Review. Processes 2023, 11, 1994. [Google Scholar] [CrossRef]
- Mahjoub, S.; Ghasempour, M.; Gharage, A.; Bijani, A.; Masrourroudsari, J. Comparison of Total Antioxidant Capacity in Saliva of Children with Severe Early Childhood Caries and Caries-Free Children. Caries Res. 2014, 48, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Sica, D.A.; Carter, B.; Cushman, W.; Hamm, L. Thiazide and Loop Diuretics. J. Clin. Hypertens. 2011, 13, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.W.; Han, S.Y. Loop Diuretics in Clinical Practice. Electrolytes Blood Press. 2015, 13, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Khorasani-Zadeh, A. Thiazide Diuretics. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023; pp. 539–545. ISBN 9780123864543. [Google Scholar]
- Cunha, T.d.S.; Gomes, S.A.; Heilberg, I.P. Thiazide and Thiazide-like Diuretics in Nephrolithiasis. Braz. J. Nephrol. 2020, 43, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Arumugham, V.B.; Shahin, M.H. Therapeutic Uses of Diuretic Agents. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Curry, J.N.; Yu, A.S.L. Magnesium Handling in the Kidney. Adv. Chronic Kidney Dis. 2018, 25, 236–243. [Google Scholar] [CrossRef]
- Jo, W.; Koh, E.S.; Chung, S. Therapeutic Roles of Thiazides and Loop Diuretics in Blood Pressure Control and Renal Protection against Chronic Kidney Disease. Clin. Hypertens. 2023, 29, 14. [Google Scholar] [CrossRef]
- Hanna, R.M.; Ahdoot, R.S.; Kalantar-Zadeh, K.; Ghobry, L.; Kurtz, I. Calcium Transport in the Kidney and Disease Processes. Front. Endocrinol. 2022, 12, 762130. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Huang, J.; Lin, J.L.; Song, C.L. Pathophysiological Mechanism of Acute Bone Loss after Fracture. J. Adv. Res. 2023, 49, 63–80. [Google Scholar] [CrossRef]
- Prasanthi, B.; Kannan, N.; Patil, R. Effect of Diuretics on Salivary Flow, Composition and Oral Health Status: A Clinico-Biochemical Study. Ann. Med. Health Sci. Res. 2014, 4, 549–553. [Google Scholar] [CrossRef]
- Griebeler, M.L.; Kearns, A.E.; Ryu, E.; Thapa, P.; Hathcock, M.A.; Joseph Melton, L.; Wermers, R.A. Thiazide-Associated Hypercalcemia: Incidence and Association with Primary Hyperparathyroidism over Two Decades. J. Clin. Endocrinol. Metab. 2016, 101, 1166–1173. [Google Scholar] [CrossRef]
- Aung, K.; Htay, T. Thiazide Diuretics and the Risk of Hip Fracture. Cochrane Database Syst. Rev. 2011, 5, CD005185. [Google Scholar] [CrossRef] [PubMed]
- Rieck, J.; Halkin, H.; Almog, S.; Seligman, H.; Lubetsky, A.; Olchovsky, D.; Ezra, D. Urinary Loss of Thiamine Is Increased by Low Doses of Furosemide in Healthy Volunteers. J. Lab. Clin. Med. 1999, 134, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Katta, N.; Balla, S.; Alpert, M.A. Does Long-Term Furosemide Therapy Cause Thiamine Deficiency in Patients with Heart Failure? A Focused Review. Am. J. Med. 2016, 753.e7–753.e11. [Google Scholar] [CrossRef]
- Bicer, I.; Dizdar, O.S.; Dondurmacı, E.; Ozcetin, M.; Yılmaz, R.; Gundogan, K.; Gunal, A.I. Furosemide-Related Thiamine Deficiency in Hospitalized Hypervolemic Patients with Renal Failure and Heart Failure. Nefrol. Engl. Ed. 2023, 43, 111–119. [Google Scholar] [CrossRef]
- Ochs, H.R.; Greenblatt, D.J.; Bodem, G.; Smith, T.W. Spironolactone. Am. Heart J. 1978, 96, 389–400. [Google Scholar] [CrossRef]
- Herman, L.L.; Bashir, K. Hydrochlorothiazide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023; ISBN 9780080552323. [Google Scholar]
- Sidhom, M.B.; Velez, M.R. Monitoring the Effect of Triamterene and Hydrochlorothiazide on Dihydrofolate Reductase Activity Using a New Spectrophotometric Method. J. Pharm. Biomed. Anal. 1989, 7, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.A.; Bell, S.J.; Guan, Y.; Yu, Y. Folic Acid Supplementation and Pregnancy: More than just Neural Tube Defect Prevention. Rev. Obstet. Gynecol. 2011, 4, 52. [Google Scholar] [CrossRef]
- Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Liberman, A.C.; Budziñski, M.L.; Sokn, C.; Gobbini, R.P.; Steininger, A.; Arzt, E. Regulatory and Mechanistic Actions of Glucocorticoids on T and Inflammatory Cells. Front. Endocrinol. 2018, 9, 344260. [Google Scholar] [CrossRef]
- Noetzlin, S.; Breville, G.; Seebach, J.D.; Gastaldi, G. Short-Term Glucocorticoid-Related Side Effects and Adverse Reactions: A Narrative Review and Practical Approach. Swiss Med. Wkly. 2022, 152, w30088. [Google Scholar] [CrossRef]
- Van Staa, T.P.; Leufkens, H.G.M.; Cooper, C. The Epidemiology of Corticosteroid-Induced Osteoporosis: A Meta-Analysis. Osteoporos. Int. 2002, 13, 777–787. [Google Scholar] [CrossRef]
- Briot, K.; Roux, C. Glucocorticoid-Induced Osteoporosis. RMD Open 2015, 1, e000014. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.S.; Zhou, H.; Seibel, M.J.; Cooper, M.S. Glucocorticoids and Bone: Consequences of Endogenous and Exogenous Excess and Replacement Therapy. Endocr. Rev. 2018, 39, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, A.; Balawender, K. Structural and Metabolic Changes in Bone. Animals 2022, 12, 1946. [Google Scholar] [CrossRef] [PubMed]
- Sunyecz, J.A. The Use of Calcium and Vitamin D in the Management of Osteoporosis. Ther. Clin. Risk Manag. 2008, 4, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Jan, B.M.; Khayat, M.A.; Bushnag, A.I.; Zahid, A.I.; Alkarim, A.S.; Alshehri, M.T.; Almasoudi, F.M.; Zahran, M.; Almazrooa, S.A.; Mawardi, H.H. The Association Between Long-Term Corticosteroids Use and Dental Caries: A Systematic Review. Cureus 2023, 15, e44600. [Google Scholar] [CrossRef] [PubMed]
- Alcázar Navarrete, B.; Gómez-Moreno, G.; Aguilar-Salvatierra, A.; Guardia, J.; Romero Palacios, P.J. Xerostomia Relates to the Degree of Asthma Control. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2015, 44, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.C.; Jamelli, S.; Costa, L.; Baracho Filho, C.; Medeiros, D.; Rizzo, J.A.; Sarinho, E. Assessing Caries, Dental Plaque and Salivary Flow in Asthmatic Adolescents Using Inhaled Corticosteroids. Allergol. Immunopathol. 2012, 40, 220–224. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.-M.; Jaramillo, J.; Sarmiento-Ordoñez, J.; Cuenca-León, K. Drugs Prescribed for Asthma and Their Adverse Effects on Dental Health. Dent. J. 2023, 11, 113. [Google Scholar] [CrossRef]
- Maupomé, G.; Shulman, J.D.; Medina-Solis, C.E.; Ladeinde, O. Is There a Relationship between Asthma and Dental Caries?: A Critical Review of the Literature. J. Am. Dent. Assoc. 1939 2010, 141, 1061–1074. [Google Scholar] [CrossRef]
- Shah, P.D.; Badner, V.M.; Rastogi, D.; Moss, K.L. Association between Asthma and Dental Caries in US (United States) Adult Population. J. Asthma Off. J. Assoc. Care Asthma 2021, 58, 1329–1336. [Google Scholar] [CrossRef]
- Gani, F.; Caminati, M.; Bellavia, F.; Baroso, A.; Faccioni, P.; Pancera, P.; Batani, V.; Senna, G. Oral Health in Asthmatic Patients: A Review: Asthma and Its Therapy May Impact on Oral Health. Clin. Mol. Allergy CMA 2020, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Shashikiran, N.D.; Reddy, V.V.S.; Raju, P.K. Effect of Antiasthmatic Medication on Dental Disease: Dental Caries and Periodontal Disease. J. Indian Soc. Pedod. Prev. Dent. 2007, 25, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Levings, J.L.; Gunn, J.P. The Imbalance of Sodium and Potassium Intake: Implications for Dietetic Practice. J. Acad. Nutr. Diet. 2014, 114, 838–839. [Google Scholar] [CrossRef] [PubMed]
- Felípez, L.; Sentongo, T.A. Drug-Induced Nutrient Deficiencies. Pediatr. Clin. N. Am. 2009, 56, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Maphosa, S.; Moleleki, L.N.; Motaung, T.E. Bacterial Secretion System Functions: Evidence of Interactions and Downstream Implications. Microbiology 2023, 169, 001326. [Google Scholar] [CrossRef]
- Paszynska, E.; Pawinska, M.; Enax, J.; Meyer, F.; Schulze Zur Wiesche, E.; May, T.W.; Amaechi, B.T.; Limeback, H.; Hernik, A.; Otulakowska-Skrzynska, J.; et al. Caries-preventing effect of a hydroxyapatite-toothpaste in adults: A 18-month double-blinded randomized clinical trial. Front. Public Health 2023, 11, 1199728. [Google Scholar] [CrossRef] [PubMed]
- Grocholewicz, K.; Matkowska-Cichocka, G.; Makowiecki, P.; Droździk, A.; Ey-Chmielewska, H.; Dziewulska, A.; Tomasik, M.; Trybek, G.; Janiszewska-Olszowska, J. Effect of nano-hydroxyapatite and ozone on approximal initial caries: A randomized clinical trial. Sci. Rep. 2020, 10, 11192. [Google Scholar] [CrossRef]
- Pérez-Nicolás, C.; Pecci-Lloret, M.P.; Guerrero-Gironés, J. Use and efficacy of mouthwashes in elderly patients: A systematic review of randomized clinical trials. Ann. Anat. 2023, 246, 152026. [Google Scholar] [CrossRef]
- Deutsch, A.; Jay, E. Optimising oral health in frail older people. Aust. Prescr. 2021, 44, 153–160. [Google Scholar] [CrossRef]
- Tahmasbi, S.; Mousavi, S.; Behroozibakhsh, M.; Badiee, M. Prevention of white spot lesions using three remineralizing agents: An in vitro comparative study. J. Dent. Res. Dent. Clin. Dent. Prospect. 2019, 13, 36–42. [Google Scholar] [CrossRef]
- Shih, Y.L.; Lin, Y.; Chen, J.Y. The Association between High-Sensitivity C-Reactive Protein and Metabolic Syndrome in an Elderly Population Aged 50 and Older in a Community Receiving Primary Health Care in Taiwan. Int. J. Environ. Res. Public Health 2022, 19, 13111. [Google Scholar] [CrossRef] [PubMed]
| Common Prescription Drugs | Nutrient | |
|---|---|---|
| Atorvastatin | ||
| Fluvastatin | ||
| Lovastatin | Coenzyme Q10 | |
| Statins | Pitavastatin | Vitamin D |
| [62,63,64,65,66,67,68,69,70,71] | Pravastatin | |
| Rosuvastatin | ||
| Simvastatin | ||
| Dexlansoprazole | Vitamin B12 | |
| Esomeprazole | Vitamin C | |
| Lansoprazole | Calcium | |
| PPIs * | Omeprazole | Iron |
| [72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91] | Pantoprazole | Magnesium |
| Rabeprazole | ||
| Oral Hypoglycaemic Agents [92,93,94,95,96,97,98,99,100] | Metformin | Vitamin B12 |
| Vitamin D | ||
| SSRIs ** [101,102,103,104,105,106,107,108,109,110,111] | Calcium | |
| Sodium | ||
| ACE Inhibitors *** | Captopril | Zinc |
| [112,113,114,115,116,117] | Captopril/enalapril | Potassium |
| CCB **** | Amlodipine | Vitamin B9 |
| [118,119,120,121,122,123] | Nifedipine | |
| Vitamin B1 | ||
| Calcium | ||
| Loop Diuretics | Furosemide | Magnesium |
| [124,125,126,127,128,129,130,131,132,133,134] | Potassium | |
| Zinc | ||
| Vitamin B1 | ||
| Chlorthalidone | Calcium | |
| Thiazide Diuretics | Hydrochlorothiazide | Magnesium |
| [124,125,126,127,128,129,135,136,137] | Indapamide | Potassium |
| Zinc | ||
| Thiazide Diuretics | Hydrochlorothiazide | Vitamin B9 |
| [138,139,140,141,142] | ||
| Potassium Sparing Diuretics | Triamterene | Vitamin B9 |
| [138,139,140,141,142] | ||
| Corticosteroids | Vitamin D | |
| [143,144,145,146,147,148,149,150] | Calcium | |
| Drug Class | Frequency of Dental Caries |
|---|---|
| Antidepressants | High |
| Antipsychotics | Moderate |
| Antihypertensives | Low |
| Analgesics/opioids | High |
| Antihistamines | Moderate |
| Antacids | Low |
| Immunosuppressants | Moderate |
| Anti-diabetic Medications | Moderate |
| Antibiotics | Low |
| Corticosteroids | High |
| Active Agents | Oral Care Products |
|---|---|
| Calcium-phosphate | Non-fluoride toothpaste and synthetic hydroxyapatite products |
| Fluoride | Fluoride toothpaste |
| Saliva Substitutes | Artificial saliva products |
| Sugar-free Chewing Gum | Xylitol-containing gum or lozenges |
| Alcohol-free Mouthwashes | Mild, alcohol-free mouthwashes |
| Antimicrobial Mouthwashes | Prescription-based mouthwashes with antimicrobial agents |
| Protocol for the Long-Term Use of Chlorhexidine in the Elderly |
|---|
Concentration and Frequency:
|
Oral Health Assessment:
|
Patient Education:
|
| Protocol for the Long-Term Use of Fluoride in the Elderly |
Fluoride Toothpaste:
|
Fluoride Mouthwash:
|
Professional Application:
|
Regular Monitoring:
|
Patient Compliance:
|
| Protocol for Xerostomia Management in the Elderly |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bell, V.; Rodrigues, A.R.; Antoniadou, M.; Peponis, M.; Varzakas, T.; Fernandes, T. An Update on Drug–Nutrient Interactions and Dental Decay in Older Adults. Nutrients 2023, 15, 4900. https://doi.org/10.3390/nu15234900
Bell V, Rodrigues AR, Antoniadou M, Peponis M, Varzakas T, Fernandes T. An Update on Drug–Nutrient Interactions and Dental Decay in Older Adults. Nutrients. 2023; 15(23):4900. https://doi.org/10.3390/nu15234900
Chicago/Turabian StyleBell, Victoria, Ana Rita Rodrigues, Maria Antoniadou, Marios Peponis, Theodoros Varzakas, and Tito Fernandes. 2023. "An Update on Drug–Nutrient Interactions and Dental Decay in Older Adults" Nutrients 15, no. 23: 4900. https://doi.org/10.3390/nu15234900
APA StyleBell, V., Rodrigues, A. R., Antoniadou, M., Peponis, M., Varzakas, T., & Fernandes, T. (2023). An Update on Drug–Nutrient Interactions and Dental Decay in Older Adults. Nutrients, 15(23), 4900. https://doi.org/10.3390/nu15234900









