The Association between Glucose 6-Phosphate Dehydrogenase Deficiency and Attention Deficit/Hyperactivity Disorder
Abstract
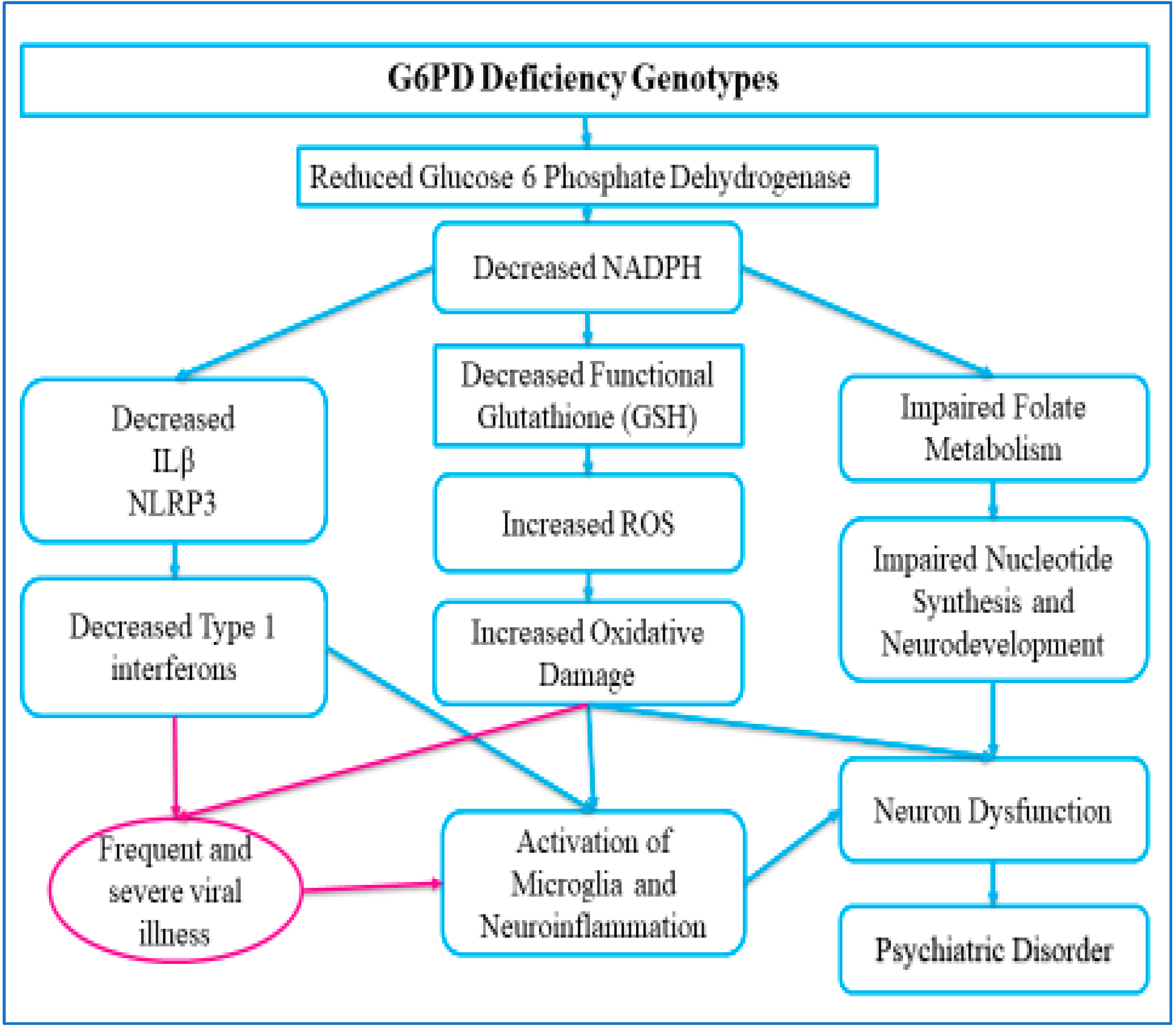
:1. Introduction
2. Materials and Methods
2.1. Methods
2.2. Study Population
2.3. Study Design
2.4. Definitions
3. Results
3.1. Physician Visits
3.2. Stimulant Agents Prescribed
3.3. Healthcare Utilization
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takahashi, N.; Nishimura, T.; Harada, T.; Okumura, A.; Iwabuchi, T.; Rahman, S.; Kuwabara, H.; Takagai, S.; Usui, N.; Makinodan, M.; et al. Interaction of genetic liability for attention deficit hyperactivity disorder (ADHD) and perinatal inflammation contributes to ADHD symptoms in children. Brain Behav. Immun. Health 2023, 30, 100630. [Google Scholar] [CrossRef] [PubMed]
- Koç, S.; Güler, E.M.; Derin, S.; Gültekin, F.; Aktaş, S. Oxidative and Inflammatory Parameters in Children and Adolescents With ADHD. J. Atten. Disord. 2023, 27, 880–886. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.G.; Scheible, K.; Sefair, A.V.; Gilchrist, M.; Blackmore, E.R.; Winter, M.A.; Gunnar, M.R.; Wyman, C.; Carnahan, J.; Moynihan, J.A.; et al. Immune and neuroendocrine correlates of temperament in infancy. Dev. Psychopathol. 2017, 29, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Morales-Muñoz, I.; Upthegrove, R.; Lawrence, K.; Thayakaran, R.; Kooij, S.; Gregory, A.M.; Marwaha, S. The role of inflammation in the prospective associations between early childhood sleep problems and ADHD at 10 years: Findings from a UK birth cohort study. J. Child. Psychol. Psychiatry 2023, 64, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Corominas-Roso, M.; Armario, A.; Palomar, G.; Corrales, M.; Carrasco, J.; Richarte, V.; Ferrer, R.; Casas, M.; Ramos-Quiroga, J. IL-6 and TNF-α in unmedicated adults with ADHD: Relationship to cortisol awakening response. Psychoneuroendocrinology 2017, 79, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mordelt, A.; de Witte, L.D. Microglia-mediated synaptic pruning as a key deficit in neurodevelopmental disorders: Hype or hope? Curr. Opin. Neurobiol. 2023, 79, 102674. [Google Scholar] [CrossRef] [PubMed]
- Çetin, F.H.; Uçaryılmaz, H.; Uçar, H.N.; Artaç, H.; Güler, H.A.; Duran, S.A.; Kılınç, K.; Türkoğlu, S. Regulatory T cells in children with attention deficit hyperactivity disorder: A case-control study. J. Neuroimmunol. 2022, 367, 577848. [Google Scholar] [CrossRef]
- Joseph, N.; Zhang-James, Y.; Perl, A.; Faraone, S.V. Oxidative Stress and ADHD: A Meta-Analysis. J. Atten. Disord. 2015, 19, 915–924. [Google Scholar] [CrossRef]
- Leffa, D.T.; Torres, I.L.S.; Rohde, L.A. A Review on the Role of Inflammation in Attention-Deficit/Hyperactivity Disorder. Neuroimmunomodulation 2018, 25, 328–333. [Google Scholar] [CrossRef]
- Sa-Carneiro, F.; Calhau, C.; Coelho, R.; Figueiredo-Braga, M. Putative shared mechanisms in autism spectrum disorders and attention deficit hyperactivity disorder, a systematic review of the role of oxidative stress. Acta Neurobiol. Exp. 2020, 80, 129–138. [Google Scholar] [CrossRef]
- Luzzatto, L.; Ally, M.; Notaro, R. Glucose-6-phosphate dehydrogenase deficiency. Blood 2020, 136, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Georgakouli, K.; Fatouros, I.G.; Draganidis, D.; Papanikolaou, K.; Tsimeas, P.; Deli, C.K.; Jamurtas, A.Z. Exercise in Glucose-6-Phosphate Dehydrogenase Deficiency: Harmful or Harmless? A Narrative Review. Oxidative Med. Cell. Longev. 2019, 2019, 8060193. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.L.; Van Noorden, C.J. Glucose-6-phosphate dehydrogenase deficiency and malaria: Cytochemical detection of heterozygous G6PD deficiency in women. J. Histochem. Cytochem. 2009, 57, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyev, A.M.; Bagirova, M.; Elcicek, S.; Koc, R.C.; Ates, S.C.; Baydar, S.Y.; Yaman, S.; Abamor, E.S.; Oztel, O.N. Glucose-6-Phosphate Dehydrogenase Deficiency and Malaria: A Method to Detect Primaquine-Induced Hemolysis in vitro. In Dehydrogenases; IntechOpen: London, UK, 2012; pp. 65–90. [Google Scholar]
- Abu Omar, R.; Algur, N.; Megged, O.; Hammerman, C.; Kaplan, M. Glucose-6-Phosphate Dehydrogenase Screening in Israel-Arab and Palestinian-Arab Neonates. J. Pediatr. 2015, 167, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, C.; Huang, W.; He, L.; Liu, Y.; Huang, H.; Chen, W.; Zheng, Y.; Xu, G.; Lin, L.; et al. Simultaneous detection of G6PD mutations using SNPscan in a multiethnic minority area of Southwestern China. Front. Genet. 2022, 13, 1000290. [Google Scholar] [CrossRef] [PubMed]
- Le Pichon, J.B.; Riordan, S.M.; Watchko, J.; Shapiro, S.M. The Neurological Sequelae of Neonatal Hyperbilirubinemia: Definitions, Diagnosis and Treatment of the Kernicterus Spectrum Disorders (KSDs). Curr. Pediatr. Rev. 2017, 13, 199–209. [Google Scholar] [PubMed]
- Corona, J.C.; Duchen, M.R. Impaired mitochondrial homeostasis and neurodegeneration: Towards new therapeutic targets? J. Bioenerg. Biomembr. 2015, 47, 89–99. [Google Scholar] [CrossRef]
- Moniczewski, A.; Gawlik, M.; Smaga, I.; Niedzielska, E.; Krzek, J.; Przegaliński, E.; Pera, J.; Filip, M. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 1. Chemical aspects and biological sources of oxidative stress in the brain. Pharmacol. Rep. 2015, 67, 560–568. [Google Scholar] [CrossRef]
- Smaga, I.; Niedzielska, E.; Gawlik, M.; Moniczewski, A.; Krzek, J.; Przegaliński, E.; Pera, J.; Filip, M. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol. Rep. 2015, 67, 569–580. [Google Scholar] [CrossRef]
- Ghanizadeh, A.; Namazi, M.R.; Davami, M.H. G6PD Deficiency as a predisposing factor for attention deficit/hyperactivity disorder: A hypothesis. Arch. Med. Res. 2010, 41, 391. [Google Scholar] [CrossRef]
- Petra, A.I.; Panagiotidou, S.; Hatziagelaki, E.; Stewart, J.M.; Conti, P.; Theoharides, T.C. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders with Suspected Immune Dysregulation. Clin. Ther. 2015, 37, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.P.; Su, K.P.; Mondelli, V.; Pariante, C.M. Omega-3 Polyunsaturated Fatty Acids in Youths with Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Clinical Trials and Biological Studies. Neuropsychopharmacology 2018, 43, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.J.; Francis, L.; Dawood, M.; Lai, Z.; Faraone, S.V.; Perl, A. Attention deficit and hyperactivity disorder scores are elevated and respond to N-acetylcysteine treatment in patients with systemic lupus erythematosus. Arthritis Rheum. 2013, 65, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Merzon, E.; Gutbir, Y.; Vinker, S.; Cohen, A.G.; Horwitz, D.; Ashkenazi, S.; Sadaka, Y. Early Childhood Shigellosis and Attention Deficit Hyperactivity Disorder: A Population-Based Cohort Study with a Prolonged Follow-up. J. Atten. Disord. 2021, 25, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Merzon, E.; Israel, A.; Ashkenazi, S.; Rotem, A.; Schneider, T.; Faraone, S.V.; Biederman, J.; Green, I.; Golan-Cohen, A.; Vinker, S.; et al. Attention-Deficit/Hyperactivity Disorder Is Associated with Increased Rates of Childhood Infectious Diseases: A Population-Based Case-Control Study. J. Am. Acad. Child. Adolesc. Psychiatry 2023, 62, 253–260.e1. [Google Scholar] [CrossRef] [PubMed]
- Merzon, E.; Weiss, M.; Krone, B.; Cohen, S.; Ilani, G.; Vinker, S.; Cohen-Golan, A.; Green, I.; Israel, A.; Schneider, T.; et al. Clinical and Socio-Demographic Variables Associated with the Diagnosis of Long COVID Syndrome in Youth: A Population-Based Study. Int. J. Environ. Res. Public. Health 2022, 19, 5993. [Google Scholar] [CrossRef]
- He, H.; Yu, Y.; Liew, Z.; Gissler, M.; László, K.D.; Valdimarsdóttir, U.A.; Zhang, J.; Li, F.; Li, J. Association of Maternal Autoimmune Diseases with Risk of Mental Disorders in Offspring in Denmark. JAMA Netw. Open 2022, 5, e227503. [Google Scholar] [CrossRef]
- Merzon, E.; Weiss, M.D.; Cortese, S.; Rotem, A.; Schneider, T.; Craig, S.G.; Vinker, S.; Cohen, A.G.; Green, I.; Ashkenazi, S.; et al. The Association between ADHD and the Severity of COVID-19 Infection. J. Atten. Disord. 2022, 26, 491–501. [Google Scholar] [CrossRef]
- Tylee, D.S.; Lee, Y.K.; Wendt, F.R.; Pathak, G.A.; Levey, D.F.; De Angelis, F.; Gelernter, J.; Polimanti, R. An Atlas of Genetic Correlations and Genetically Informed Associations Linking Psychiatric and Immune-Related Phenotypes. JAMA Psychiatry 2022, 79, 667–676. [Google Scholar] [CrossRef]
- Vázquez-González, D.; Carreón-Trujillo, S.; Alvarez-Arellano, L.; Abarca-Merlin, D.M.; Domínguez-López, P.; Salazar-García, M.; Corona, J.C. A Potential Role for Neuroinflammation in ADHD. Adv. Exp. Med. Biol. 2023, 1411, 327–356. [Google Scholar]
- Lauden, A.; Geishin, A.; Merzon, E.; Korobeinikov, A.; Green, I.; Golan-Cohen, A.; Vinker, S.; Manor, I.; Weizman, A.; Magen, E. Higher rates of allergies, autoimmune diseases and low-grade inflammation markers in treatment-resistant major depression. Brain Behav. Immun. Health 2021, 16, 100313. [Google Scholar] [CrossRef]
- Dunn, G.A.; Nigg, J.T.; Sullivan, E.L. Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacol. Biochem. Behav. 2019, 182, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, N. The role of oxidative stress in cardiovascular disease caused by social isolation and loneliness. Redox Biol. 2020, 37, 101585. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Faraone, S.V.; Banaschewski, T.; Coghill, D.; Zheng, Y.; Biederman, J.; Bellgrove, M.A.; Newcorn, J.H.; Gignac, M.; Al Saud, N.M.; Manor, I.; et al. The World Federation of ADHD International Consensus Statement: 208 Evidence-based conclusions about the disorder. Neurosci. Biobehav. Rev. 2021, 128, 789–818. [Google Scholar] [CrossRef] [PubMed]
- Vinker-Shuster, M.; Golan-Cohen, A.; Merhasin, I.; Merzon, E. Attention-Deficit Hyperactivity Disorder in Pediatric Patients with Type 1 Diabetes Mellitus: Clinical Outcomes and Diabetes Control. J. Dev. Behav. Pediatr. 2019, 40, 330–334. [Google Scholar] [CrossRef]
- Vinker-Shuster, M.; Eldor, R.; Green, I.; Golan-Cohen, A.; Manor, I.; Merzon, E. Glycemic Control and Diabetes Related Complications in Adults with Type 1 Diabetes Mellitus and ADHD. J. Atten. Disord. 2022, 26, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Schäffer, A.A.; Berkovitch, M.; Ozeri, D.J.; Merzon, E.; Green, I.; Golan-Cohen, A.; Ruppin, E.; Vinker, S.; Magen, E. Glucose-6-phosphate dehydrogenase deficiency and long-term risk of immune-related disorders. Front. Immunol. 2023, 14, 1232560. [Google Scholar] [CrossRef] [PubMed]
- Alrahmany, D.; Omar, A.F.; Al-Maqbali, S.R.; Harb, G.; Ghazi, I.M. Infections in G6PD-Deficient Hospitalized Patients-Prevalence, Risk Factors, and Related Mortality. Antibiotics 2022, 11, 934. [Google Scholar] [CrossRef]
- Alrahmany, D.; Omar, A.F.; Hafez, W.; Albaloshi, S.; Harb, G.; Ghazi, I.M. Infections in Glucose-6-Phosphate Dehydrogenase G6PD-Deficient Patients; Predictors for Infection-Related Mortalities and Treatment Outcomes. Antibiotics 2023, 12, 494. [Google Scholar] [CrossRef]
- Lopez-Lopez, A.; Villar-Cheda, B.; Quijano, A.; Garrido-Gil, P.; Garcia-Garrote, M.; Díaz-Ruiz, C.; Muñoz, A.; Labandeira-Garcia, J.L. NADPH-Oxidase, Rho-Kinase and Autophagy Mediate the (Pro)renin-Induced Pro-Inflammatory Microglial Response and Enhancement of Dopaminergic Neuron Death. Antioxidants 2021, 10, 1340. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, S.; Chu, C.; Wang, S.; Oyarzabal, E.; Wilson, B.; Sanders, V.; Xie, K.; Wang, Q.; Hong, J. A novel role of microglial NADPH oxidase in mediating extra-synaptic function of norepinephrine in regulating brain immune homeostasis. Glia 2015, 63, 1057–1072. [Google Scholar] [CrossRef]
- Begieneman, M.P.V.; ter Horst, E.N.; Rijvers, L.; Meinster, E.; Leen, R.; Pankras, J.E.; Fritz, J.; Kubat, B.; Musters, R.J.P.; van Kuilenburg, A.B.P.; et al. Dopamine induces lipid accumulation, NADPH oxidase-related oxidative stress, and a proinflammatory status of the plasma membrane in H9c2 cells. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1097–H1107. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Mukherjee, S.; Dar, W.; Upadhyay, P.; Ranganathan, A.; Pati, S.; Singh, S. G6PD deficiency: Imbalance of functional dichotomy contributing to the severity of COVID-19. Future Microbiol. 2022, 17, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Berkovitch, M.; Merzon, E.; Golan-Cohen, A.; Green, I.; Ruppin, E.; Vinker, S.; Magen, E. Glucose-6-Phosphate Dehydrogenase Deficiency and Coronavirus Disease 2019. Clin. Infect. Dis. 2023, 77, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Sadaka, Y.; Freedman, J.; Ashkenazi, S.; Vinker, S.; Golan-Cohen, A.; Green, I.; Israel, A.; Eran, A.; Merzon, E. The Effect of Antibiotic Treatment of Early Childhood Shigellosis on Long-Term Prevalence of Attention Deficit/Hyperactivity Disorder. Children 2021, 8, 880. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, R.; Volkow, N.D. Increased risk of COVID-19 infection and mortality in people with mental disorders: Analysis from electronic health records in the United States. World Psychiatry 2020, 20, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.S.; Brito, M.A.M.; Sampaio, V.S.; Monteiro, W.M.; Costa, M.R.F.; Alecrim, M.G.C.; Lacerda, M.V.G. High frequency of diabetes and impaired fasting glucose in patients with glucose-6-phosphate dehydrogenase deficiency in the Western brazilian Amazon. Am. J. Trop. Med. Hyg. 2014, 91, 74–76. [Google Scholar] [CrossRef]
- Heymann, A.D.; Cohen, Y.; Chodick, G. Glucose-6-phosphate dehydrogenase deficiency and type 2 diabetes. Diabetes Care 2012, 35, e58. [Google Scholar] [CrossRef]
- Pinna, A.; Contini, E.L.; Carru, C.; Solinas, G. Glucose-6-phosphate dehydrogenase deficiency and diabetes mellitus with severe retinal complications in a Sardinian population, Italy. Int. J. Med. Sci. 2013, 10, 1907–1913. [Google Scholar] [CrossRef]
- Kweka, B.; Lyimo, E.; Jeremiah, K.; Filteau, S.; Rehman, A.M.; Friis, H.; Manjurano, A.; Faurholt-Jepsen, D.; Krogh-Madsen, R.; PrayGod, G.; et al. Influence of hemoglobinopathies and glucose-6-phosphate dehydrogenase deficiency on diagnosis of diabetes by HbA1c among Tanzanian adults with and without HIV: A cross-sectional study. PLoS ONE 2020, 15, e0244782. [Google Scholar] [CrossRef]
- Leong, A.; Lim, V.J.Y.; Wang, C.; Chai, J.-F.; Dorajoo, R.; Heng, C.-K.; van Dam, R.M.; Koh, W.-P.; Yuan, J.-M.; Jonas, J.B.; et al. Association of G6PD variants with hemoglobin A1c and impact on diabetes diagnosis in East Asian individuals. BMJ Open Diabetes Res. Care 2020, 8, e001091. [Google Scholar] [CrossRef] [PubMed]
- Kwok, M.K.; Leung, C.M.; Schooling, C.M. Glucose-6-Phosphate Dehydrogenase Deficiency and Physical and Mental Health until Adolescence. PLoS ONE 2016, 11, e0166192. [Google Scholar] [CrossRef] [PubMed]
- Sinningen, K.; Emons, B.; Böhme, P.; Juckel, G.; Hanusch, B.; Beckmann, B.; Tsikas, D.; Lücke, T. l-Arginine/nitric oxide pathway and oxidative stress in adults with ADHD: Effects of methylphenidate treatment. Nitric Oxide 2023, 138–139, 64–69. [Google Scholar] [CrossRef]
- Miniksar, D.Y.; Cansız, M.A.; Göçmen, A.Y.; Kılıç, M.; Miniksar, Ö.H. The Effect of Drug Use, Body Mass Index and Blood Pressure on Oxidative Stress Levels in Children and Adolescents with Attention Deficit and Hyperactivity Disorder. Clin. Psychopharmacol. Neurosci. 2023, 21, 88–98. [Google Scholar] [CrossRef]
- Sangouni, A.A.; Mirhosseini, H.; Hosseinzadeh, M. Effect of vitamin D supplementation on brain waves, behavioral performance, nitric oxide, malondialdehyde, and high-sensitivity C-reactive protein in children with attention deficit/hyperactivity disorder: Study protocol for a randomized clinical trial. Trials 2022, 23, 890. [Google Scholar] [CrossRef]
| G6PD-Deficient Group | Matched Controls | p | Odds Ratio [95% CI] | ||
|---|---|---|---|---|---|
| N | 7473 | 29,892 | |||
| Gender, N (%) | Female | 2327 (31.1%) | 9308 (31.1%) | 1 | 1.00 [0.94–1.06] |
| Male | 5146 (68.9%) | 20,584 (68.9%) | 1 | 1.00 [0.94–1.06] | |
| Age in years M(SD) | 29.2 (22.3) | 29.2 (22.3) | 0.984 | 1.00 [0.94–1.06] | |
| Age in years N (%) | 0–2 | 378 (5.06%) | 1512 (5.06%) | 1 | 1.00 [0.91–1.10] |
| 3–9 | 1331 (17.81%) | 5324 (17.81%) | 1 | 1.00 [0.93–1.07] | |
| 10–18 | 1268 (16.97%) | 5072 (16.97%) | 1 | 1.00 [0.93–1.08] | |
| 19–29 | 1355 (18.13%) | 5420 (18.13%) | 1 | 1.00 [0.93–1.07] | |
| 30–39 | 1053 (14.09%) | 4212 (14.09%) | 1 | 1.00 [0.92–1.08] | |
| 40–49 | 623 (8.34%) | 2488 (8.32%) | 1 | 1.00 [0.91–1.10] | |
| 50–59 | 531 (7.11%) | 2128 (7.12%) | 1 | 1.00 [0.91–1.10] | |
| 60–69 | 460 (6.16%) | 1841 (6.16%) | 1 | 1.00 [0.90–1.11] | |
| 70–79 | 260 (3.48%) | 1039 (3.48%) | 1 | 1.00 [0.86–1.16] | |
| 80–89 | 152 (2.03%) | 608 (2.03%) | 1 | 1.00 [0.80–1.24] | |
| ≥90 | 62 (0.83%) | 248 (0.83%) | 1 | 1.00 [0.42–2.07] | |
| Ethnic group N (%) | Arab | 729 (9.76%) | 2916 (9.76%) | 1 | 1.00 [0.91–1.09] |
| General | 5096 (68.19%) | 20,384 (68.19%) | 1 | 1.00 [0.94–1.06] | |
| Ultra-Orthodox | 1648 (22.05%) | 6592 (22.05%) | 1 | 1.00 [0.94–1.07] | |
| Region N (%) | Center | 2160 (28.9%) | 8408 (28.13%) | 0.212 | 1.04 [0.98–1.10] |
| Jerusalem | 2330 (31.18%) | 7239 (24.22%) | 0.001 | 1.42 [1.34–1.51] | |
| North | 1466 (19.62%) | 5406 (18.09%) | 0.002 | 1.11 [1.03–1.18] | |
| South | 1517 (20.3%) | 8835 (29.56%) | 0.001 | 0.61 [0.57–0.65] | |
| SES | M (SD) | 9.2 (3.6) | 9.2 (3.6) | 1 | 1.00 [0.91–1.09] |
| Missing | N (%) | 652 (9.2%) | 2608 (9.2%) | 1 | 1.00 [0.91–1.09] |
| SES N (%) | Low | 1457(34.5%) | 9828(34.5%) | 1 | 1.00 [0.94–1.07] |
| Low-Middle | 1357 (19.1%) | 5428 (19.1%) | 1 | 1.00 [0.94–1.07] | |
| Middle-High | 1564 (22.0%) | 6256 (22.0%) | 1 | 1.00 [0.94–1.07] | |
| High | 1092 (15.3%) | 4368 (15.3%) | 1 | 1.00 [0.93–1.08] | |
| ~Missing~ | 652 (9.2%) | 2608 (9.2%) | 1 | 1.00 [0.91–1.09] | |
| G6PD Deficient Group | Matched Controls | p | Odds Ratio [95% CI] | ||
|---|---|---|---|---|---|
| N | 7473 | 29,892 | |||
| Smoking status | Non-smoker | 3505 (76.93%) | 13,073 (74.77%) | 0.001 | 1.12 [1.07–1.19] |
| Past smoker | 83 (1.8%) | 305 (1.8%) | 0.489 | 1.09 [0.84–1.40] | |
| Smoker | 968 (21.25%) | 4096 (23.43%) | 0.186 | 0.94 [0.87–1.01] | |
| Missing | 2917 (39.03%) | 12,407 (41.51%) | 0.001 | 0.90 [0.85–0.95] | |
| BMI (kg/m2) | 23.33 (6.14) | 23.45 (6.32) | 0.104 | ||
| Obesity, n (%) | 915 (13.6%) | 3934 (15.0%) | 0.042 | 0.92 [0.85–0.99] | |
| Hypertension, n (%) | 804 (10.8%) | 3365 (11.3%) | 0.971 | 0.97 [0.89–1.05] | |
| Diabetes Mellitus, n (%) | 418 (5.59%) | 1952 (6.53%) | 0.035 | 0.85 [0.76–0.95] | |
| ADHD, n (%) | 1040 (13.9%) | 3650 (12.2%) | 0.001 | 1.16 [1.08–1.25] | |
| G6PD-Deficient Group | Matched Controls | p | Odds Ratio [95% CI] | |
|---|---|---|---|---|
| N | 7473 | 29,892 | ||
| Adult Neurologist Visits, n (%) | 1746 (23.4%) | 5687 (19.1%) | 0.001 | 1.30 [1.22–1.38] |
| Child Neurologist Visits, n (%) | 604 (8.1%) | 2253 (7.5%) | 0.073 | 1.08 [0.98–1.19] |
| Adult Psychiatrist Visits, n (%) | 514 (6.9%) | 1846 (6.2%) | 0.048 | 1.12 [1.01–1.24] |
| Child Psychiatrist Visits, n (%) | 161 (2.2%) | 615 (2.1%) | 0.586 | 1.05 [0.87–1.25] |
| ADHD-trained PCP Visits, n (%) | 115 (1.5%) | 410 (1.3%) | 0.054 | 1.12 [0.98–1.39] |
| Methylphenidate Use, n (%) | 929 (12.4%) | 3233 (10.8%) | 0.001 | 1.17 [1.08–1.27] |
| Amphetamine, n (%) | 195 (2.6%) | 675 (2.2%) | 0.041 | 1.16 [1.03–1.37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merzon, E.; Magen, E.; Ashkenazi, S.; Weizman, A.; Manor, I.; Krone, B.; Green, I.; Golan-Cohen, A.; Vinker, S.; Faraone, S.V.; et al. The Association between Glucose 6-Phosphate Dehydrogenase Deficiency and Attention Deficit/Hyperactivity Disorder. Nutrients 2023, 15, 4948. https://doi.org/10.3390/nu15234948
Merzon E, Magen E, Ashkenazi S, Weizman A, Manor I, Krone B, Green I, Golan-Cohen A, Vinker S, Faraone SV, et al. The Association between Glucose 6-Phosphate Dehydrogenase Deficiency and Attention Deficit/Hyperactivity Disorder. Nutrients. 2023; 15(23):4948. https://doi.org/10.3390/nu15234948
Chicago/Turabian StyleMerzon, Eugene, Eli Magen, Shai Ashkenazi, Abraham Weizman, Iris Manor, Beth Krone, Ilan Green, Avivit Golan-Cohen, Shlomo Vinker, Stephen V. Faraone, and et al. 2023. "The Association between Glucose 6-Phosphate Dehydrogenase Deficiency and Attention Deficit/Hyperactivity Disorder" Nutrients 15, no. 23: 4948. https://doi.org/10.3390/nu15234948
APA StyleMerzon, E., Magen, E., Ashkenazi, S., Weizman, A., Manor, I., Krone, B., Green, I., Golan-Cohen, A., Vinker, S., Faraone, S. V., & Israel, A. (2023). The Association between Glucose 6-Phosphate Dehydrogenase Deficiency and Attention Deficit/Hyperactivity Disorder. Nutrients, 15(23), 4948. https://doi.org/10.3390/nu15234948









