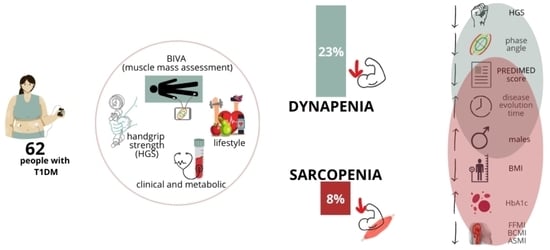
Prevalence of Sarcopenia and Dynapenia and Related Clinical Outcomes in Patients with Type 1 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Variables
2.3. Lifestyle Parameters
2.4. Anthropometric Measurements
2.5. Body Composition Parameters
2.6. Muscle Strength
2.7. Biochemical and Metabolic Variables
2.8. Sarcopenia Diagnosis
2.9. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Sarcopenia and Dynapenia
3.2. Correlation between Study Variables
3.3. Risk Factors for Sarcopenia and Dynapenia in T1DM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holt, R.I.G.; DeVries, J.H.; Hess-Fischl, A.; Hirsch, I.B.; Kirkman, M.S.; Klupa, T.; Ludwig, B.; Nørgaard, K.; Pettus, J.; Renard, E.; et al. The Management of Type 1 Diabetes in Adults. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2021, 44, 2589–2625. [Google Scholar] [CrossRef]
- Mobasseri, M.; Shirmohammadi, M.; Amiri, T.; Vahed, N.; Hosseini Fard, H.; Ghojazadeh, M. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot. Perspect. 2020, 10, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Travis, C.; Srivastava, P.S.; Hawke, T.J.; Kalaitzoglou, E. Diabetic Bone Disease and Diabetic Myopathy: Manifestations of the Impaired Muscle-Bone Unit in Type 1 Diabetes. J. Diabetes Res. 2022, 2022, 2650342. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Gunendi, Z.; Meray, J.; Yetkin, İ. The evaluation of muscle strength and architecture in type 1 diabetes mellitus: A cross-sectional study. BMC Endocr. Disord. 2022, 22, 153. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Gao, Q.; Hu, K.; Wu, M.; Wang, Z.; Chen, F.; Mei, F.; Zhao, L.; Ma, B. Prevalence and Risk Factors of Sarcopenia in Patients With Diabetes: A Meta-analysis. J. Clin. Endocrinol. Metab. 2022, 107, 1470–1483. [Google Scholar] [CrossRef] [PubMed]
- Monaco, C.M.F.; Gingrich, M.A.; Hawke, T.J. Considering Type 1 Diabetes as a Form of Accelerated Muscle Aging. Exerc. Sport Sci. Rev. 2019, 47, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Santilli, V. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Maratova, K.; Soucek, O.; Matyskova, J.; Hlavka, Z.; Petruzelkova, L.; Obermannova, B.; Pruhova, S.; Kolouskova, S.; Sumnik, Z. Muscle functions and bone strength are impaired in adolescents with type 1 diabetes. Bone 2018, 106, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Fu, J.; Mu, Z.; Duan, X.; Chan, P.; Xiu, S. Association between body fat and sarcopenia in older adults with type 2 diabetes mellitus: A cross-sectional study. Front. Endocrinol. 2023, 14, 1094075. [Google Scholar] [CrossRef]
- Pacifico, J.; Geerlings, M.A.J.; Reijnierse, E.M.; Phassouliotis, C.; Lim, W.K.; Maier, A.B. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta-analysis. Exp. Gerontol. 2020, 131, 110801. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Kuroda, A.; Ishizu, M.; Ohishi, M.; Takashi, Y.; Otsuka, Y.; Taniguchi, S.; Tamaki, M.; Kurahashi, K.; Yoshida, S.; et al. Association of accumulated advanced glycation end-products with a high prevalence of sarcopenia and dynapenia in patients with type 2 diabetes. J. Diabetes Investig. 2019, 10, 1332–1340. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef]
- Vergara Ruiz, J.C.; Triviño, M.P.M.; Alcalá, I.C.; Sánchez, S. Sarcopenia y dinapenia en pacientes con diabetes mellitus tipo 2 en un área rural de Castilla-La Mancha. Rev. Clínica Med. Fam. 2017, 10, 86–95. [Google Scholar]
- Hiromine, Y.; Noso, S.; Rakugi, H.; Sugimoto, K.; Takata, Y.; Katsuya, T.; Fukuda, M.; Akasaka, H.; Osawa, H.; Tabara, Y.; et al. Poor glycemic control rather than types of diabetes is a risk factor for sarcopenia in diabetes mellitus: The MUSCLES-DM study. J. Diabetes Investig. 2022, 13, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Pollakova, D.; Tubili, C.; Di Folco, U.; De Giuseppe, R.; Battino, M.; Giampieri, F. Muscular involvement in long-term type 1 diabetes: Does it represent an underestimated complication? Nutrition 2023, 112, 112060. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Piccoli, A.; Rossi, B.; Pillon, L.; Bucciante, G. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994, 46, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef]
- Kyle, U.G.; Genton, L.; Karsegard, L.; Slosman, D.O.; Pichard, C. Single Prediction Equation for Bioelectrical Impedance Analysis in Adults Aged 20–94 Years. Nutrition 2001, 17, 248–253. [Google Scholar] [CrossRef]
- Sánchez Torralvo, F.J.; Porras, N.; Abuín Fernández, J.; García Torres, F.; Tapia, M.J.; Lima, F.; Soriguer, F.; Gonzalo Marín, M.; Rojo Martínez, G.; Olveira, G. Normative reference values for hand grip dynamometry in Spain. Association with lean mass. Nutr. Hosp. 2018, 35, 98–103. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Jin, C.; Yang, Y.; Wang, J.; Wang, J.; Zeng, H.; Chen, Y.; Zhou, J.; Wang, Y. Association Between Autoimmune Diseases and Sarcopenia: A Two-Sample Mendelian Randomization Study. CLEP 2023, 15, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Kuroda, A.; Araki, M.; Suzuki, R.; Taniguchi, S.; Tamaki, M.; Akehi, Y.; Matsuhisa, M. Advanced glycation end-products are a risk for muscle weakness in Japanese patients with type 1 diabetes. J. Diabetes Investig. 2017, 8, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Kuroda, A.; Matsuhisa, M. Clinical impact of sarcopenia and dynapenia on diabetes. Diabetol. Int. 2019, 10, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Dial, A.G.; Monaco, C.M.F.; Grafham, G.K.; Patel, T.P.; Tarnopolsky, M.A.; Hawke, T.J. Impaired Function and Altered Morphology in the Skeletal Muscles of Adult Men and Women With Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, 2405–2422. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Miki, A.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Osaka, T.; Hamaguchi, M.; Yamazaki, M.; Fukui, M. Shortage of energy intake rather than protein intake is associated with sarcopenia in elderly patients with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort. J. Diabetes 2019, 11, 477–483. [Google Scholar] [CrossRef]
- Antoniotti, V.; Spadaccini, D.; Ricotti, R.; Carrera, D.; Savastio, S.; Goncalves Correia, F.P.; Caputo, M.; Pozzi, E.; Bellone, S.; Rabbone, I.; et al. Adherence to the Mediterranean Diet Is Associated with Better Metabolic Features in Youths with Type 1 Diabetes. Nutrients 2022, 14, 596. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Di Somma, C.; Tramontano, G.; De Luca, V.; Illario, M.; Colao, A.; Savastano, S. Association between Mediterranean diet and hand grip strength in older adult women. Clin. Nutr. 2019, 38, 721–729. [Google Scholar] [CrossRef]
- Fougère, B.; Mazzuco, S.; Spagnolo, P.; Guyonnet, S.; Vellas, B.; Cesari, M.; Gallucci, M. Association between the Mediterranean-style dietary pattern score and physical performance: Results from TRELONG study. J. Nutr. Health Aging 2016, 20, 415–419. [Google Scholar] [CrossRef]
- Isanejad, M.; Sirola, J.; Mursu, J.; Rikkonen, T.; Kröger, H.; Tuppurainen, M.; Erkkilä, A.T. Association of the Baltic Sea and Mediterranean diets with indices of sarcopenia in elderly women, OSPTRE-FPS study. Eur. J. Nutr. 2018, 57, 1435–1448. [Google Scholar] [CrossRef]
- Mendes, J.; Afonso, C.; Borges, N.; Santos, A.; Moreira, P.; Padrão, P.; Negrão, R.; Amaral, T.F. Adherence to a Mediterranean Dietary Pattern and Functional Parameters: A Cross-Sectional Study in an Older Population. J. Nutr. Health Aging 2020, 24, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Salucci, S.; Bartoletti-Stella, A.; Bavelloni, A.; Aramini, B.; Blalock, W.L.; Fabbri, F.; Vannini, I.; Sambri, V.; Stella, F.; Faenza, I. Extra Virgin Olive Oil (EVOO), a Mediterranean Diet Component, in the Management of Muscle Mass and Function Preservation. Nutrients 2022, 14, 3567. [Google Scholar] [CrossRef] [PubMed]
- García-Esquinas, E.; Rahi, B.; Peres, K.; Colpo, M.; Dartigues, J.-F.; Bandinelli, S.; Feart, C.; Rodríguez-Artalejo, F. Consumption of fruit and vegetables and risk of frailty: A dose-response analysis of 3 prospective cohorts of community-dwelling older adults. Am. J. Clin. Nutr. 2016, 104, 132–142. [Google Scholar] [CrossRef]
- Welch, A.A.; Kelaiditi, E.; Jennings, A.; Steves, C.J.; Spector, T.D.; MacGregor, A. Dietary Magnesium Is Positively Associated With Skeletal Muscle Power and Indices of Muscle Mass and May Attenuate the Association Between Circulating C-Reactive Protein and Muscle Mass in Women. J. Bone Min. Res. 2016, 31, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Kelaiditi, E.; Jennings, A.; Steves, C.J.; Skinner, J.; Cassidy, A.; MacGregor, A.J.; Welch, A.A. Measurements of skeletal muscle mass and power are positively related to a Mediterranean dietary pattern in women. Osteoporos. Int. 2016, 27, 3251–3260. [Google Scholar] [CrossRef]
- Lauretani, F.; Semba, R.D.; Bandinelli, S.; Giacomini, V.; Corsi, A.M.; Ferrucci, L. Low Plasma Carotenoids and Skeletal Muscle Strength Decline over Six Years. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 376–383. [Google Scholar] [CrossRef]
- Cervo, M.M.C.; Scott, D.; Seibel, M.J.; Cumming, R.G.; Naganathan, V.; Blyth, F.M.; Le Couteur, D.G.; Handelsman, D.J.; Ribeiro, R.V.; Waite, L.M.; et al. Adherence to Mediterranean Diet and Its Associations with Circulating Cytokines, Musculoskeletal Health and Incident Falls in Community-Dwelling Older Men: The Concord Health and Ageing in Men Project. Clin. Nutr. 2021, 40, 5753–5763. [Google Scholar] [CrossRef]
- Dhindsa, S.; Ghanim, H.; Green, K.; Abuaysheh, S.; Batra, M.; Makdissi, A.; Chaudhuri, A.; Sandhu, S.; Dandona, P. Acute effects of insulin on skeletal muscle growth and differentiation genes in men with type 2 diabetes. Eur. J. Endocrinol. 2019, 181, K55–K59. [Google Scholar] [CrossRef]
- Abdulla, H.; Smith, K.; Atherton, P.J.; Idris, I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: A systematic review and meta-analysis. Diabetologia 2016, 59, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.H.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C.; Kyle, U.G.; Kondrup, J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: Phase angle and impedance ratio. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kimura, Y.; Ishiyama, D.; Nishio, N.; Otobe, Y.; Tanaka, T.; Ohji, S.; Koyama, S.; Sato, A.; Suzuki, M.; et al. Phase Angle is a Useful indicator for Muscle Function in Older Adults. J. Nutr. Health Aging 2019, 23, 251–255. [Google Scholar] [CrossRef]
- Więch, P.; Bazaliński, D.; Sałacińska, I.; Binkowska-Bury, M.; Korczowski, B.; Mazur, A.; Kózka, M.; Dąbrowski, M. Decreased Bioelectrical Impedance Phase Angle in Hospitalized Children and Adolescents with Newly Diagnosed Type 1 Diabetes: A Case-Control Study. JCM 2018, 7, 516. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Blunda, G.; Maneri, R.; Verga, S. Bioelectrical characteristics of type 1 and type 2 diabetic subjects with reference to body water compartments. Acta Diabetol. 1998, 35, 220–223. [Google Scholar] [CrossRef]
- Murata, Y.; Kadoya, Y.; Yamada, S.; Sanke, T. Sarcopenia in elderly patients with type 2 diabetes mellitus: Prevalence and related clinical factors. Diabetol. Int. 2018, 9, 136–142. [Google Scholar] [CrossRef]
- Fukuoka, Y.; Narita, T.; Fujita, H.; Morii, T.; Sato, T.; Sassa, M.H.; Yamada, Y. Importance of physical evaluation using skeletal muscle mass index and body fat percentage to prevent sarcopenia in elderly Japanese diabetes patients. J. Diabetes Investig. 2019, 10, 322–330. [Google Scholar] [CrossRef]
- Chen, F.; Xu, S.; Wang, Y.; Chen, F.; Cao, L.; Liu, T.; Huang, T.; Wei, Q.; Ma, G.; Zhao, Y.; et al. Risk Factors for Sarcopenia in the Elderly with Type 2 Diabetes Mellitus and the Effect of Metformin. J. Diabetes Res. 2020, 2020, 3950404. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Harashima, S.; Hosoda, K.; Arai, H.; Inagaki, N. Sex-related differences in frailty factors in older persons with type 2 diabetes: A cross-sectional study. Ther. Adv. Endocrinol. 2019, 10, 204201881983330. [Google Scholar] [CrossRef] [PubMed]
- Snow, L.M.; Thompson, L.V. Influence of Insulin and Muscle Fiber Type in Nε-(Carboxymethyl)-Lysine Accumulation in Soleus Muscle of Rats with Streptozotocin-Induced Diabetes Mellitus. Pathobiology 2009, 76, 227–234. [Google Scholar] [CrossRef]
- Haus, J.M.; Carrithers, J.A.; Trappe, S.W.; Trappe, T.A. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J. Appl. Physiol. 2007, 103, 2068–2076. [Google Scholar] [CrossRef]
- Hughes, V.A.; Frontera, W.R.; Roubenoff, R.; Evans, W.J.; Singh, M.A. Longitudinal changes in body composition in older men and women: Role of body weight change and physical activity. Am. J. Clin. Nutr. 2002, 76, 473–481. [Google Scholar] [CrossRef]
- Orlando, G.; Balducci, S.; Bazzucchi, I.; Pugliese, G.; Sacchetti, M. The impact of type 1 diabetes and diabetic polyneuropathy on muscle strength and fatigability. Acta Diabetol. 2017, 54, 543–550. [Google Scholar] [CrossRef]
- Monaco, C.M.F.; Hughes, M.C.; Ramos, S.V.; Varah, N.E.; Lamberz, C.; Rahman, F.A.; McGlory, C.; Tarnopolsky, M.A.; Krause, M.P.; Laham, R.; et al. Altered mitochondrial bioenergetics and ultrastructure in the skeletal muscle of young adults with type 1 diabetes. Diabetologia 2018, 61, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Pechmann, L.M.; Jonasson, T.H.; Canossa, V.S.; Trierweiler, H.; Kisielewicz, G.; Petterle, R.R.; Moreira, C.A.; Borba, V.Z.C. Sarcopenia in Type 2 Diabetes Mellitus: A Cross-Sectional Observational Study. Int. J. Endocrinol. 2020, 2020, 7841390. [Google Scholar] [CrossRef]
- Gupta, P.; Aravindhan, A.; Gan, A.T.L.; Man, R.E.K.; Fenwick, E.K.; Mitchell, P.; Tan, N.; Sabanayagam, C.; Wong, T.Y.; Cheng, C.-Y.; et al. Association Between the Severity of Diabetic Retinopathy and Falls in an Asian Population With Diabetes: The Singapore Epidemiology of Eye Diseases Study. JAMA Ophthalmol. 2017, 135, 1410. [Google Scholar] [CrossRef]
- Fukuda, T.; Bouchi, R.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izumiyama, H.; Hashimoto, K.; Yoshimoto, T.; Ogawa, Y. Association of diabetic retinopathy with both sarcopenia and muscle quality in patients with type 2 diabetes: A cross-sectional study. BMJ Open Diabetes Res. Care 2017, 5, e000404. [Google Scholar] [CrossRef] [PubMed]




| Total (n = 62) | No Sarcopenia (n = 57) | Sarcopenia (n = 5) | p-Value | No Dynapenia (n = 48) | Dynapenia (n = 14) | p- Value | |
|---|---|---|---|---|---|---|---|
| Clinical variables | |||||||
| Sex | 0.02 | 0.04 | |||||
| Male | 21 (33.9) | 17 (29.8) | 4 (80) | 13 (27.1) | 8 (57.1) | ||
| Female | 41 (66.1) | 40 (70.2) | 1 (20) | 35 (72.9) | 6 (42.9) | ||
| Age (years) | 38 ± 14 | 39 ± 14 | 36 ± 19 | 0.83 | 37.4 ± 13.6 | 38.5 ± 16.9 | 0.81 |
| Diabetes duration (years) | 20.5 ± 13.9 | 21.9 ± 13.5 | 14.8 ± 8.6 | 0.01 | 22.7 ± 13.9 | 13.2 ± 11.6 | 0.02 |
| Microvascular complications | |||||||
| Diabetic nephropathy | 7 (11.3) | 7 (12.3) | 0 (0) | 0.41 | 6 (12.5) | 1 (7.1) | 0.58 |
| Diabetic polyneuropathy | 13 (21) | 12 (21.1) | 1 (20) | 0.96 | 10 (20.8) | 3 (21.4) | 0.96 |
| Diabetic retinopathy | 12 (19.4) | 11 (19.3) | 1 (20) | 0.97 | 10 (20.8) | 2 (14.3) | 0.56 |
| Lifestyle variables | |||||||
| PREDIMED score | 9 ± 2 | 9 ± 2 | 7 ± 2 | 0.01 | 9 ± 2 | 7 ± 2 | 0.01 |
| Adherence to MedDiet | 36 (58.1) | 35 (61.4) | 1 (20.0) | 0.07 | 31 (64.6) | 5 (35.7) | 0.05 |
| IPAQ | 0.41 | 0.81 | |||||
| Low | 17 (29.3) | 15 (27.8) | 2 (50.0) | 13 (28.3) | 4 (33.3) | ||
| Moderate | 33 (56.9) | 32 (59.3) | 1 (25.0) | 26 (56.5) | 7 (58.3) | ||
| High | 8 (13.8) | 7 (13.0) | 1 (25.0) | 7 (15.2) | 1 (8.3) | ||
| Metabolic and biochemical variables | |||||||
| Fasting glucose (mg/dL) | 156 ± 72 | 156 ± 70 | 154 ± 99 | 0.94 | 158 ± 72 | 147 ± 72 | 0.60 |
| HbA1c (%) | 8.4 ± 1.5 | 8.2 ± 1.7 | 10.0 ± 2.4 | 0.03 | 8.2 ± 1.7 | 8.7 ± 2.2 | 0.37 |
| TID (UI) | 46 ± 21 | 47 ± 22 | 41 ± 12 | 0.36 | 46 ± 22 | 48 ± 16 | 0.77 |
| ISF | 44 ± 21 | 43 ± 20 | 38 ± 13 | 0.38 | 44 ± 21 | 40 ± 20 | 0.48 |
| TID/kg (UI/kg) | 0.66 ± 0.30 | 0.66 ± 0.28 | 0.72 ± 0.34 | 0.65 | 0.64 ± 0.30 | 0.76 ± 0.29 | 0.14 |
| Pre-albumin (mg/dL) | 18.8 ± 3.6 | 19.2 ± 3.4 | 12.6 ± 3.6 | 0.08 | 19.2 ± 3.5 | 15.9 ± 4.7 | 0.24 |
| CRP (mg/L) | 4.7 ± 6.1 | 4.6 ± 6.1 | 5.1 ± 6.7 | 0.88 | 4.6 ± 6.1 | 4.9 ± 6.4 | 0.87 |
| Muscle strength | |||||||
| HGS (kg) | 29 ± 11 | 30 ± 12 | 26 ± 8 | 0.41 | 31 ± 11 | 22 ± 10 | 0.01 |
| Total (n = 62) | No Sarcopenia (n = 57) | Sarcopenia (n = 5) | p-Value | No Dynapenia (n = 48) | Dynapenia (n = 14) | p-Value | |
|---|---|---|---|---|---|---|---|
| BIA and body composition variables | |||||||
| Rz (Ohm) | 568.6 ± 78.4 | 561.2 ± 75.6 | 650.2 ± 66.7 | 0.01 | 565.8 ± 74.5 | 577.9 ± 92.6 | 0.61 |
| Xc (Ohm) | 57.7 ± 8.1 | 57.7 ± 8.1 | 64.4 ± 4.2 | 0.05 | 58.5 ± 7.4 | 55.4 ± 9.9 | 0.21 |
| PhA (°) | 5.8 ± 0.7 | 5.8 ± 0.7 | 5.7 ± 0.3 | 0.56 | 5.9 ± 0.6 | 5.5 ± 0.7 | 0.02 |
| SPhA | 0.4 ± 1.4 | 0.5 ± 1.5 | −0.3 ± 0.5 | 0.23 | 0.6 ± 1.5 | −0.3 ± 0.8 | 0.01 |
| TBW (%) | 53.6 ± 7.9 | 53.1 ± 8.0 | 59.6 ± 1.3 | <0.001 | 52.8 ± 8.1 | 56.2 ± 6.6 | 0.16 |
| ECW (%) | 46.7 ± 3.0 | 46.6 ± 3.1 | 47.3 ± 1.8 | 0.66 | 46.2 ± 2.8 | 48.4 ± 3.3 | 0.02 |
| FFM (%) | 73.1 ± 10.4 | 72.3 ± 10.5 | 81.8 ± 1.8 | <0.001 | 72.0 ± 10.6 | 76.7 ± 9.2 | 0.14 |
| FFMI (kg/m2) | 17.7 ± 2.0 | 17.9 ± 1.9 | 15.4 ± 1.3 | 0.01 | 17.8 ± 1.8 | 17.5 ± 2.6 | 0.71 |
| FM (%) | 26.9 ± 10.4 | 27.7 ± 10.5 | 18.2 ± 1.8 | <0.001 | 28.0 ± 10.7 | 23.3 ± 9.2 | 0.14 |
| BCMI (kg/m2) | 9.3 ± 1.3 | 9.5 ± 1.3 | 8.0 ± 0.9 | 0.02 | 9.5 ± 1.4 1.2 | 5.9 ± 1.5 | 0.17 |
| BMI (kg/m2) | 24.9 ± 4.7 | 25.3 ± 4.5 | 18.8 ± 1.8 | 0.003 | 25.3 ± 4.5 | 23.3 ± 5.1 | 0.16 |
| SMI (kg/m2) | 8.6 ± 1.5 | 8.6 ± 1.0 | 7.8 ± 0.8 | 0.26 | 8.5 ± 1.4 | 8.6 ± 1.6 | 0.91 |
| ASMI (kg/m2) | 6.9 ± 1.0 | 7.0 ± 1.3 | 5.9 ± 0.9 | 0.03 | 6.9 ± 0.8 | 6.7 ± 1.2 | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreo-López, M.C.; Zarco-Martín, M.T.; Contreras-Bolívar, V.; Fernández-Soto, M.L. Prevalence of Sarcopenia and Dynapenia and Related Clinical Outcomes in Patients with Type 1 Diabetes Mellitus. Nutrients 2023, 15, 4914. https://doi.org/10.3390/nu15234914
Andreo-López MC, Zarco-Martín MT, Contreras-Bolívar V, Fernández-Soto ML. Prevalence of Sarcopenia and Dynapenia and Related Clinical Outcomes in Patients with Type 1 Diabetes Mellitus. Nutrients. 2023; 15(23):4914. https://doi.org/10.3390/nu15234914
Chicago/Turabian StyleAndreo-López, María Carmen, María Teresa Zarco-Martín, Victoria Contreras-Bolívar, and María Luisa Fernández-Soto. 2023. "Prevalence of Sarcopenia and Dynapenia and Related Clinical Outcomes in Patients with Type 1 Diabetes Mellitus" Nutrients 15, no. 23: 4914. https://doi.org/10.3390/nu15234914