Weight Management during Pregnancy and the Postpartum Period in Women with Gestational Diabetes Mellitus: A Systematic Review and Summary of Current Evidence and Recommendations
Abstract
:1. Introduction
2. Methods
2.1. Establishment of the Problem
2.2. Evidence Sources and Retrieval Strategies
2.3. Inclusion Criteria and Study Selection
2.4. Quality Assessment
2.4.1. Evaluation of Guidelines
2.4.2. Evaluation of Systematic Reviews
2.4.3. Evaluation of Clinical Decisions, Recommended Practice, and Expert Consensus
2.4.4. Evaluation of Evidence Summaries
2.5. Data Extraction
2.6. Data Synthesis and Classification
3. Results
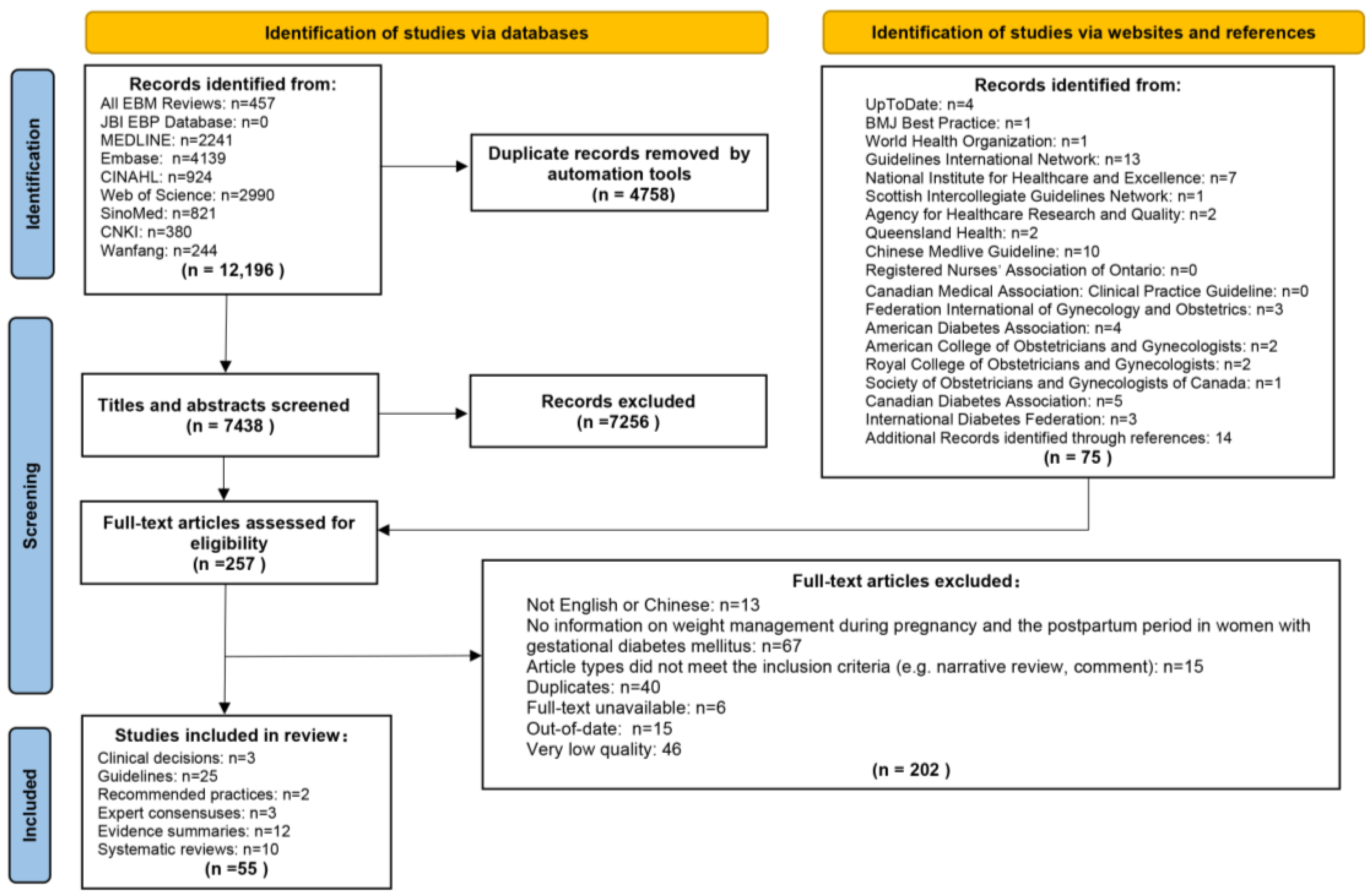
3.1. Search Results
3.2. Quality Assessment
3.2.1. Quality Evaluation Results of Guidelines
3.2.2. Quality Evaluation Results of Systematic Reviews
3.2.3. Quality Evaluation Results of Evidence Summaries
3.2.4. Quality Evaluation Results of Other Studies
3.3. Synthesis of Evidence
3.4. The Proportion of Carbohydrates, Proteins, and Fats in Daily Energy Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Queensland Health. Queensland Clinical Guidelines: Gestational Diabetes Mellitus (GDM). Available online: http://health.qld.gov.au/qcg (accessed on 14 April 2023).
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas. Available online: https://diabetesatlas.org/data/en/indicators/14/ (accessed on 14 April 2023).
- Manrique-Acevedo, C.; Chinnakotla, B.; Padilla, J.; Martinez-Lemus, L.A.; Gozal, D. Obesity and cardiovascular disease in women. Int. J. Obes. 2020, 44, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- Attali, E.; Yogev, Y. The impact of advanced maternal age on pregnancy outcome. Best. Pract. Res. Clin. Obs. Obstet. Gynaecol. 2021, 70, 2–9. [Google Scholar] [CrossRef]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational diabetes mellitus and adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2022, 377, e067946. [Google Scholar] [CrossRef]
- Choudhury, A.A.; Devi Rajeswari, V. Gestational diabetes mellitus-A metabolic and reproductive disorder. Biomed. Pharmacother. 2021, 143, 112183. [Google Scholar] [CrossRef]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Dehmer, E.W.; Phadnis, M.A.; Gunderson, E.P.; Lewis, C.E.; Bibbins-Domingo, K.; Engel, S.M.; Jonsson Funk, M.; Kramer, H.; Kshirsagar, A.V.; Heiss, G. Association Between Gestational Diabetes and Incident Maternal CKD: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am. J. Kidney Dis. 2018, 71, 112–122. [Google Scholar] [CrossRef]
- Gou, B.H.; Guan, H.M.; Bi, Y.X.; Ding, B.J. Gestational diabetes: Weight gain during pregnancy and its relationship to pregnancy outcomes. Chin. Med. J. 2019, 132, 154–160. [Google Scholar] [CrossRef]
- Bao, W.; Yeung, E.; Tobias, D.K.; Hu, F.B.; Vaag, A.A.; Chavarro, J.E.; Mills, J.L.; Grunnet, L.G.; Bowers, K.; Ley, S.H.; et al. Long-term risk of type 2 diabetes mellitus in relation to BMI and weight change among women with a history of gestational diabetes mellitus: A prospective cohort study. Diabetologia 2015, 58, 1212–1219. [Google Scholar] [CrossRef]
- Minschart, C.; Myngheer, N.; Maes, T.; De Block, C.; Van Pottelbergh, I.; Abrams, P.; Vinck, W.; Leuridan, L.; Driessens, S.; Mathieu, C.; et al. Weight retention and glucose intolerance in early postpartum after gestational diabetes. Eur. J. Endocrinol. 2023, 19, lvad053. [Google Scholar] [CrossRef]
- Shekelle, P.G. Clinical Practice Guidelines: What’s Next? JAMA 2018, 320, 757–758. [Google Scholar] [CrossRef]
- Graham, R.; Mancher, M.; Miller Wolman, D.; Greenfield, S.; Steinberg, E. Clinical Practice Guidelines We Can Trust; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Harrison, C.L.; Teede, H.; Khan, N.; Lim, S.; Chauhan, A.; Drakeley, S.; Moran, L.; Boyle, J. Weight management across preconception, pregnancy, and postpartum: A systematic review and quality appraisal of international clinical practice guidelines. Obes. Rev. 2021, 22, e13310. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, M.C.; Kho, M.E.; Browman, G.P.; Burgers, J.S.; Cluzeau, F.; Feder, G.; Fervers, B.; Graham, I.D.; Grimshaw, J.; Hanna, S.E.; et al. AGREE II: Advancing guideline development, reporting and evaluation in health care. CMAJ 2010, 182, E839–E842. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- McArthur, A.; Klugarova, J.; Yan, H.; Florescu, S. Chapter 4: Systematic reviews of text and opinion. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; 2020; Available online: https://doi.org/10.46658/JBIMES-20-05 (accessed on 14 April 2023).
- Foster, M.J.; Shurtz, S. Making the Critical Appraisal for Summaries of Evidence (CASE) for evidence-based medicine (EBM): Critical appraisal of summaries of evidence. J. Med. Libr. Assoc. 2013, 101, 192–198. [Google Scholar] [CrossRef]
- The Joanna Briggs Institute. JBI Levels of Evidence. Available online: https://jbi.global/sites/default/files/2019-05/JBI-Levels-of-evidence_2014_0.pdf (accessed on 14 April 2023).
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S254–S266. [Google Scholar] [CrossRef]
- Duarte-Gardea, M.O.; Gonzales-Pacheco, D.M.; Reader, D.M.; Thomas, A.M.; Wang, S.R.; Gregory, R.P.; Piemonte, T.A.; Thompson, K.L.; Moloney, L. Academy of Nutrition and Dietetics Gestational Diabetes Evidence-Based Nutrition Practice Guideline. J. Acad. Nutr. Diet. 2018, 118, 1719–1742. [Google Scholar] [CrossRef]
- The American College of Obstetricians and Gynecologists Committee. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol. 2018, 131, e49–e64. [Google Scholar] [CrossRef]
- Blonde, L.; Umpierrez, G.E.; Reddy, S.S.; McGill, J.B.; Berga, S.L.; Bush, M.; Chandrasekaran, S.; DeFronzo, R.A.; Einhorn, D.; Galindo, R.J.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline: Developing a Diabetes Mellitus Comprehensive Care Plan—2022 Update. Endocr. Pract. 2022, 28, 923–1049. [Google Scholar] [CrossRef]
- Brown, F.M.; Anderson-Haynes, S.E.; Blair, E.; Serdy, S.; Halprin, E.; Feldman, A.; O’Brien, K.E.; Ghiloni, S.; Suhl, E.; Rizzotto, J.A.; et al. CHAPTER 3. Guideline for detection and management of diabetes in pregnancy. Am. J. Manag. Care 2018, 24, SP232–SP239. [Google Scholar]
- Yang, H.X. Guideline for the diagnosis and management of hyperglycemia in pregnancy (2022) [Part I]. Chin. J. Obstet. Gynecol. 2022, 57, 3–12. Available online: https://rs.yiigle.com/CN112141202201/1349426.htm (accessed on 14 April 2023).
- Branch of Women’s Health Care, C.P.M.A. Guideline to postpartum health services. Chin. J. Woman Child. Health Res. 2021, 32, 767–781. [Google Scholar]
- Huang, N.; Zhou, Y.; Zhang, M.; Wang, K.; Li, L. Update of clinical nursing practice guideline for gestational diabetes mellitus. J. Nurses Train. 2021, 36, 1937–1943. [Google Scholar]
- National Institute for Health and Care Excellence. Diabetes in Pregnancy: Management from Preconception to the Postnatal Period. Available online: https://www.nice.org.uk/guidance/ng3 (accessed on 14 April 2023).
- Dyson, P.A.; Twenefour, D.; Breen, C.; Duncan, A.; Elvin, E.; Goff, L.; Hill, A.; Kalsi, P.; Marsland, N.; McArdle, P.; et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet. Med. 2018, 35, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.S.; Berger, H.; Donovan, L.; Godbout, A.; Kader, T.; Keely, E.; Sanghera, R. Diabetes and Pregnancy. Can. J. Diabetes 2018, 42, S255–S282. [Google Scholar] [CrossRef]
- Mottola, M.F.; Davenport, M.H.; Ruchat, S.M.; Davies, G.A.; Poitras, V.J.; Gray, C.E.; Jaramillo Garcia, A.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. 2019 Canadian guideline for physical activity throughout pregnancy. Br. J. Sports Med. 2018, 52, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K.; et al. Japanese Clinical Practice Guideline for Diabetes 2019. J. Diabetes Investig. 2020, 11, 1020–1076. [Google Scholar] [CrossRef] [PubMed]
- Itakura, A.; Shoji, S.; Shigeru, A.; Kotaro, F.; Junichi, H.; Hironobu, H.; Kamei, Y.; Eiji, K.; Shintaro, M.; Ryu, M.; et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology and Japan Association of Obstetricians and Gynecologists 2020 edition. J. Obstet. Gynaecol. Res. 2023, 49, 5–53. [Google Scholar] [CrossRef]
- Hassabi, M.; Esteghamati, A.; Halabchi, F.; Abedi-Yekta, A.H.; Mahdaviani, B.; Hassanmirzaie, B.; Hosseinpanah, F.; Valizadeh, M. Iranian National Clinical Practice Guideline for Exercise in Patients with Diabetes. Int. J. Endocrinol. Metab. 2021, 19, e109021. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, M.; Hosseinpanah, F.; Tehrani, F.R.; Abdi, H.; Mehran, L.; Hadaegh, F.; Amouzegar, A.; Sarvghadi, F.; Azizi, F.; Iranian Endocrine Society Task Force. Iranian Endocrine Society Guidelines for Screening, Diagnosis, and Management of Gestational Diabetes Mellitus. Int. J. Endocrinol. Metab. 2021, 19, e107906. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, E.; Farmakidis, G.; Gerede, A.; Goulis, D.G.; Koukkou, E.; Kourtis, A.; Mamopoulos, A.; Papadimitriou, K.; Papadopoulos, V.; Stefos, T. Clinical practice guidelines on diabetes mellitus and pregnancy: II. Gestational diabetes mellitus. Hormones 2020, 19, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Ranjan, P.; Vikram, N.K.; Kaur, D.; Balsalkar, G.; Malhotra, A.; Puri, M.; Batra, A.; Madan, J.; Tyagi, S.; et al. Executive summary of evidence and consensus-based clinical practice guideline for management of obesity and overweight in postpartum women: An AIIMS-DST initiative. Diabetes Metab. Syndr.-Clin. Res. Rev. 2022, 16, 102425. [Google Scholar] [CrossRef]
- Bayram, M.; Asyali Biri, A.; Esim Büyükbayrak, E.; Daglar, H.K.; Ercan, F.; Gürsoy Erzincan, S.; Çorbacioglu Esmer, A.; Inan, C.; Kanit, H.; Kara, Ö.; et al. Guideline on pregnancy and diabetes by the society of specialists in perinatology (PUDER), Turkey. J. Clin. Obstet. Gynecol. 2020, 30, 35–42. [Google Scholar] [CrossRef]
- Masood, S.N.; Baqai, S.; Naheed, F.; Masood, Y.; Sikandar, R.; Chaudhri, R.; Yasmin, H.; Korejo, R.; Comm, G.D.M.G.; Soc Obstetricians, G. Guidelines for management of hyperglycemia in pregnancy (HIP) by Society of Obstetricians & Gynaecologists of Pakistan (SOGP). J. Diabetol. 2021, 12, 83–98. [Google Scholar]
- Masood, S.N.; Shegem, N.; Baqai, S.; Suliman, M.; Alromaihi, D.; Sultan, M.; Salih, B.T.; Ram, U.; Ahmad, Z.; Aljufairi, Z.; et al. IDF-MENA region guidelines for management of hyperglycemia in pregnancy. J. Diabetol. 2021, 12, S3–S45. [Google Scholar] [CrossRef]
- Wender-Ozegowska, E.; Bomba-Opon, D.; Brazert, J.; Celewicz, Z.; Czajkowski, K.; Gutaj, P.; Malinowska-Polubiec, A.; Zawiejska, A.; Wielgos, M. Standards of Polish Society of Gynecologists and Obstetricians in management of women with diabetes. Ginekol. Pol. 2018, 89, 341–350. [Google Scholar] [CrossRef]
- Ministry of Public Health Qatar. National Clinical Guideline: The Diagnosis and Management of Diabetes in Pregnancy; Ministry of Public Health Qatar: Doha, Qatar, 2021.
- Schafer-Graf, U.M.; Gembruch, U.; Kainer, F.; Groten, T.; Hummel, S.; Hosli, I.; Grieshop, M.; Kaltheuner, M.; Buhrer, C.; Kautzky-Willer, A.; et al. Gestational Diabetes Mellitus (GDM)-Diagnosis, Treatment and Follow-Up. Guideline of the DDG and DGGG (S3 Level, AWMF Registry Number 057/008, February 2018). Geburtshilfe Und Frauenheilkd. 2018, 78, 1219–1231. [Google Scholar] [CrossRef]
- Martis, R.; Crowther, C.A.; Shepherd, E.; Alsweiler, J.; Downie, M.R.; Brown, J. Treatments for women with gestational diabetes mellitus: An overview of Cochrane systematic reviews. Cochrane Database Syst. Rev. 2018, 8, Cd012327. [Google Scholar] [CrossRef]
- Hewage, S.S.; Wu, S.; Neelakantan, N.; Yoong, J. Systematic review of effectiveness and cost-effectiveness of lifestyle interventions to improve clinical diabetes outcome measures in women with a history of GDM. Clin. Nutr. ESPEN 2020, 35, 20–29. [Google Scholar] [CrossRef]
- Retnakaran, M.; Viana, L.V.; Kramer, C.K. Lifestyle intervention for the prevention of type 2 diabetes in women with prior gestational diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2023, 25, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.M.; Kellett, J.E.; Balsells, M.; García-Patterson, A.; Hadar, E.; Solà, I.; Gich, I.; van der Beek, E.M.; Castañeda-Gutiérrez, E.; Heinonen, S.; et al. Gestational Diabetes Mellitus and Diet: A Systematic Review and Meta-analysis of Randomized Controlled Trials Examining the Impact of Modified Dietary Interventions on Maternal Glucose Control and Neonatal Birth Weight. Diabetes Care 2018, 41, 1346–1361. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.R.K.; Lima, S.A.M.; da Silvia Mazeto, G.M.F.; Calderon, I.M.P.; Magalhães, C.G.; Ferraz, G.A.R.; Molina, A.C.; de Araújo Costa, R.A.; Nogueira, V.D.S.N.; Rudge, M.V.C. Efficacy of Vitamin D supplementation in gestational diabetes mellitus: Systematic review and meta-analysis of randomized trials. PLoS ONE 2019, 14, e0213006. [Google Scholar] [CrossRef]
- Yefet, E.; Bar, L.; Izhaki, I.; Iskander, R.; Massalha, M.; Younis, J.S.; Nachum, Z. Effects of Probiotics on Glycemic Control and Metabolic Parameters in Gestational Diabetes Mellitus: Systematic Review and Meta-Analysis. Nutrients 2023, 15, 1633. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, E.; Rayner, J.; Hodge, A.; Ross, L.J.; Schoenaker, D.A.J.M. The Role of Diet in the Prevention of Diabetes among Women with Prior Gestational Diabetes: A Systematic Review of Intervention and Observational Studies. J. Acad. Nutr. Diet. 2020, 120, 69. [Google Scholar] [CrossRef]
- Dingena, C.F.; Arofikina, D.; Campbell, M.D.; Holmes, M.J.; Scott, E.M.; Zulyniak, M.A. Nutritional and Exercise-Focused Lifestyle Interventions and Glycemic Control in Women with Diabetes in Pregnancy: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 2023, 15, 323. [Google Scholar] [CrossRef]
- Mu, J.; Guo, X.; Zhou, Y.; Cao, G. The Effects of Probiotics/Synbiotics on Glucose and Lipid Metabolism in Women with Gestational Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2023, 15, 1375. [Google Scholar] [CrossRef]
- Goveia, P.; Cañon-Montañez, W.; De Paula Santos, D.; Lopes, G.W.; Ma, R.C.W.; Duncan, B.B.; Ziegelman, P.K.; Schmidt, M.I. Lifestyle intervention for the prevention of diabetes in women with previous gestational diabetes mellitus: A systematic review and meta-analysis. Front. Endocrinol. 2018, 9, 583. [Google Scholar] [CrossRef]
- Lopes, G. Evidence Summary. Gestational Diabetes Mellitus: Antenatal Care. The JBI EBP Database. 2023. JBI-ES-524-4. Available online: https://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=jbi&AN=JBI17950 (accessed on 14 April 2023).
- Lopes, G. Evidence Summary. Gestational Diabetes Mellitus: Management (Diet). The JBI EBP Database. 2023. JBI-ES-472-4. Available online: https://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=jbi&AN=JBI20937 (accessed on 14 April 2023).
- Lopes, G. Evidence Summary. Gestational Diabetes Mellitus: Management (Education). The JBI EBP Database. 2023. JBI-ES-474-4. Available online: https://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=jbi&AN=JBI21004 (accessed on 14 April 2023).
- Lopes, G. Evidence Summary. Gestational Diabetes Mellitus: Management (Exercise). The JBI EBP Database. 2023. JBI-ES-486-3. Available online: https://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=jbi&AN=JBI21005 (accessed on 14 April 2023).
- Whitehorn, A. Evidence Summary. Gestational Diabetes: Management (Dietary Supplements). The JBI EBP Database. 2023. JBI-ES-629-2. Available online: https://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=jbi&AN=JBI21076 (accessed on 14 April 2023).
- Moola, S. Evidence Summary. Gestational Diabetes: Management (Lifestyle Interventions). The JBI EBP Database. 2023. JBI-ES-647-2. Available online: https://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=jbi&AN=JBI21120 (accessed on 14 April 2023).
- Aginga, C. Evidence Summary. Gestational Diabetes: Management (Omega-3 Fatty Acids). The JBI EBP Database. 2023. JBI-ES-657-2. Available online: https://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=jbi&AN=JBI21074 (accessed on 14 April 2023).
- Aginga, C. Evidence Summary. Gestational Diabetes: Management (Probiotics). The JBI EBP Database. 2023. JBI-ES-652-2. Available online: https://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=jbi&AN=JBI21075 (accessed on 14 April 2023).
- Wang, S.; Chen, Y.; Li, S.; Geng, X. Evidence summary for dietary management of gestational diabetes. Chin. J. Clin. Res. 2022, 35, 284–288. [Google Scholar]
- Cao, Y.; Zhu, Y.; Wang, N.; Zhong, S.; Lu, Y.; Zhong, L.; Dong, L.; Zhai, Y. Evidence summary of diet management for patients with gestational diabetes mellitus. Chin. J. Nurs. Educ. 2021, 18, 1040–1046. [Google Scholar]
- Liu, T.; Tan, X.; Xu, N.; Mi, Y.; Chen, Y.; Xu, M. Evidence summary of exercise regimens for patients with gestational diabetes mellitus. Chin. J. Nurs. 2020, 55, 1514–1519. [Google Scholar]
- Li, X.; Zhao, Y.; Yao, Q. Summary of best evidence for dietary nutrition management after cesarean section in pregnant women with gestational diabetes mellitus. J. Women Child. Health Guide 2022, 1, 77–82. [Google Scholar]
- Eleanor Scott, R.S. Gestational Diabetes Mellitus 2022. Available online: https://bestpractice.bmj.com/topics/zh-cn/665 (accessed on 14 April 2023).
- Durnwald, C. Gestational Diabetes Mellitus Glucose Management and Maternal Prognosis 2022. Available online: https://www.uptodate.com/contents/zh-Hans/gestational-diabetes-mellitus-glucose-management-and-maternal-prognosis?search=Gestational%20diabetes%20mellitus%20Glucose%20management%20and%20maternal%20prognosis&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 14 April 2023).
- Durnwald, C. Gestational Diabetes Mellitus Screening, Diagnosis, and Prevention 2022. Available online: https://www.uptodate.com/contents/zh-Hans/gestational-diabetes-mellitus-screening-diagnosis-and-prevention?search=Gestational%20diabetes%20mellitus%20Screening,%20diagnosis,%20and%20prevention%20&source=search_result&selectedTitle=1~27&usage_type=default&display_rank=1 (accessed on 14 April 2023).
- Abbas Raza, S. Gdm: Safes Recommendations and Action Plan-2017. JPMA-J. Pak. Med. Assoc. 2018, 68, S1–S23. [Google Scholar]
- Benhalima, K.; Minschart, C.; Van Crombrugge, P.; Calewaert, P.; Verhaeghe, J.; Vandamme, S.; Theetaert, K.; Devlieger, R.; Pierssens, L.; Ryckeghem, H.; et al. The 2019 Flemish consensus on screening for overt diabetes in early pregnancy and screening for gestational diabetes mellitus. Acta Clin. Belg. 2020, 75, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.; McIntyre, H.D.; Tsoi, K.Y.; Kapur, A.; Ma, R.C.; Dias, S.; Okong, P.; Hod, M.; Poon, L.C.; Smith, G.N.; et al. Pregnancy as an opportunity to prevent type 2 diabetes mellitus: FIGO Best Practice Advice. Int. J. Gynaecol. Obstet. 2023, 160 (Suppl. S1), 56–67. [Google Scholar] [CrossRef]
- Chinese Maternal and Child Health Association. Expert consensus on exercise during pregnancy (draft). Chin. J. Perinat. Med. 2021, 24, 641–645. [Google Scholar]
- Shawe, J.; Ceulemans, D.; Akhter, Z.; Neff, K.; Hart, K.; Heslehurst, N.; Štotl, I.; Agrawal, S.; Steegers-Theunissen, R.; Taheri, S.; et al. Pregnancy after bariatric surgery: Consensus recommendations for periconception, antenatal and postnatal care. Obes. Rev. 2019, 20, 1507–1522. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Zhang, M.X.; Li, L.; Zhong, J.; Pan, X.H.; Zhao, X.Z.; Guo, N.F. Expert consensus study on recommendations of clinical nursing practice guidelines for gestational diabetes mellitus. Chin. Nurs. Res. 2020, 34, 4313–4318. [Google Scholar]
- Yang, H. Guideline for the diagnosis and management of hyperglycemia in pregnancy (2022) [Part II]. Chin. J. Obstet. Gynecol. 2022, 57, 81–90. Available online: https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjMwMzIxEg56aGZjazIwMjIwMjAwMRoIbjN2Nm9hdHE%3D (accessed on 14 April 2023).
| Type of Evidence | Serial Number | Evidence | Level of Evidence | |
|---|---|---|---|---|
| Gestational | ||||
| Weight control | Target population | 1 | All women diagnosed with GDM should undergo diet modification, regular exercise, and weight management [1,34,38,47,50,60,62,67,76]. | I |
| Benefits | 2 | Weight management during pregnancy in women with GDM can: (1) help achieve weight gain goals and reduce the risk of excessive gestational weight gain and postpartum weight retention; (2) increase tissue sensitivity to insulin, improve glucose tolerance, facilitate the achievement of glycemic control goals, and reduce insulin requirements; and (3) decrease the risk of adverse outcomes such as pre-eclampsia, cesarean section, large for gestational age, macrosomia, preterm labor, shoulder dystocia, and Neonatal Intensive Care Unit admission [23,24,28,33,37,46,47,50,54,67,70]. | I | |
| Principles | 3 | Women with GDM should control their weight according to the recommended weight gain goals during pregnancy, and aggressive weight loss during pregnancy is not recommended [45,70]. | V | |
| Weight control goals | 4 | Weight management goals during pregnancy (Institute of Medicine): (1) BMI < 18.5 before pregnancy: the total weight gain during pregnancy ranged from approximately 12.5 to 18.0 kg and the weight gain per week in the 2nd and 3rd trimester ranged from approximately 0.5 to 0.6 kg; (2) 18.5 < BMI < 24.9 before pregnancy: the total weight gain during pregnancy ranged from approximately 11.5 to 16.0 kg and the weight gain per week in the 2nd and 3rd trimester ranged from approximately 0.4 to 0.5 kg; (3) 25.0 < BMI < 29.9 before pregnancy: the total weight gain during pregnancy ranged from approximately 7.0 to 11.5 kg and the weight gain per week in the 2nd and 3rd trimester ranged from approximately 0.2 to 0.3 kg; (4) BMI ≥ 30.0 before pregnancy: the total weight gain during pregnancy ranged from approximately 5.0 to 9.0 kg and the weight gain per week in the 2nd and 3rd trimester ranged from approximately 0.2 to 0.3 kg [27,39,46,66,70]. | V | |
| 5 | Weight management goals during pregnancy (Chinese Medical Association): (1) BMI < 18.5 before pregnancy: the total weight gain during pregnancy ranged from approximately 11.0 to 16.0 kg, the weight gain in the first trimester should be ≤2 kg, and the weekly weight gain in the 2nd and 3rd trimester was 0.46(0.37~0.56) kg; (2) 18.5 < BMI < 24.0 before pregnancy: the total weight gain during pregnancy ranged from approximately 8.0 to 14.0 kg, the weight gain in the first trimester should be ≤2 kg, and the weekly weight gain in the 2nd and 3rd trimester was 0.37(0.26~0.48) kg; (3) 24.0 < BMI < 28.0 before pregnancy: the total weight gain during pregnancy ranged from approximately 7.0 to 11.0 kg, the weight gain in the first trimester should be ≤2 kg, and the weekly weight gain in the 2nd and 3rd trimester was 0.30(0.22~0.37) kg; (4) BMI ≥ 28.0 before pregnancy: the total weight gain during pregnancy should be ≤9.0 kg, the weight gain in the first trimester should be ≤2 kg, and the weekly weight gain in the 2nd and 3rd trimester should be ≤0.30 kg [28]. | V | ||
| Weight monitoring | 6 | Professionals should record the pre-pregnancy weight, height, and BMI of women with GDM, measure their current weight at each visit and assess the change in weight from the previous visit [1,24,27]. | V | |
| 7 | Women with GDM should monitor and record fasting weight early in the morning each week [46]. | V | ||
| Diet management | Nutrition assessment and counseling | 8 | Women with GDM should receive nutritional assessment and counseling from professionals (e.g., diabetes educators, dietitians) to develop an individualized dietary management plan based on the nutritional needs of the mother and child, pre-pregnancy BMI, blood glucose levels, dietary habits, personal and socio-cultural preferences, economic levels, etc. [1,24,30,32,33,43,44,45,46,57,58,65,69,70,77]. | I |
| 9 | Nutritional assessments provided by professionals for women with GDM include but are not limited to [24,66]: (1) food, beverage, and nutrient intake, including types and amount of carbohydrate (including fiber), fat, and protein, serving sizes, and meal and snack patterns; (2) appetite and changes in appetite; (3) eating environment and meals eaten away from home; (4) diet history and behavior; (5) factors influencing access to food; (6) method of food preparation and food safety; (7) pharmacologic therapy (including insulin or oral glucose-lowering agent); (8) substance use: alcohol, tobacco, caffeine, and recreational drugs; (9) use of dietary supplements, prenatal vitamins, over-the-counter medications, complementary and/or herbal medicine; (10) knowledge, beliefs, and attitudes: motivation, readiness to change, self-efficacy, and willingness and ability to make lifestyle changes; and (11) physical activity and function: exercise patterns, functions of activities of daily living, and sleep patterns. | V | ||
| 10 | The types of nutritional counseling provided by professionals for women with GDM include knowledge education, skill instruction, and individualized advice, and written educational materials should be distributed; nutritional counseling includes topics such as weight control, carbohydrate counting, Palma rule, healthy food types, healthy cooking methods, dietary records, food label reading, and physical activity [26,27,45,57,59,65,66,72,77]. | I | ||
| 11 | Women with GDM should receive at least three nutritional counseling sessions before delivery: (1) the 1st session (60~90 min) takes place after the diagnosis of GDM to assess and develop a dietary management plan; (2) the 2nd session (30~45 min) takes place within 1 week to assess and modify the plan; and (3) the 3rd session (15~45 min) takes place after 2~3 weeks to further assess and modification of the plan. Thereafter, counseling should be conducted every 2~3 weeks or as needed during pregnancy; and one counseling should be conducted 6~8 weeks after delivery [1,24,27,30,66]. | V | ||
| 12 | To assess the effectiveness of the dietary management plan, in addition to the components of the initial nutritional assessment, professionals should add the following components to the assessment at each visit for women with GDM, including but not limited to [24]: (1) daily food intake in relation to postmeal glucose readings; (2) understanding of the treatment plan for GDM; (3) weight changes compared with a previous visit; (4) self-monitoring of blood glucose records, ketone testing records (when previously recommended because of weight loss or inadequate calorie intake), and updated fetal and maternal testing or lab values. | V | ||
| 13 | The form of nutritional counseling should take into account available resources and the mother’s preferences and can be performed by telemedicine if necessary, such as telephone, e-mail, and smartphone [1,59,65,66]. | V | ||
| Energy intake | 14 | Women with GDM should control their daily energy intake, and the total daily energy intake can be determined according to pre-pregnancy BMI. In early pregnancy, pregnant women with low weight before pregnancy need 35 kcal/kg/d, pregnant women with normal weight before pregnancy need 30 kcal/kg/d, pregnant women who are overweight before pregnancy need 25 kcal/kg/d; in the middle and third trimester of pregnancy, pregnant women with low weight before pregnancy need 40 kcal/kg/d, pregnant women with normal weight before pregnancy need 35 kcal/kg/d, and pregnant women who are overweight before pregnancy need 30 kcal/kg/d [27,28,39,41,45,69,72,73]. | V | |
| 15 | Excessive restriction of energy intake (less than 1500 kcal/d) can lead to ketosis and malnutrition, which can adversely affect both the mother and the fetus; therefore, energy intake should not be less than 1600 kcal in early pregnancy, and 1800 kcal in the second and third trimesters [28,42,45,66,72]. | V | ||
| 16 | Women with GDM who are obese before pregnancy should reduce their energy intake by approximately 30% of the recommended intake, but ensure a minimum intake of 1600~1800 kcal/d to meet weight gain recommendations and not cause ketosis [66,70]. | I | ||
| 17 | The energy of breakfast, lunch, and dinner should be controlled at 10%~15%, 30%, and 30% of the total daily energy intake, respectively, and the energy of each snack can account for 5%~10% of the daily energy intake [28,65,66]. | V | ||
| Carbohydrate intake | 18 | Carbohydrate intake in women with GDM should not be less than 175 g per day [1,23,24,28,43,44,46,70]. | I | |
| 19 | Breakfast carbohydrate intake for women with GDM should be lower than lunch and dinner. Recommended carbohydrate intake per meal: 15~30 g for breakfast, 45 g for lunch, 45 g for dinner, 15~20 g for the morning snack, 15~20 g for the afternoon snack, and 15~30 g for the bedtime snack [27,38,45,46]. | V | ||
| 20 | Women with GDM should give priority to complex and diverse carbohydrates, rich in dietary fiber and low GI. For example, vegetables such as lotus root, sweet potato, taro, and yam; whole grains such as brown rice, whole-wheat flour, oatmeal, buckwheat, and corn; and miscellaneous beans such as peas, red beans, and mung beans; and avoid monosaccharides such as honey [42,44,45,46,50,66,72]. | V | ||
| Protein intake | 21 | Daily protein intake in women with GDM should not be less than 71 or 1.1 g/kg [23,24,27,28,43,44,45,66,70]. | V | |
| 22 | Women with GDM should consume equal proportions of animal and plant proteins each day. Chicken, fish, egg whites, low-fat/skimmed dairy products, legumes, and nuts are all sources of high-quality protein [43,44]. | V | ||
| Fat intake | 23 | Women with GDM should appropriately limit foods high in saturated fats, such as animal fats, red meats, egg yolks, coconut milk, full-fat dairy products, coconut oil, palm oil, fried foods, and butter [23,27,28,43,45,66,70]. | V | |
| 24 | Unsaturated fats intake in women with GDM should account for more than 1/3 of total fat energy intake, of which 90% should be monounsaturated fats, such as olive oil, canola oil, camellia oil, and peanut oil, and 10% should be polyunsaturated fats, such as sunflower oil, corn oil, soybean oil, seeds (e.g., chia seeds, flaxseeds), nuts (e.g., walnuts), and high-fat fish (e.g., salmon, tuna, sardines) [23,27,28,43,44,45,66]. | V | ||
| 25 | Women with GDM should avoid foods high in trans fats such as baked goods, cakes, snacks, cookies, chips, fried foods, processed foods, and margarine [23,28,43,45,66,72]. | V | ||
| Fiber intake | 26 | Women with GDM should consume 25~30 g (14 g/1000 kcal/d) of dietary fiber per day. Fruits, kelp, nori, oatmeal, buckwheat noodles, konjac flour, and fresh vegetables are all foods rich in dietary fiber [23,24,27,28,38,42,43,45,66,70]. | V | |
| Vitamin and mineral intake | 27 | Women with GDM should eat a varied diet, such as lean meat, chicken, fish, shrimp, low-fat/skimmed dairy products, fresh vegetables, and fruits, to ensure an adequate supply of micronutrients such as folic acid, vitamin A, vitamin B, vitamin C, vitamin D, calcium, magnesium, iron, iodine, potassium, zinc, selenium, phosphorus, and choline [1,24,28,46,66]. | V | |
| 28 | Women with food deficiencies, dependence on tobacco, alcohol, or other substances, anemia, strict vegetarianism, and poor dietary habits can meet the body’s vitamin and mineral needs with dietary supplements [24,65,66]. | V | ||
| Water intake | 29 | It is recommended that women with GDM intake three liters of water or about ten cups per day to ensure adequate hydration; fluid intake should be increased appropriately when there is increased activity or in hot environments [27,43]. | V | |
| Other dietary issues (dietary supplements, sugar-sweetened beverages, sweeteners, alcohol, coffee, and food safety) | 30 | Dietary supplements such as Omega-3 fatty acids, probiotics, and vitamin D can be used as an adjunctive management strategy for GDM [52,54,55,61,63,64,65]. | I | |
| 31 | Women with GDM should reduce or avoid sugar-sweetened beverages (e.g., soft drinks and juice drinks) [66,70]. | V | ||
| 32 | High-intensity sweeteners can be used appropriately in women with GDM. Women are advised to choose sweeteners that are approved or generally considered safe by the US Food and Drug Administration and limit their intake to the acceptable daily intake, such as saccharin, aspartame, acesulfame potassium, sucralose, neotame, and advantame. In addition, steviol glycosides and luo han guo extracts are also generally recognized as safe when consumed within the acceptable daily intake [24,46,65,66,70,72]. | V | ||
| 33 | Women with GDM are advised to abstain from alcohol intake, and those who are unable to stop drinking should receive further health counseling or behavioral treatment [24,27,43,66,72]. | V | ||
| 34 | Caffeine should be limited in women with GDM, with a recommended daily caffeine intake of <200 mg (equivalent to 1 cup of coffee or 4 cups of black tea) [27,72]. | V | ||
| 35 | Women with GDM should avoid foods such as raw meat, raw eggs, and unpasteurized milk to prevent bacterial foodborne illnesses [72]. | V | ||
| Meal arrangements | 36 | Total daily energy, carbohydrate intake, and protein intake in women with GDM should be distributed among 3 main meals (small to medium portions) and 2~3 or more snacks (e.g., between breakfast and lunch, between lunch and dinner, and at bedtime). There should be a 2~3 h interval between meals and snacks [24,25,27,28,30,38,39,42,43,44,45,46,65,66,70,72]. | V | |
| 37 | Women with GDM should eat regularly, and if a meal is missed, women are advised to test their blood glucose and choose appropriate foods to compensate [43]. | V | ||
| 38 | Women on insulin therapy should maintain consistency of carbohydrate intake at meals and snacks to facilitate insulin dose adjustment [70]. | V | ||
| Dietary patterns | 39 | Women with GDM should avoid diets that severely restrict any macronutrient, such as ketogenic diets that restrict carbohydrates, and paleolithic diets that restrict dairy products [23]. | V | |
| 40 | Women with GDM should replace high-GI foods with low-GI foods. Low GI: ≤55, medium GI: 56–69, high GI: ≥70 [1,28,31,33,43,45,50,53,65,69]. | I | ||
| 41 | There is not enough evidence to recommend a specific dietary pattern, and professionals should provide individualized dietary plans for women with GDM [58]. | I | ||
| Exercise management | Exercise assessment and counseling | 42 | Women diagnosed with GDM should receive a medical evaluation and exercise counseling by a professional to develop an exercise program that is tailored to the individual’s physical condition and preferences. The exercise program should include aspects such as type, frequency, duration, and intensity of exercise, and should be revised by the professional as the pregnancy progresses [1,25,37,43,60,67]. | I |
| 43 | Exercise is not recommended for women with absolute contraindications to exercise, and women with relative contraindications to exercise need to exercise moderately under the guidance of a professional [34,38,47,60,62,67,76]. | V | ||
| 44 | Absolute contraindications to exercise include (but are not necessarily limited to): hemodynamically significant heart conditions, restrictive lung disease, incompetent cervix/cerclage, multiple pregnancies (three or more), placenta previa after 28 weeks’ gestation, unexplained persistent vaginal bleeding, threatened preterm labor, ruptured membranes, pre-eclampsia, intrauterine growth restriction, severe anemia, uncontrolled hypertension, uncontrolled thyroid disease, and other serious cardiovascular, respiratory, or systemic disorders [1,28,34,43,67,77]. | V | ||
| 45 | Relative contraindications to exercise include (but are not necessarily limited to): recurrent pregnancy loss, gestational hypertension, a history of spontaneous preterm birth, mild/moderate cardiovascular or respiratory disease, symptomatic anemia, malnutrition, morbid obesity, eating disorder, twin pregnancy after the 28th week, orthopedic limitations, and other significant medical conditions [34,43,67]. | V | ||
| Exercise preparation | 46 | Women with GDM should exercise in a well-ventilated environment with appropriate temperature and humidity, limit exposure to ambient temperatures >32 °C, and maintain hydrotherapy pool temperatures ≤ 35 °C [28,43,67]. | V | |
| 47 | Cotton socks, jogging pants, and loose clothing are recommended for exercise [28,43]. | V | ||
| Type of exercise | 48 | Both aerobic and resistance exercises are appropriate for women with GDM, such as walking, brisk walking, stationary bicycling, swimming (no diving), modified yoga, Pilates, stretching, jogging (for previously athletically active women), exercises using elastic bands, and pelvic floor muscle exercises. Exercise should be preceded by a warm-up and concluded with stretching exercises [27,28,30,34,39,41,42,43,44,46,67,69,72,77]. | I | |
| Intensity of exercise | 49 | Moderate-intensity exercise is recommended for women with GDM [23,24,25,27,28,30,37,38,41,69]. | I | |
| 50 | Women with GDM should not exercise beyond the recommended intensity. The assessment methods of exercise intensity: (1) talk test: when exercising, women can talk but not sing, suggesting that the intensity of exercise is moderate; (2) target heart rate: when exercising, the heart rate reaches 40~59% of the heart rate range (220-age), suggesting that the exercise is moderate-intensity; (3) rating of perceived exertion: when exercising at moderate intensity, the score of the rating of perceived exertion scale should not be more than 12~14 points [1,28,43,67]. | V | ||
| Frequency of exercise | 51 | Women without contraindications to exercise should exercise at least 5 days per week, encourage daily exercise, and avoid two or more consecutive days of inactivity [25,28,34,37,38,41,43,67,75,77]. | V | |
| Duration of exercise | 52 | Women without contraindications to exercise should exercise at least 30 min per day and 150 min per week cumulatively [25,27,28,30,31,32,34,37,38,41,43,44,67,69,70,72,73]. | I | |
| 53 | The 30 min of exercise per day can be divided into several 10~15 min periods [1,42,43,72]. | V | ||
| 54 | Exercise one hour after a meal is appropriate [31,42,46,67,72]. | V | ||
| 55 | Women with GDM should avoid being sedentary and spread out long periods (≥90 min) of sedentary time [77]. | V | ||
| 56 | For women who exercise regularly before pregnancy, it is recommended to continue to maintain appropriate exercise during pregnancy, or reduce the intensity of exercise to a moderate level; for pregnant women who do not exercise regularly before pregnancy or who are overweight/obese, it is recommended that exercise during pregnancy should start from a short period (10~15 min per day) of low-intensity exercise, and then gradually reach the recommended exercise time and intensity [1,24,28,37,38,43,44,67,77]. | V | ||
| Exercise risk prevention | 57 | Inappropriate exercise may lead to adverse maternal and fetal outcomes, and women with GDM should avoid: (1) positions that cause decreased venous return and hypotension, such as supine exercise (after three months of pregnancy); (2) exercises that are prone to falls, abdominal trauma, collisions, and that requires frequent changes of direction, such as boxing, basketball, soccer, horseback riding, ice skating, surfing, and cross-country bicycling; (3) exercises that can cause excessive maternal temperature, such as hot yoga and hot Pilates; (4) exercises that adds extra load to the pelvic floor, such as heavy duty strength training, bouncing, and jumping; (5) exercises at extreme altitudes, such as scuba diving, parachute jumping, mountaineering; and (6) other strenuous exercise [1,24,28,34,43,67,72]. | V | |
| 58 | Exercise is not recommended for women with GDM who are hungry, unwell, or have an elevated body temperature [1]. | V | ||
| 59 | Women with GDM should stop exercising if they experience: high heart rate, difficulty breathing, dizziness, headache, nausea, decreased fetal movement, regular and painful contractions, vaginal bleeding, vaginal discharge, abdominal pain, back or pelvic pain, chest pain, muscle weakness that interferes with balance, or pain or swelling in the lower legs [1,28,34,43,67]. | V | ||
| 60 | Women who use insulin should be alert to exercise-induced hypoglycemia and avoid exercise in the early morning on an empty stomach without insulin injections. Women should carry cookies or candies with them during exercise and consume them promptly if they have symptoms of hypoglycemia [1,43,67,75]. | V | ||
| 61 | It is recommended that women monitor their blood sugar before and after exercise, and those with blood glucose <3.3 mmol/L or >13.9 mmol/L should stop exercising and have their urine tested for ketones [28,67]. | V | ||
| Precautions during pregnancy | 62 | Women whose blood glucose does not reach the standard after 1~2 weeks of diet plus exercise management, or those who develop starvation ketosis after dietary adjustments, increase caloric intake, resulting in blood glucose above gestational control standards; then, insulin or medication should be added promptly [28,31,33,35,36,44,45,57,66,70,72,73,76,77]. | V | |
| Postpartum | ||||
| Postpartum management | Target population | 63 | Women with GDM should continue to practice weight management, dietary modification, and regular exercise in the postnatal period, and professionals should provide them with health education on dietary modification, physical activity, and weight control [40,43,48,49,56,74]. | I |
| Benefits | 64 | Weight management during postpartum in women with GDM can: (1) improve women’s anthropometric markers such as weight, waist circumference, and hip circumference, (2) increase insulin sensitivity and reduce women’s lifelong risk of T2DM; and (3) reduce the risk of recurrence of GDM at the time of re-pregnancy [23,25,30,31,33,45,46,48,49,56,70,71,72]. | I | |
| Postpartum weight control goals | 65 | For women who were overweight or obese before pregnancy, the goal of postpartum weight management is to lose 5~7% of their pre-pregnancy weight [27,38]. | V | |
| Postpartum weight monitoring | 66 | Women with GDM should undergo blood glucose screening and lipid testing at 4~12 weeks postpartum and have their blood pressure, height, weight, BMI, waist circumference, and hip circumference measured. For those with normal blood glucose results, follow-up should be conducted once every 1~3 years thereafter; for women diagnosed with pre-diabetes and diabetes mellitus, they should be treated in the endocrinology department in time [29,40,68,74,78]. | V | |
| Dietary recommendations | 67 | (1) The proportion of coarse grains in staple foods should be appropriately increased, such as buckwheat, oats, and black rice; (2) cut back on sugar-rich foods, such as brown sugar, longan, red dates, and glutinous rice; (3) limit the intake of fat; 25~30 g of cooking oil per day is recommended; (4) ensure the intake of high-quality protein foods; and (5) limit the intake of fruit, about 250 g per day is recommended [29]. | V | |
| Exercise recommendations | 68 | Women should get out of bed as soon as possible after delivery according to their physical condition and wound recovery, puerperal health exercises can be carried out, aerobic and resistance exercises need to be gradually increased, and it is recommended to resume strenuous exercise three months after cesarean section [29,43]. | V | |
| Postpartum precautions | 69 | When preparing for a second pregnancy, women with previous GDM are advised to receive preconception care and counseling from professionals to ensure adequate nutrition, healthy weight, and glucose control before, during, and after pregnancy [26]. | V | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Wu, Y.; Li, H.; Cui, H.; Zhang, Q.; Long, T.; Zhang, Y.; Li, M. Weight Management during Pregnancy and the Postpartum Period in Women with Gestational Diabetes Mellitus: A Systematic Review and Summary of Current Evidence and Recommendations. Nutrients 2023, 15, 5022. https://doi.org/10.3390/nu15245022
Huang J, Wu Y, Li H, Cui H, Zhang Q, Long T, Zhang Y, Li M. Weight Management during Pregnancy and the Postpartum Period in Women with Gestational Diabetes Mellitus: A Systematic Review and Summary of Current Evidence and Recommendations. Nutrients. 2023; 15(24):5022. https://doi.org/10.3390/nu15245022
Chicago/Turabian StyleHuang, Jing, Yi Wu, Hua Li, Hangyu Cui, Qi Zhang, Tianxue Long, Yiyun Zhang, and Mingzi Li. 2023. "Weight Management during Pregnancy and the Postpartum Period in Women with Gestational Diabetes Mellitus: A Systematic Review and Summary of Current Evidence and Recommendations" Nutrients 15, no. 24: 5022. https://doi.org/10.3390/nu15245022
APA StyleHuang, J., Wu, Y., Li, H., Cui, H., Zhang, Q., Long, T., Zhang, Y., & Li, M. (2023). Weight Management during Pregnancy and the Postpartum Period in Women with Gestational Diabetes Mellitus: A Systematic Review and Summary of Current Evidence and Recommendations. Nutrients, 15(24), 5022. https://doi.org/10.3390/nu15245022






