Theabrownin from Wuniuzao Dark Tea Regulates Hepatic Lipid Metabolism and Gut Microbiota in Mice Fed High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Theabrownin Preparation
2.2. Animal Experimental Design and Sample Collection
2.3. Detection of Serum Lipid Levels and Enzyme Activity
2.4. Liver Section Staining
2.5. RNA Extraction and Quantitative PCR Analysis
2.6. Protein Extraction and Western Blotting
2.7. High-Throughput Sequencing of Gut Microbiota
2.8. Statistical Analysis
3. Results
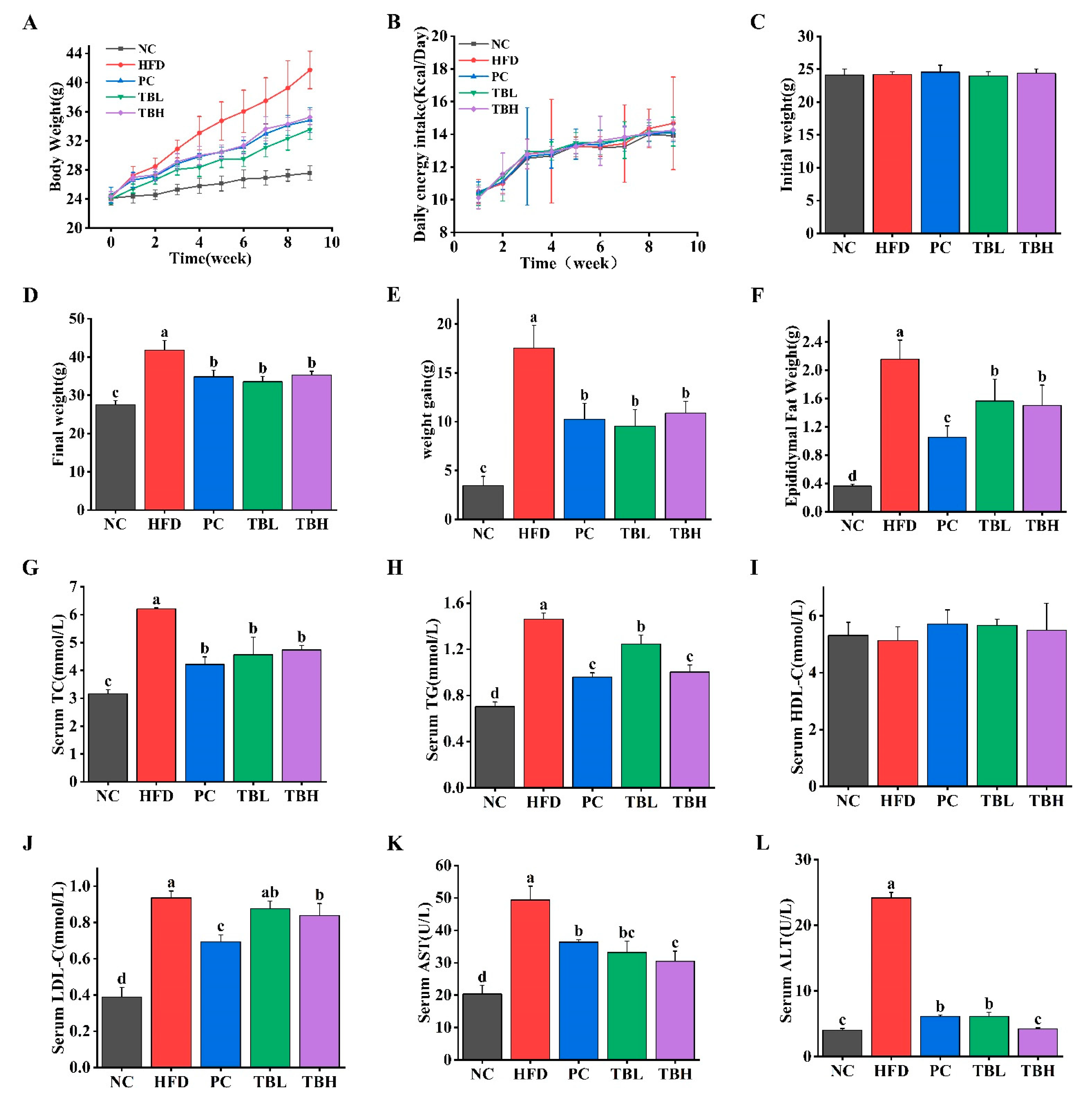
3.1. Theabrownin Reduced HFD-Induced Weight Gain and Hyperlipidemia
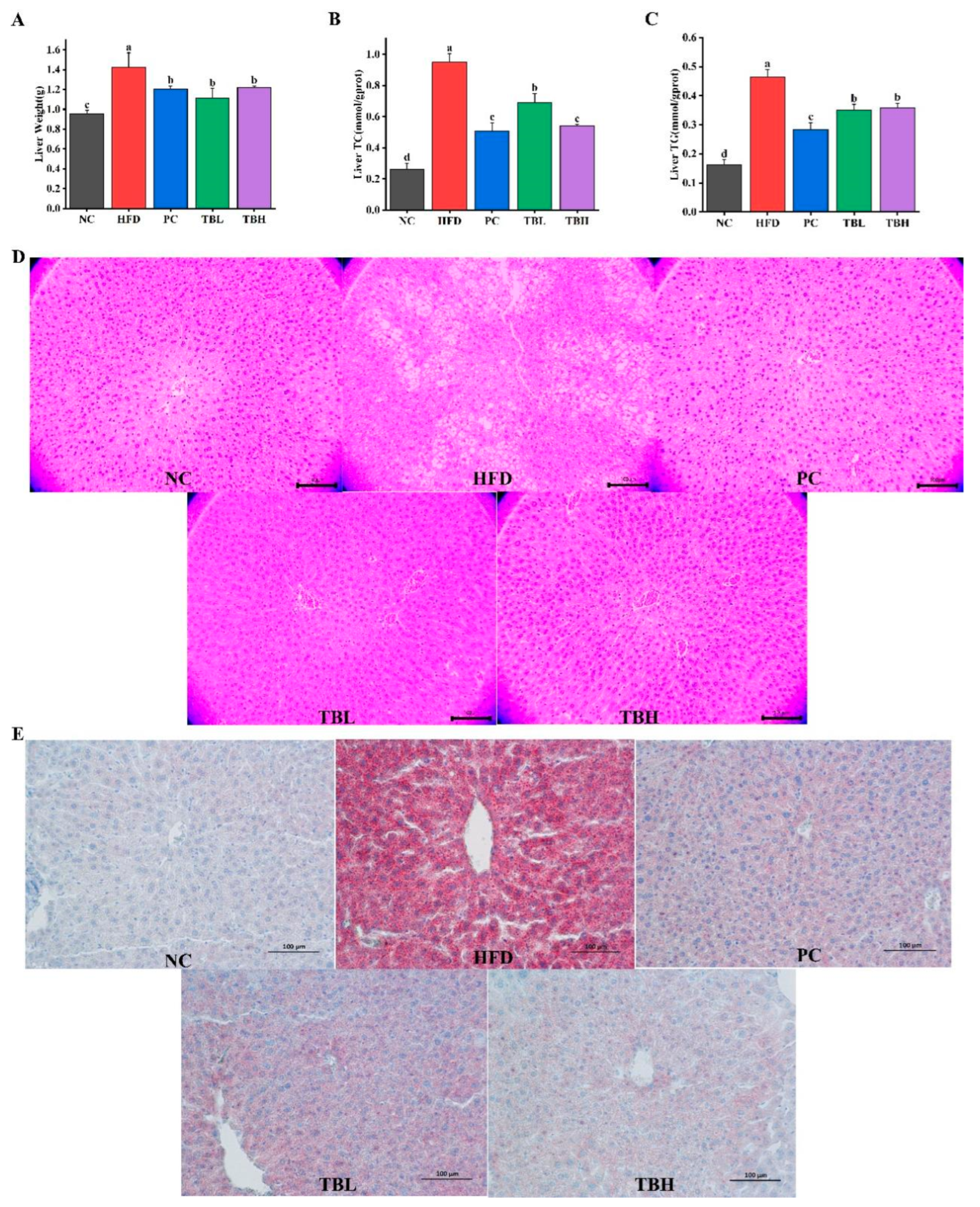
3.2. Theabrownin Reduced HFD-Induced Lipid Deposition in Liver
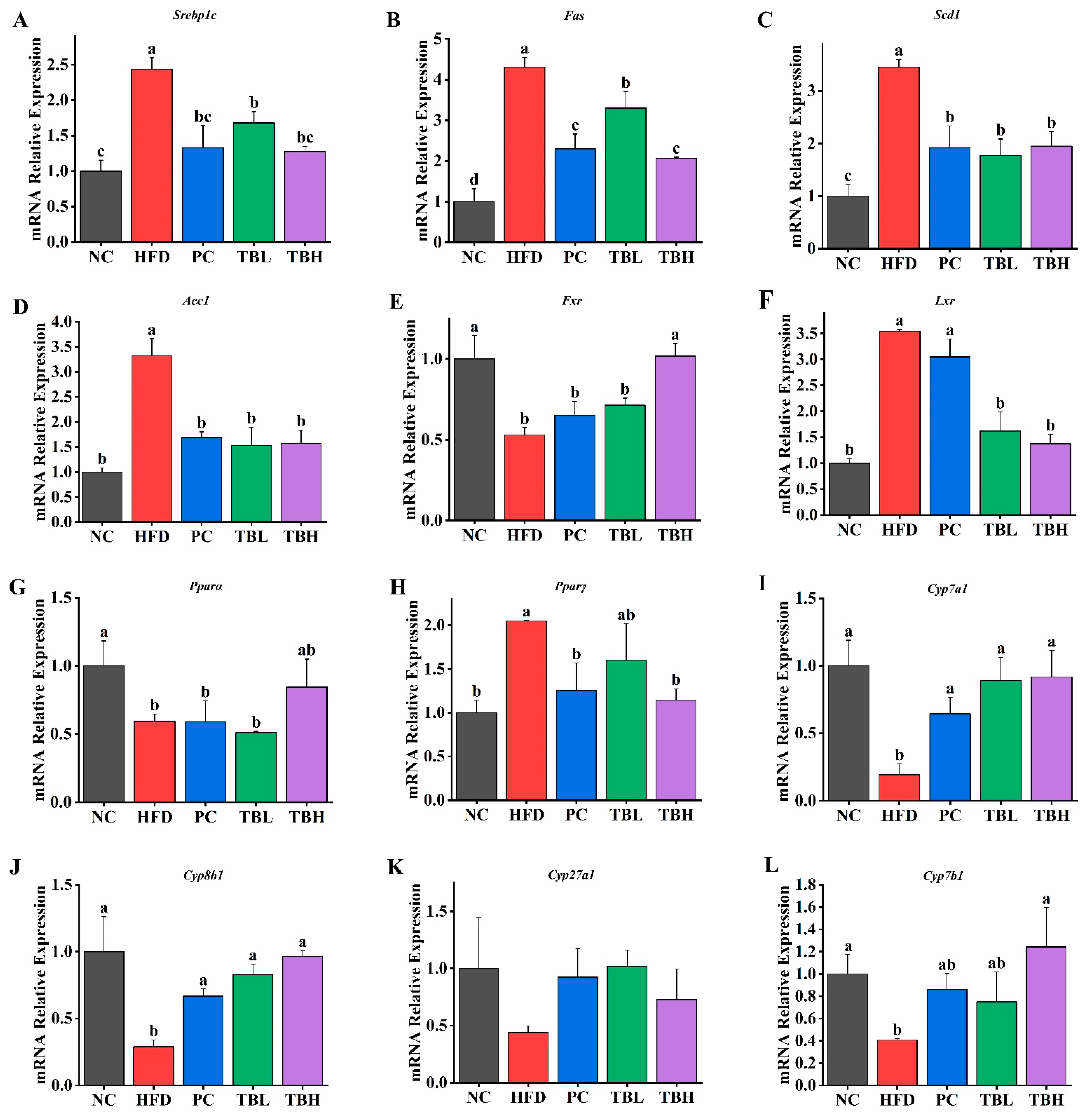
3.3. Theabrownin Regulated Relative Expression of Lipid Metabolism-Related Genes in Mice
3.4. Theabrownin-Regulated Relative Expression of Lipid Metabolism-Related Key Proteins in Mice
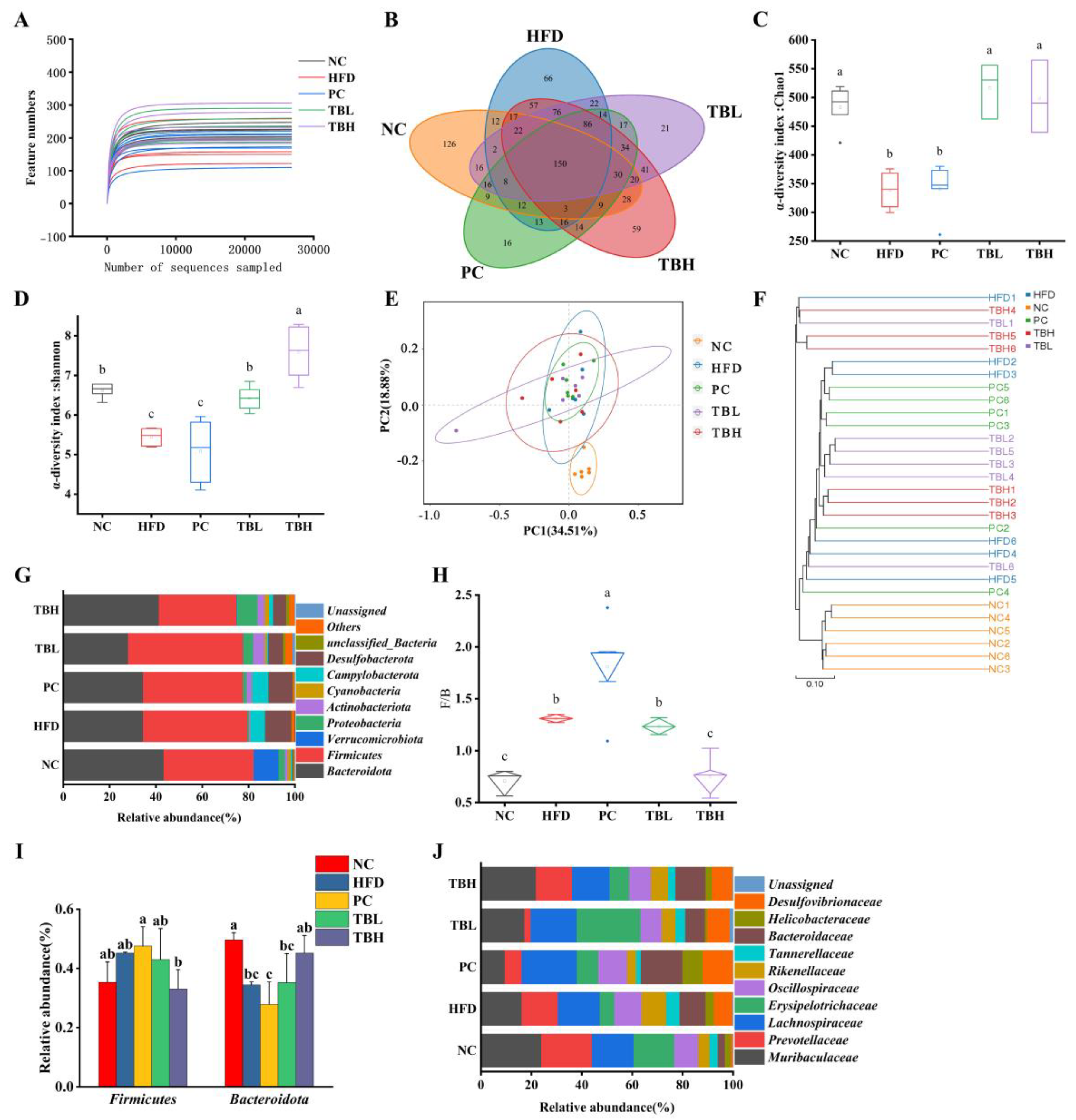
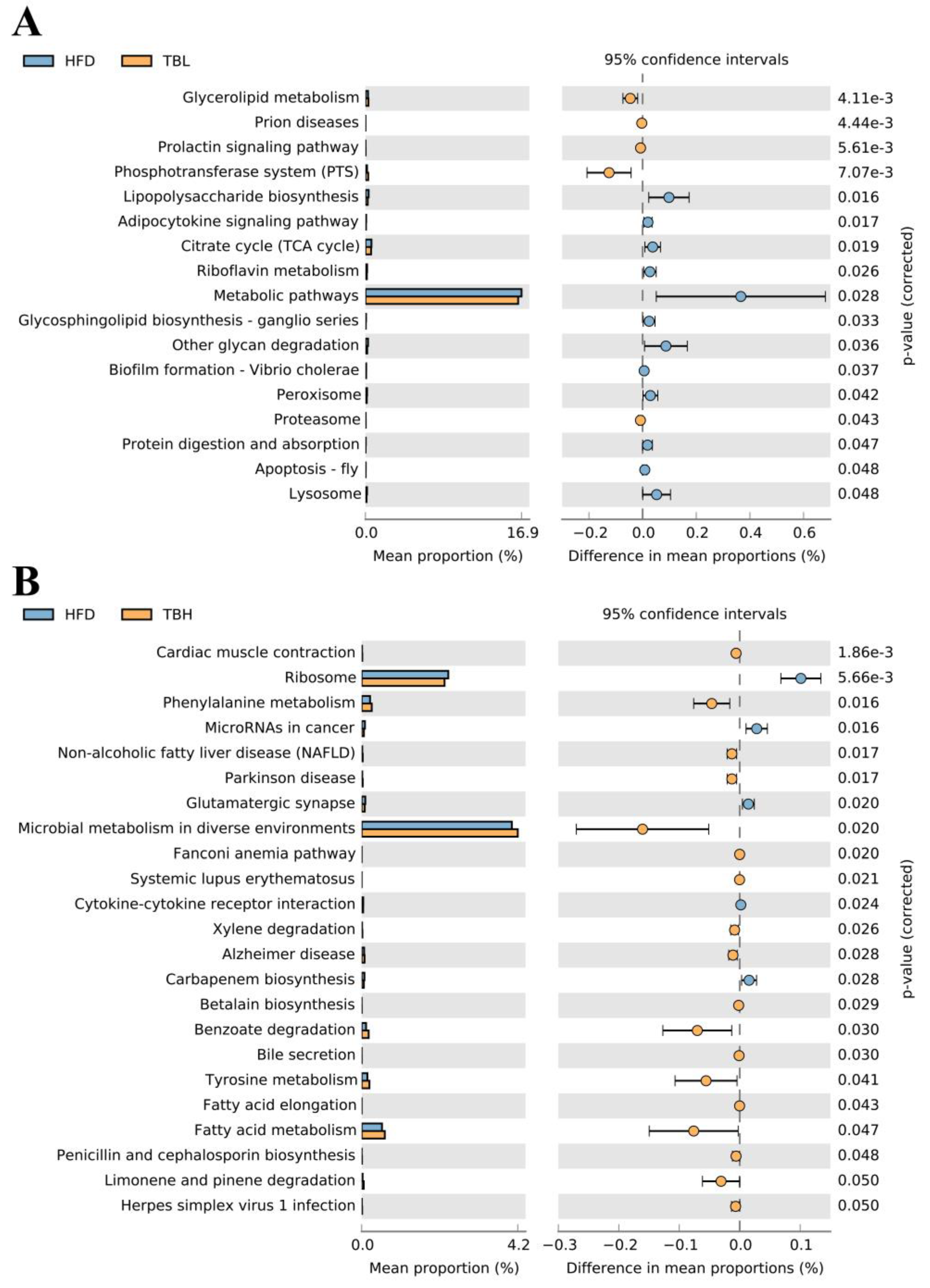
3.5. Theabrownin-Adjusted Gut Microbiota Composition in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Upadhyay, J.; Farr, O.; Perakakis, N.; Ghaly, W.; Mantzoros, C. Obesity as a disease. Med. Clin. N. Am. 2018, 102, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Rask, L.J.; Dima, L.; Correll, C.U.; Manu, P. The pharmacological management of metabolic syndrome. Expert Rev. Clin. Pharmacol. 2018, 11, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.N.; Liu, Q.; Feng, Y.B.; Li, S.; Wei, X.L.; Atanasov, A.G.; Corke, H.; et al. Health functions and related molecular mechanisms of tea components: An update review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef]
- Zhu, M.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.; Xu, W.; Li, J.; Lin, H.; Zhang, Z.; Xiao, J.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.L.; Weng, M.S.; Chiang, C.T.; Tsai, Y.J.; Lin-Shiau, S.Y.; Lin, J.K. Comparative studies on the hypolipidemic and growth suppressive effects of oolong, black, pu-erh, and green tea leaves in rats. J. Agric. Food Chem. 2005, 53, 480–489. [Google Scholar] [CrossRef]
- Zheng, W.J.; Wan, X.C.; Bao, G.H. Brick dark tea: A review of the manufacture, chemical constituents and bioconversion of the major chemical components during fermentation. Phytochem. Rev. 2015, 14, 499–523. [Google Scholar] [CrossRef]
- Li, L.; Shi, M.; Salerno, S.; Tang, M.; Guo, F.; Liu, J.; Feng, Y.; Fu, M.; Huang, Q.; Ma, L.; et al. Microbial and metabolomic remodeling by a formula of Sichuan dark tea improves hyperlipidemia in apoE-deficient mice. PLoS ONE 2019, 14, e0219010. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, B.; Zheng, W.; Chen, X.; Zhang, J.; Yan, R.; Zhang, T.; Yu, L.; Dong, Y.; Ma, B. Liupao tea extract alleviates diabetes mellitus and modulates gut microbiota in rats induced by streptozotocin and high-fat, high-sugar diet. Biomed. Pharmacother. 2019, 118, 109262. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef]
- Wang, Q.; Gong, J.; Chisti, Y.; Sirisansaneeyakul, S. Production of theabrownins using a crude fungal enzyme concentrate. J. Biotechnol. 2016, 231, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, Y.; Sarkar, D. Association of adipose tissue and adipokines with development of obesity-induced liver cancer. Int. J. Mol. Sci. 2021, 22, 2163. [Google Scholar] [CrossRef] [PubMed]
- Balsan, G.; Pellanda, L.C.; Sausen, G.; Galarraga, T.; Zaffari, D.; Pontin, B.; Portal, V.L. Effect of yerba mate and green tea on paraoxonase and leptin levels in patients affected by overweight or obesity and dyslipidemia: A randomized clinical trial. Nutr. J. 2019, 18, 5. [Google Scholar] [CrossRef]
- Sirichaiwetchakoon, K.; Lowe, G.M.; Kupittayanant, S.; Churproong, S.; Eumkeb, G. Pluchea indica (L.) Less. tea ameliorates hyperglycemia, dyslipidemia, and obesity in high fat diet-fed mice. J. Evid.-Based Complement. Altern. Med. 2020, 2020, 8746137. [Google Scholar] [CrossRef]
- Mou, H.; Wu, S.; Zhao, G.; Wang, J. Changes of Th17/Treg ratio in the transition of chronic hepatitis B to liver cirrhosis and correlations with liver function and inflammation. Exp. Ther. Med. 2019, 17, 2963–2968. [Google Scholar] [CrossRef]
- Ren, D.; Wang, Q.; Yang, Y.; Hu, Y.; Song, Y.; He, Y.; Liu, S.; Wu, L. Hypolipidemic effects of fucoidan fractions from Saccharina sculpera (Laminariales, Phaeophyceae). Int. J. Biol. Macromol. 2019, 140, 188–195. [Google Scholar] [CrossRef]
- Ferré, P.; Phan, F.; Foufelle, F. SREBP-1c and lipogenesis in the liver: An update. Biochem. J. 2021, 478, 3723–3739. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.M.; Pham, S.C.D.L.; Grishok, A. Nonautonomous regulation of neuronal migration by insulin signaling, DAF-16/FOXO, and PAK-1. Cell Rep. 2013, 4, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S. Regulation of body fat in Caenorhabditis elegans. Annu. Rev. Physiol. 2015, 77, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, Y.; Yoda, M.; Sakakibara, H.; Matsunaga, K.; Masuda, S. Pu-erh tea suppresses diet-Induced body fat accumulation in C57BL/6J mice by down-regulating SREBP-1c and related molecules. Biosci. Biotechnol. Biochem. 2013, 77, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Cao, W.; Yu, B.; Chen, H.; Gong, R.; Luorong, Q.; Luo, J.; Yao, L.; Zhang, D. Hawk tea prevents high-fat diet-induced obesity in mice by activating the AMPK/ACC/SREBP1c signaling pathways and regulating the gut microbiota. Food Funct. 2022, 13, 6056–6071. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, S.Y.; Lee, J.H. Berteroin ameliorates lipid accumulation through AMPK-mediated regulation of hepatic lipid metabolism and inhibition of adipocyte differentiation. Life Sci. 2021, 282, 119668. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Li, H. Role of farnesoid X receptor in hepatic steatosis in nonalcoholic fatty liver disease. Biomed. Pharmacother. 2020, 121, 109609. [Google Scholar] [CrossRef]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as metabolic regulators in the liver: Lessons from liver-specific PPAR-null mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, D.; Huang, F.; Zhao, A.; Kuang, J.; Ren, Z.; Chen, T.; Lei, J.; Lin, J.; Wang, X.; et al. Theabrownin and poria cocos polysaccharide improve lipid metabolism via modulation of bile acid and fatty acid metabolism. Front. Pharmacol. 2022, 13, 875549. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Zhou, F.; Ouyang, J.; Wang, Q.Y.; Li, Y.L.; Wu, J.L.; Huang, J.A.; Liu, Z.H. Combined use of epigallocatechin-3-gallate (EGCG) and caffeine in low doses exhibits marked anti-obesity synergy through regulation of gut microbiota and bile acid metabolism. Food Funct. 2021, 12, 4105–4116. [Google Scholar] [CrossRef]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; de Vos, W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wilkes, S.; Rogers, T.; Khanal, R.C.; Wu, X.; Hager, T.J.; Hager, A.; Howard, L. Dietary black raspberry anthocyanins do not alter development of obesity in mice fed an obesogenic high-fat diet. J. Agric. Food Chem. 2010, 58, 3977–3983. [Google Scholar] [CrossRef] [PubMed]
- Soto-Martin, E.C.; Warnke, I.; Farquharson, F.M.; Christodoulou, M.; Horgan, G.; Derrien, M.; Faurie, J.M.; Flint, H.J.; Duncan, S.H.; Louis, P. Vitamin biosynthesis by human gut butyrate-producing bacteria and cross-feeding in synthetic microbial communities. mBio 2020, 11, e00886-20. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Jeon, C.O. Promotion and induction of liver cancer by gut microbiome-mediated modulation of bile acids. PLoS Pathog. 2019, 15, e1007954. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]

| Gene | Primer Sequences (5′ to 3′) | Size (bp) | Accession Number |
|---|---|---|---|
| Gapdh | GAAGGTCGGTGTGAACGGATTTG | 127 | NM_001289726.2 |
| CATGTAGACCATGTAGTTGAGGTCA | |||
| Scd1 | GCAAGCTCTACACCTGCCTCTTC | 110 | NM_009127.4 |
| CAGCCGTGCCTTGTAAGTTCTG | |||
| Cd36 | GGAGCCATCTTTGAGCCTTCA | 217 | NM_001159556.2 |
| GAACCAAACTGAGGAATGGATCT | |||
| Acc1 | CGTGCAATCCGATTTGTTGTCATG | 86 | NM_133360.3 |
| GGAACATAGTGGTCTGCCATCT | |||
| Srebp1c | CCCGGCTATTCCGTGAACAT | 111 | XM_006532716.4 |
| GCAGATATCCAAGGGCATCTGA | |||
| Fas | GCTTTGCTGCCGTGTCCTTCTA | 102 | NM_007988.3 |
| CTGTCTTGGCACGCAGCAGT | |||
| Fxr | CTGGCATCTATGAACTCAGGC | 96 | NM_001385711.1 |
| CCATTCGCGGCTTCTTTGT | |||
| Lxr | ACAGAGCTTCGTCCACAAAAG | 165 | XM_006499168.4 |
| GCGTGCTCCCTTGATGACA | |||
| Pparα | AACATCGAGTGTCGCGAATATGTGG | 99 | XM_030248424.2 |
| CCGAATAGTTCGCCGAAAGAA | |||
| Pparγ | CTCCAAGAATACCAAAGTGCGA | 150 | NM_011146.4 |
| GCCTGATGCTTTATCCCCACA | |||
| Cyp7a1 | GGGATTGCTGTGGTAGTGAGC | 100 | NM_007824.3 |
| GGTATGGAATCAACCCGTTGTC | |||
| Cyp7b1 | GGAGCCACGACCCTAGATG | 164 | NM_007825.5 |
| GCCATGCCAAGATAAGGAAGC | |||
| Cyp8b1 | CTAGGGCCTAAAGGTTCGAGT | 111 | NM_010012.3 |
| GTAGCCGAATAAGCTCAGGAAG | |||
| Cyp27a1 | CCAGGCACAGGAGAGTACG | 139 | NM_024264.5 |
| GGGCAAGTGCAGCACATAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Ye, J.; Gong, M.; Zhang, Y.; Yuan, Y.; Zhao, J. Theabrownin from Wuniuzao Dark Tea Regulates Hepatic Lipid Metabolism and Gut Microbiota in Mice Fed High-Fat Diet. Nutrients 2023, 15, 4912. https://doi.org/10.3390/nu15234912
Xu Q, Ye J, Gong M, Zhang Y, Yuan Y, Zhao J. Theabrownin from Wuniuzao Dark Tea Regulates Hepatic Lipid Metabolism and Gut Microbiota in Mice Fed High-Fat Diet. Nutrients. 2023; 15(23):4912. https://doi.org/10.3390/nu15234912
Chicago/Turabian StyleXu, Qianqian, Jiangcheng Ye, Mingxiu Gong, Yifan Zhang, Yiwei Yuan, and Jin Zhao. 2023. "Theabrownin from Wuniuzao Dark Tea Regulates Hepatic Lipid Metabolism and Gut Microbiota in Mice Fed High-Fat Diet" Nutrients 15, no. 23: 4912. https://doi.org/10.3390/nu15234912
APA StyleXu, Q., Ye, J., Gong, M., Zhang, Y., Yuan, Y., & Zhao, J. (2023). Theabrownin from Wuniuzao Dark Tea Regulates Hepatic Lipid Metabolism and Gut Microbiota in Mice Fed High-Fat Diet. Nutrients, 15(23), 4912. https://doi.org/10.3390/nu15234912







