A Comprehensive Review of the Effects of Glycemic Carbohydrates on the Neurocognitive Functions Based on Gut Microenvironment Regulation and Glycemic Fluctuation Control
Abstract
:1. Introduction
2. Glycemic Carbohydrate Digestion Rate and Glycemic Fluctuation Patterns
3. Susceptible Population for Diet-Induced Neurocognitive Impairments
3.1. Early and Late Life of Healthy Individuals
3.2. Patients with Metabolic Diseases
4. Microbiota Remodeling and Neurocognitive Functions
4.1. Glycemic Carbohydrate Diet-Induced Microbiota Remodeling
4.2. Microbial Metabolites and Neurocognitive Functions
4.2.1. SCFAs
4.2.2. Neurotransmitters
4.2.3. Glycerophospholipids
5. Glycemic Fluctuations and Neurocognitive Functions
5.1. Glycemic Fluctuation-Induced Glucose Metabolic Disorders
5.2. Neurotoxic Substances and Neurocognitive Functions
5.2.1. Glycometabolites
5.2.2. AGEs
5.2.3. ROS
5.2.4. Proinflammatory Cytokines
5.3. Calcium Overload and Neurocognitive Functions
5.3.1. Disrupt Energy Metabolisms Associated with Ca2+
5.3.2. Trigger Neuron Injury and Apoptosis
5.3.3. Promote Neurotoxic Protein Accumulation
5.4. Glucose Fluctuations Trigger More Severe Impairments
5.5. The Interaction between Glycemic Fluctuations and Gut Microbiota
6. Carbohydrate Dietary Strategies Beneficial for Neurocognitive Functions
6.1. RS Selection
6.2. SDS Selection
6.3. Sweetener Selection
6.4. Research Models on Diet-Induced Changes in Neuro Functions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lim, J.; Pullicin, A.J. Oral carbohydrate sensing: Beyond sweet taste. Physiol. Behav. 2019, 202, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M. Nutrition Transition and the Global Diabetes Epidemic. Curr. Diabetes Rep. 2015, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M. Personalized nutrition by prediction of glycemic responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Hasek, L.Y.; Phillips, R.J.; Zhang, G.; Kinzig, K.P.; Kim, C.Y.; Powley, T.L.; Hamaker, B.R. Dietary slowly digestible starch triggers the gut–brain axis in obese rats with accompanied reduced food intake. Mol. Nutr. Food Res. 2018, 62, 1700117. [Google Scholar] [CrossRef]
- Kroemer, G.; López-Otín, C.; Madeo, F.; de Cabo, R. Carbotoxicity-noxious effects of carbohydrates. Cell 2018, 175, 605–614. [Google Scholar] [CrossRef]
- Kim, C.; Sohn, J.-H.; Jang, M.U.; Kim, S.-H.; Choi, M.-G.; Ryu, O.-H.; Lee, S.; Choi, H.-C. Association between visit-to-visit glucose variability and cognitive function in aged type 2 diabetic patients: A cross-sectional study. PLoS ONE 2015, 10, e0132118. [Google Scholar] [CrossRef]
- Geijselaers, S.L.; Sep, S.J.; Claessens, D.; Schram, M.T.; Van Boxtel, M.P.; Henry, R.M.; Verhey, F.R.; Kroon, A.A.; Dagnelie, P.C.; Schalkwijk, C.G. The role of hyperglycemia, insulin resistance, and blood pressure in diabetes-associated differences in cognitive performance—The Maastricht Study. Diabetes Care 2017, 40, 1537–1547. [Google Scholar] [CrossRef]
- Ingwersen, J.; Defeyter, M.A.; Kennedy, D.O.; Wesnes, K.A.; Scholey, A.B. A low glycaemic index breakfast cereal preferentially prevents children’s cognitive performance from declining throughout the morning. Appetite 2007, 49, 240–244. [Google Scholar] [CrossRef]
- Smith, M.A.; Foster, J.K. The impact of a high versus a low glycaemic index breakfast cereal meal on verbal episodic memory in healthy adolescents. Nutr. Neurosci. 2008, 11, 219–227. [Google Scholar] [CrossRef]
- Mahoney, C.R.; Taylor, H.A.; Kanarek, R.B.; Samuel, P. Effect of breakfast composition on cognitive processes in elementary school children. Physiol. Behav. 2005, 85, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.A.; Gunstad, J.; Calvo, D.; Spitznagel, M.B. Higher fasting glucose is associated with poorer cognition among healthy young adults. Health Psychol. 2016, 35, 199. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Ding, N.; Ren, J.; Gu, Z.; Li, C.; Hong, Y.; Cheng, L.; Holler, T.P.; Li, Z. Maltooligosaccharide-forming amylase: Characteristics, preparation, and application. Biotechnol. Adv. 2017, 35, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-B.; Lamothe, L.M.; Rodriguez, N.E.N.; Rose, D.R.; Lee, B.-H. New insights suggest isomaltooligosaccharides are slowly digestible carbohydrates, rather than dietary fibers, at constitutive mammalian α-glucosidase levels. Food Chem. 2022, 383, 132456. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Kingman, S.; Cummings, J. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [PubMed]
- Englyst, K.N.; Englyst, H.N.; Hudson, G.J.; Cole, T.J.; Cummings, J.H. Rapidly available glucose in foods: An in vitro measurement that reflects the glycemic response. Am. J. Clin. Nutr. 1999, 69, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Jiang, B.; Cui, S.W.; Zhang, T.; Jin, Z. Slowly digestible starch—A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1642–1657. [Google Scholar] [CrossRef]
- Lee, B.-H.; Hamaker, B.R. Number of branch points in α-limit dextrins impact glucose generation rates by mammalian mucosal α-glucosidases. Carbohydr. Polym. 2017, 157, 207–213. [Google Scholar] [CrossRef]
- Kong, H.; Yu, L.; Li, C.; Ban, X.; Gu, Z.; Li, Z. Short-Clustered Maltodextrin Activates Ileal Glucose-Sensing and Induces Glucagon-like Peptide 1 Secretion to Ameliorate Glucose Homeostasis in Type 2 Diabetic Mice. J. Agric. Food Chem. 2022, 70, 12604–12619. [Google Scholar] [CrossRef]
- Lennerz, B.; Lennerz, J.K. Food addiction, high-glycemic-index carbohydrates, and obesity. Clin. Chem. 2018, 64, 64–71. [Google Scholar] [CrossRef]
- Della Pepa, G.; Vetrani, C.; Lupoli, R.; Massimino, E.; Lembo, E.; Riccardi, G.; Capaldo, B. Uncooked cornstarch for the prevention of hypoglycemic events. Crit. Rev. Food Sci. Nutr. 2022, 62, 3250–3263. [Google Scholar] [CrossRef] [PubMed]
- Nadia, J.; Bronlund, J.; Singh, R.P.; Singh, H.; Bornhorst, G.M. Structural breakdown of starch-based foods during gastric digestion and its link to glycemic response: In vivo and in vitro considerations. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2660–2698. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.H.; Liu, Q.; Yada, R.Y. Methodologies for increasing the resistant starch content of food starches: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1219–1234. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Brian, C.; Cheng, S.; Mir, H.; Amil, S.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Yun, D.; Li, L.; Zhao, W.; Li, Y.; Liu, X.; Liu, Z. Alternate-day fasting alleviates diabetes-induced glycolipid metabolism disorders: Roles of FGF21 and bile acids. J. Nutr. Biochem. 2020, 83, 108403. [Google Scholar] [CrossRef]
- Włodarek, D. Role of ketogenic diets in neurodegenerative diseases (Alzheimer’s disease and Parkinson’s disease). Nutrients 2019, 11, 169. [Google Scholar] [CrossRef]
- Atabilen, B.; Akdevelioğlu, Y. Effects of different dietary interventions in multiple sclerosis: A systematic review of evidence from 2018 to 2022. Nutr. Neurosci. 2022, 26, 1279–1291. [Google Scholar] [CrossRef]
- Janssen, H.; Kahles, F.; Liu, D.; Downey, J.; Koekkoek, L.L.; Roudko, V.; D’Souza, D.; McAlpine, C.S.; Halle, L.; Poller, W.C. Monocytes re-enter the bone marrow during fasting and alter the host response to infection. Immunity 2023, 56, 783–796. [Google Scholar] [CrossRef]
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic diets and chronic disease: Weighing the benefits against the risks. Front. Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef]
- Sünram-Lea, S.I.; Owen, L. The impact of diet-based glycaemic response and glucose regulation on cognition: Evidence across the lifespan. Proc. Nutr. Soc. 2017, 76, 466–477. [Google Scholar] [CrossRef]
- Noble, E.E.; Hsu, T.M.; Liang, J.; Kanoski, S.E. Early-life sugar consumption has long-term negative effects on memory function in male rats. Nutr. Neurosci. 2019, 22, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.E.; Kanoski, S.E. Early life exposure to obesogenic diets and learning and memory dysfunction. Curr. Opin. Behav. Sci. 2016, 9, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.E.; Olson, C.A.; Davis, E.; Tsan, L.; Chen, Y.-W.; Schade, R.; Liu, C.; Suarez, A.; Jones, R.B.; de La Serre, C. Gut microbial taxa elevated by dietary sugar disrupt memory function. Transl. Psychiatry 2021, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.M.; Konanur, V.R.; Taing, L.; Usui, R.; Kayser, B.D.; Goran, M.I.; Kanoski, S.E. Effects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent rats. Hippocampus 2015, 25, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Blok, E.; Barroso, M.; Jansen, P.W.; White, T.; Voortman, T. Dietary patterns, brain morphology and cognitive performance in children: Results from a prospective population-based study. Eur. J. Epidemiol. 2023, 38, 669–687. [Google Scholar] [CrossRef] [PubMed]
- Henn, R.E.; Elzinga, S.E.; Glass, E.; Parent, R.; Guo, K.; Allouch, A.A.; Mendelson, F.E.; Hayes, J.; Webber-Davis, I.; Murphy, G.G. Obesity-induced neuroinflammation and cognitive impairment in young adult versus middle-aged mice. Immun. Ageing 2022, 19, 67. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of brain aging: Adaptive and pathological modification by metabolic states. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef]
- Liu, L.; Volpe, S.L.; Ross, J.A.; Grimm, J.A.; Van Bockstaele, E.J.; Eisen, H.J. Dietary sugar intake and risk of Alzheimer’s disease in older women. Nutr. Neurosci. 2022, 25, 2302–2313. [Google Scholar] [CrossRef]
- Fu, J.; Qiu, W.; Zheng, H.; Qi, C.; Hu, S.; Wu, W.; Wang, H.; Wu, G.; Cao, P.; Ma, Z. Ageing trajectory of the gut microbiota is associated with metabolic diseases in a chronological age-dependent manner. Gut 2023, 72, 1431–1433. [Google Scholar] [CrossRef]
- Zhou, R.; Qian, S.; Cho, W.C.; Zhou, J.; Jin, C.; Zhong, Y.; Wang, J.; Zhang, X.; Xu, Z.; Tian, M. Microbiota-microglia connections in age-related cognition decline. Aging Cell 2022, 21, e13599. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Ida, M.; Peterson, V.L.; Prenderville, J.A.; Moloney, G.M.; Izumo, T.; Murphy, K.; Murphy, A.; Ross, R.P.; Stanton, C. Revisiting Metchnikoff: Age-related alterations in microbiota-gut-brain axis in the mouse. Brain Behav. Immun. 2017, 65, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ning, L.; Yin, Y.; Wang, R.; Zhang, Z.; Hao, L.; Wang, B.; Zhao, X.; Yang, X.; Yin, L. Age-related shifts in gut microbiota contribute to cognitive decline in aged rats. Aging 2020, 12, 7801. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, X.; Zhang, H.; Shi, R.; Hui, Y.; Jin, X.; Zhang, W.; Wang, L.; Wang, Q.; Wang, D. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 2020, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xu, P.; Jiang, Q.; Xu, Q.; Zheng, Y.; Yan, J.; Ji, H.; Ning, J.; Zhang, X.; Li, C. Depletion of acetate-producing bacteria from the gut microbiota facilitates cognitive impairment through the gut-brain neural mechanism in diabetic mice. Microbiome 2021, 9, 145. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.; Bekhit, A.E.-D.A.; Bhat, H.F. Obesity and neurological disorders: Dietary perspective of a global menace. Crit. Rev. Food Sci. Nutr. 2019, 59, 1294–1310. [Google Scholar] [CrossRef]
- Dye, L.; Boyle, N.B.; Champ, C.; Lawton, C. The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 2017, 76, 443–454. [Google Scholar] [CrossRef]
- Li, H.; Ren, J.; Li, Y.; Wu, Q.; Wei, J. Oxidative stress: The nexus of obesity and cognitive dysfunction in diabetes. Front. Endocrinol. 2023, 14, 1134025. [Google Scholar] [CrossRef]
- Cope, E.C.; LaMarca, E.A.; Monari, P.K.; Olson, L.B.; Martinez, S.; Zych, A.D.; Katchur, N.J.; Gould, E. Microglia play an active role in obesity-associated cognitive decline. J. Neurosci. 2018, 38, 8889–8904. [Google Scholar] [CrossRef]
- Bocarsly, M.E.; Fasolino, M.; Kane, G.A.; LaMarca, E.A.; Kirschen, G.W.; Karatsoreos, I.N.; McEwen, B.S.; Gould, E. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc. Natl. Acad. Sci. USA 2015, 112, 15731–15736. [Google Scholar] [CrossRef]
- Piatkowska-Chmiel, I.; Herbet, M.; Gawronska-Grzywacz, M.; Ostrowska-Lesko, M.; Dudka, J. The role of molecular and inflammatory indicators in the assessment of cognitive dysfunction in a mouse model of diabetes. Int. J. Mol. Sci. 2021, 22, 3878. [Google Scholar] [CrossRef] [PubMed]
- van der Kooij, M.A.; Jene, T.; Treccani, G.; Miederer, I.; Hasch, A.; Voelxen, N.; Walenta, S.; Müller, M.B. Chronic social stress-induced hyperglycemia in mice couples individual stress susceptibility to impaired spatial memory. Proc. Natl. Acad. Sci. USA 2018, 115, E10187–E10196. [Google Scholar] [CrossRef] [PubMed]
- Remor, A.P.; Da Silva, R.A.; de Matos, F.J.; Glaser, V.; de Paula Martins, R.; Ghisoni, K.; da Luz Scheffer, D.; Andia, D.C.; Portinho, D.; de Souza, A.P. Chronic metabolic derangement-induced cognitive deficits and neurotoxicity are associated with REST inactivation. Mol. Neurobiol. 2019, 56, 1539–1557. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Nashawaty, M.; Fatima, S.; Ennis, K.; Tkac, I. Neonatal hyperglycemia alters the neurochemical profile, dendritic arborization and gene expression in the developing rat hippocampus. NMR Biomed. 2018, 31, e3910. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, G.; Chen, D.; Ye, H.; Zeng, X. The antidiabetic effect and potential mechanisms of natural polysaccharides based on the regulation of gut microbiota. J. Funct. Foods 2020, 75, 104222. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Sun, J.; Huang, Y.; Liu, T.; Ye, Z.; Hu, J.; Zhang, G.; Chen, H.; Ye, Z. Calorie restriction ameliorates hyperglycemia, modulates the disordered gut microbiota, and mitigates metabolic endotoxemia and inflammation in type 2 diabetic rats. J. Endocrinol. Investig. 2023, 46, 699–711. [Google Scholar] [CrossRef]
- Tai, N.; Wong, F.S.; Wen, L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev. Endocr. Metab. Disord. 2015, 16, 55–65. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Modulation of gut microbiota in the management of metabolic disorders: The prospects and challenges. Int. J. Mol. Sci. 2014, 15, 4158–4188. [Google Scholar] [CrossRef]
- Walsh, S.K.; Lucey, A.; Walter, J.; Zannini, E.; Arendt, E.K. Resistant starch—An accessible fiber ingredient acceptable to the Western palate. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2930–2955. [Google Scholar] [CrossRef]
- Meenu, M.; Xu, B. A critical review on anti-diabetic and anti-obesity effects of dietary resistant starch. Crit. Rev. Food Sci. Nutr. 2019, 59, 3019–3031. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.-W.; et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 2019, 69, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.Y.; Kim, D.W.; Kim, M.J.; Choi, J.H.; Jung, H.Y.; Nam, S.M.; Kim, J.W.; Yoon, Y.S.; Choi, S.Y.; Hwang, I.K. Sodium butyrate, a histone deacetylase Inhibitor, ameliorates SIRT2-induced memory impairment, reduction of cell proliferation, and neuroblast differentiation in the dentate gyrus. Neurol. Res. 2015, 37, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Intlekofer, K.A.; Berchtold, N.C.; Malvaez, M.; Carlos, A.J.; McQuown, S.C.; Cunningham, M.J.; Wood, M.A.; Cotman, C.W. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology 2013, 38, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Laukoetter, M.G.; Bruewer, M.; Nusrat, A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr. Opin. Gastroenterol. 2006, 22, 85–89. [Google Scholar] [CrossRef] [PubMed]
- De la Cuesta-Zuluaga, J.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients 2018, 11, 51. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef]
- Milenkovic, D.; Capel, F.; Combaret, L.; Comte, B.; Dardevet, D.; Evrard, B.; Guillet, C.; Monfoulet, L.-E.; Pinel, A.; Polakof, S. Targeting the gut to prevent and counteract metabolic disorders and pathologies during aging. Crit. Rev. Food Sci. Nutr. 2023, 63, 11185–11210. [Google Scholar] [CrossRef]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Bäckhed, F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef]
- Shin, N.-R.; Lee, J.-C.; Lee, H.-Y.; Kim, M.-S.; Whon, T.W.; Lee, M.-S.; Bae, J.-W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Macchione, I.; Lopetuso, L.; Ianiro, G.; Napoli, M.; Gibiino, G.; Rizzatti, G.; Petito, V.; Gasbarrini, A.; Scaldaferri, F. Akkermansia muciniphila: Key player in metabolic and gastrointestinal disorders. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8075–8083. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.; Abbasi, A.; Somi, M.H.; Moaddab, S.Y.; Nikniaz, L.; Kafil, H.S.; Ebrahimzadeh Leylabadlo, H. Akkermansia muciniphila: From its critical role in human health to strategies for promoting its abundance in human gut microbiome. Crit. Rev. Food Sci. Nutr. 2022, 63, 7357–7377. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Lerner, A. Perspective: Prospects for nutraceutical support of intestinal barrier function. Adv. Nutr. 2021, 12, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A double-edged sword for health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-M.; Yu, R.; Zhang, L.-P.; Wen, S.-Y.; Wang, S.-J.; Zhang, X.-Y.; Xu, Q.; Kong, L.-D. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: A benefit of short-chain fatty acids. Microbiome 2019, 7, 98. [Google Scholar] [CrossRef]
- O’Leary, O.F.; Ogbonnaya, E.S.; Felice, D.; Levone, B.R.; Conroy, L.C.; Fitzgerald, P.; Bravo, J.A.; Forsythe, P.; Bienenstock, J.; Dinan, T.G. The vagus nerve modulates BDNF expression and neurogenesis in the hippocampus. Eur. Neuropsychopharmacol. 2018, 28, 307–316. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mapstone, M.; Cheema, A.K.; Fiandaca, M.S.; Zhong, X.; Mhyre, T.R.; MacArthur, L.H.; Hall, W.J.; Fisher, S.G.; Peterson, D.R.; Haley, J.M. Plasma phospholipids identify antecedent memory impairment in older adults. Nat. Med. 2014, 20, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Horrocks, L.A.; Farooqui, T. Glycerophospholipids in brain: Their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem. Phys. Lipids 2000, 106, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Solvi, C.; Zhang, F.; Qi, Z.; Chittka, L.; Zhao, W. Gut microbiome drives individual memory variation in bumblebees. Nat. Commun. 2021, 12, 6588. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
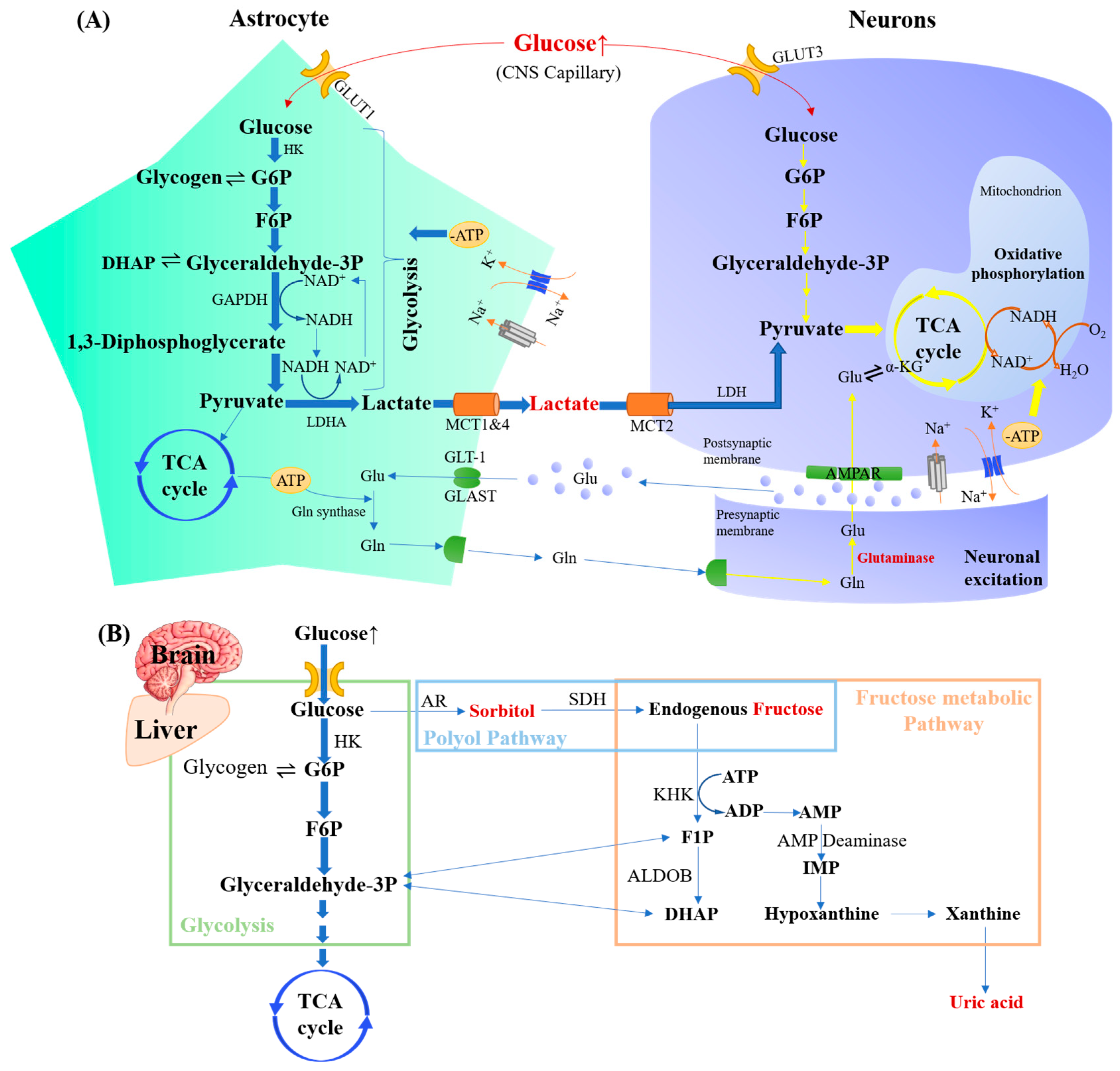
- Alberini, C.M.; Cruz, E.; Descalzi, G.; Bessières, B.; Gao, V. Astrocyte glycogen and lactate: New insights into learning and memory mechanisms. Glia 2017, 66, 1244–1262. [Google Scholar] [CrossRef]
- Sergi, D.; Renaud, J.; Simola, N.; Martinoli, M.-G. Diabetes, a contemporary risk for Parkinson’s disease: Epidemiological and cellular evidences. Front. Aging Neurosci. 2019, 11, 302. [Google Scholar] [CrossRef]
- Hubbard, J.; Binder, D. Glutamate metabolism. In Astrocytes and Epilepsy; Academic Press: San Diego, CA, USA, 2016; pp. 197–224. [Google Scholar] [CrossRef]
- Voutsinos-Porche, B.; Bonvento, G.; Tanaka, K.; Steiner, P.; Welker, E.; Chatton, J.-Y.; Magistretti, P.J.; Pellerin, L. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron 2003, 37, 275–286. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, P.; Almeida, A.; Bolaños, J.P. Brain energy metabolism in glutamate-receptor activation and excitotoxicity: Role for APC/C-Cdh1 in the balance glycolysis/pentose phosphate pathway. Neurochem. Int. 2013, 62, 750–756. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, M.; Qian, H.; Ying, H.; Li, Y.; Wang, L. Whole grains-derived functional ingredients against hyperglycemia: Targeting hepatic glucose metabolism. Crit. Rev. Food Sci. Nutr. 2023, 1–22. [Google Scholar] [CrossRef]
- Whitmer, R.A.; Gilsanz, P.; Quesenberry, C.P.; Karter, A.J.; Lacy, M.E. Association of Type 1 Diabetes and Hypoglycemic and Hyperglycemic Events and Risk of Dementia. Neurology 2021, 97, e275–e283. [Google Scholar] [CrossRef]
- Bourdel-Marchasson, I.; Schweizer, A.; Dejager, S. Incretin therapies in the management of elderly patients with type 2 diabetes mellitus. Hosp. Pract. 2011, 39, 7–21. [Google Scholar] [CrossRef]
- Mushtaq, G.; Greig, N.H.; Khan, J.A.; Kamal, M.A. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord.-Drug Targets 2014, 13, 1432–1439. [Google Scholar] [CrossRef]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.A.; Thorpe, J.E.; Testa, R.; Boemi, M.; Giugliano, D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008, 57, 1349–1354. [Google Scholar] [CrossRef]
- Shi, X.-l.; Ren, Y.-z.; Wu, J. Intermittent high glucose enhances apoptosis in INS-1 cells. Exp. Diabetes Res. 2011, 2011, 754673. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Miao, L.-F.; Qian, L.-L.; Wang, N.; Qi, M.-M.; Zhang, Y.-M.; Dang, S.-P.; Wu, Y.; Wang, R.-X. Molecular mechanisms of glucose fluctuations on diabetic complications. Front. Endocrinol. 2019, 10, 640. [Google Scholar] [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M. Resistant starch: Promise for improving human health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef]
- Owens, D.R.; Monnier, L.; Hanefeld, M. A review of glucagon-like peptide-1 receptor agonists and their effects on lowering postprandial plasma glucose and cardiovascular outcomes in the treatment of type 2 diabetes mellitus. Diabetes Obes. Metab. 2017, 19, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
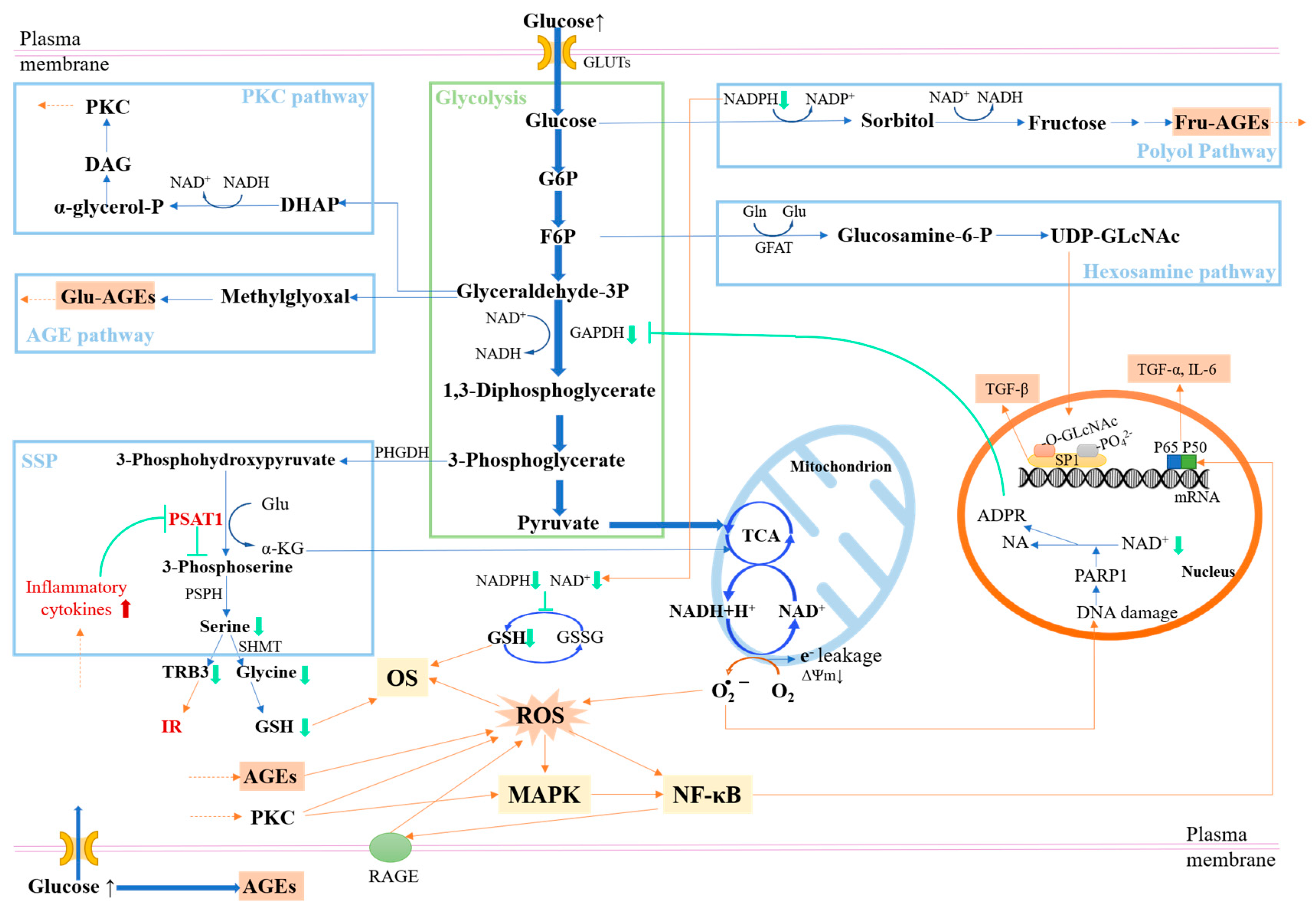
- Tomlinson, D.R.; Gardiner, N.J. Glucose neurotoxicity. Nat. Rev. Neurosci. 2008, 9, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.; Fa Va, A.; Plastino, M.; Montalcini, T.; Pujia, A. Possible implications of insulin resistance and glucose metabolism in Alzheimer’s disease pathogenesis. J. Cell. Mol. Med. 2011, 15, 1807–1821. [Google Scholar] [CrossRef]
- Ying, L.; Fei, L.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C.X. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J. Pathol. 2011, 225, 54–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, N.Q.; Yan, F.; Jin, H.; Zhou, S.Y.; Shi, J.S.; Jin, F. Diabetes mellitus and Alzheimer’s disease: GSK-3β as a potential link. Behav. Brain Res. 2017, 339, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Infante-Garcia, C.; Ramos-Rodriguez, J.J.; Galindo-Gonzalez, L.; Garcia-Alloza, M. Long-term central pathology and cognitive impairment are exacerbated in a mixed model of Alzheimer’s disease and type 2 diabetes. Psychoneuroendocrinology 2016, 65, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Bogush, M.; Heldt, N.A.; Persidsky, Y. Blood brain barrier injury in diabetes: Unrecognized effects on brain and cognition. J. Neuroimmune Pharmacol. 2017, 12, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Wang, L.; Zhang, L.; Qin, C.; Song, Y.; Ma, Y.; Chen, Y.; Chen, S.; Wang, Y.; Zhang, Z. Blood-brain barrier disruption induced cognitive impairment is associated with increase of inflammatory cytokine. Front. Aging Neurosci. 2018, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Miranda, H.V.; El-Agnaf, O.; Outeiro, T.F. Glycation in Parkinson’s disease and Alzheimer’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2016, 31, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Dong, M.; Wang, D.; Ren, M.; Zheng, Y.; Zheng, H.; Li, C.; Gao, H. Characteristic metabolic alterations identified in primary neurons under high glucose exposure. Front. Cell. Neurosci. 2018, 12, 207. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Ishimoto, T.; Li, N.; Cicerchi, C.; Orlicky, D.J.; Ruzycki, P.; Rivard, C.; Inaba, S.; Roncal-Jimenez, C.A.; Bales, E.S. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat. Commun. 2013, 4, 2434. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Jiang, L.; Hamza, M.; Dai, F.; Belfort-DeAguiar, R.; Cline, G.; Rothman, D.L.; Mason, G.; Sherwin, R.S. The human brain produces fructose from glucose. JCI Insight 2017, 2, e90508. [Google Scholar] [CrossRef]
- Lustig, R.H. Fructose: It’s “alcohol without the buzz”. Adv. Nutr. 2013, 4, 226–235. [Google Scholar] [CrossRef]
- Kendig, M.D.; Lin, C.S.; Beilharz, J.E.; Rooney, K.B.; Boakes, R.A. Maltodextrin can produce similar metabolic and cognitive effects to those of sucrose in the rat. Appetite 2014, 77, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Gomez-Pinilla, F.; Nagel, M.; Nakagawa, T.; Rodriguez-Iturbe, B.; Sanchez-Lozada, L.G.; Tolan, D.R.; Lanaspa, M.A. Cerebral fructose metabolism as a potential mechanism driving Alzheimer’s disease. Front. Aging Neurosci. 2020, 12, 560865. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.A.; Birnbaum, M.J. Molecular aspects of fructose metabolism and metabolic disease. Cell Metab. 2021, 33, 2329–2354. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Lu, W.; Gao, F.; Li, D.; Hu, J.; Li, Y.; Zuo, Z.; Jie, H.; Zhao, Y.; Cen, X. Uric acid induces cognitive dysfunction through hippocampal inflammation in rodents and humans. J. Neurosci. 2016, 36, 10990–11005. [Google Scholar] [CrossRef] [PubMed]
- Desideri, G.; Gentile, R.; Antonosante, A.; Benedetti, E.; Grassi, D.; Cristiano, L.; Manocchio, A.; Selli, S.; Ippoliti, R.; Ferri, C. Uric acid amplifies Aβ amyloid effects involved in the cognitive dysfunction/dementia: Evidences from an experimental model in vitro. J. Cell. Physiol. 2017, 232, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Yasujima, M.; Yagihashi, S. Role of advanced glycation end products in diabetic neuropathy. Curr. Pharm. Des. 2008, 14, 953–961. [Google Scholar] [CrossRef]
- Rajchgot, T.; Thomas, S.C.; Wang, J.-C.; Ahmadi, M.; Balood, M.; Crosson, T.; Dias, J.P.; Couture, R.; Claing, A.; Talbot, S. Neurons and Microglia; A Sickly-Sweet Duo in Diabetic Pain Neuropathy. Front. Neuroence 2019, 13, 25. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced glycation end-products (AGEs): Formation, chemistry, classification, receptors, and diseases related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Tups, A.; Benzler, J.; Sergi, D.; Ladyman, S.R.; Williams, L.M. Central regulation of glucose homeostasis. Compr. Physiol. 2017, 7, 741–764. [Google Scholar] [CrossRef]
- Du, X.-L.; Edelstein, D.; Rossetti, L.; Fantus, I.G.; Goldberg, H.; Ziyadeh, F.; Wu, J.; Brownlee, M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. USA 2000, 97, 12222–12226. [Google Scholar] [CrossRef]
- Lal, S.; Szwergold, B.S.; Taylor, A.H.; Randall, W.C.; Kappler, F.; Wellsknecht, K.; Baynes, J.W.; Brown, T.R. Metabolism of fructose-3-phosphate in the diabetic rat lens. Arch. Biochem. Biophys. 1995, 318, 191–199. [Google Scholar] [CrossRef]
- Sandhir, R.; Yadav, A.; Sunkaria, A.; Singhal, N. Nano-antioxidants: An emerging strategy for intervention against neurodegenerative conditions. Neurochem. Int. 2015, 89, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-m.; Sun, W.-y.; Duan, W.-j.; Gong, H.-b.; Tu, L.-f.; Li, Y.-f.; Kurihara, H.; He, R.-r. Phospholipid peroxidation: A key factor in” susceptibility” to neurodegenerative diseases. Acta Pharm. Sin. 2021, 56, 2154–2163. [Google Scholar] [CrossRef]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD (P) H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Sandireddy, R.; Yerra, V.G.; Areti, A.; Komirishetty, P.; Kumar, A. Neuroinflammation and oxidative stress in diabetic neuropathy: Futuristic strategies based on these targets. Int. J. Endocrinol. 2014, 2014, 674987. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.L.; Forbes, J.M.; Cooper, M.E. AGE, RAGE, and ROS in diabetic nephropathy. Semin. Nephrol. 2007, 27, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Snell, K. Enzymes of serine metabolism in normal, developing and neoplastic rat tissues. Adv. Enzym. Regul. 1984, 22, 325–400. [Google Scholar] [CrossRef] [PubMed]
- Ly, C.H.; Lynch, G.S.; Ryall, J.G. A metabolic roadmap for somatic stem cell fate. Cell Metab. 2020, 31, 1052–1067. [Google Scholar] [CrossRef]
- Lin, W.; Wang, M.; Chen, M.; Zheng, X.; Wu, Y.; Gao, D.; Yang, Z.; Tian, Z. Metabolomics and correlation network analyses of core biomarkers in type 2 diabetes. Amino Acids 2020, 52, 1307–1317. [Google Scholar] [CrossRef]
- Yu, J.; Xiao, F.; Guo, Y.; Deng, J.; Liu, B.; Zhang, Q.; Li, K.; Wang, C.; Chen, S.; Guo, F. Hepatic phosphoserine aminotransferase 1 regulates insulin sensitivity in mice via tribbles homolog 3. Diabetes 2015, 64, 1591–1602. [Google Scholar] [CrossRef]
- Zhang, J.; An, H.; Ni, K.; Chen, B.; Li, H.; Li, Y.; Sheng, G.; Zhou, C.; Xie, M.; Chen, S. Glutathione prevents chronic oscillating glucose intake-induced β-cell dedifferentiation and failure. Cell Death Dis. 2019, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kishore, L.; Kaur, N. Diabetic peripheral neuropathy: Current perspective and future directions. Pharmacol. Res. 2014, 80, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Brigelius-Flohé, R.; Saliou, C.; Traber, M.G.; Packer, L. Redox regulation of NF-kappa B activation. Free Radic. Biol. Med. 1997, 22, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Chan, D.C.; Lan, K.C.; Wang, C.C.; Chen, C.M.; Chao, S.C.; Tsai, K.S.; Yang, R.S.; Liu, S.H. PPARγ is involved in the hyperglycemia-induced inflammatory responses and collagen degradation in human chondrocytes and diabetic mouse cartilages. J. Orthop. Res. 2015, 33, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, Y.; Ding, L.; He, X.; Takahashi, Y.; Gao, Y.; Shen, W.; Cheng, R.; Chen, Q.; Qi, X. Pathogenic role of diabetes-induced PPAR-α down-regulation in microvascular dysfunction. Proc. Natl. Acad. Sci. USA 2013, 110, 15401–15406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Capanoglu, E.; Jiao, L.; Yin, L.; Liu, X.; Wang, R.; Xiao, J.; Lu, B. Coarse cereals modulating chronic low-grade inflammation. Crit. Rev. Food Sci. Nutr. 2022, 63, 9694–9715. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhang, D.; Chen, T.; Hu, A.; Han, X. Glycemic fluctuation exacerbates inflammation and bone loss and alters microbiota profile around implants in diabetic mice with experimental peri-implantitis. Int. J. Implant Dent. 2021, 7, 79. [Google Scholar] [CrossRef]
- Marcelo, K.L.; Means, A.R.; York, B. The Ca2+/calmodulin/CaMKK2 axis: Nature’s metabolic CaMshaft. Trends Endocrinol. Metab. 2016, 27, 706–718. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, W.; Song, Y.; Zhang, B.; Li, F.; Liu, Y. Blockade of store-operated calcium entry alleviates high glucose-induced neurotoxicity via inhibiting apoptosis in rat neurons. Chem.-Biol. Interact. 2016, 254, 63–72. [Google Scholar] [CrossRef]
- Khomula, E.V.; Viatchenko-Karpinski, V.Y.; Borisyuk, A.L.; Duzhyy, D.E.; Belan, P.V.; Voitenko, N.V. Specific functioning of Cav3. 2 T-type calcium and TRPV1 channels under different types of STZ-diabetic neuropathy. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 636–649. [Google Scholar] [CrossRef]
- Hou, X.; Pedi, L.; Diver, M.M.; Long, S.B. Crystal structure of the calcium release–activated calcium channel Orai. Science 2012, 338, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-N.; Wu, P.-F.; Zhou, J.; Guan, X.-L.; Zhang, Z.; Yang, Y.-J.; Long, L.-H.; Xie, N.; Chen, J.-G.; Wang, F. Orexin-A activates hypothalamic AMP-activated protein kinase signaling through a Ca2+-dependent mechanism involving voltage-gated L-type calcium channel. Mol. Pharmacol. 2013, 84, 876–887. [Google Scholar] [CrossRef]
- Walkon, L.L.; Strubbe-Rivera, J.O.; Bazil, J.N. Calcium Overload and Mitochondrial Metabolism. Biomolecules 2022, 12, 1891. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Saikumar, P.; Weinberg, J.M.; Venkatachalam, M.A. Calcium in cell injury and death. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 405–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, L.; He, D.; Ling, S. Endoplasmic reticulum stress-mediated hippocampal neuron apoptosis involved in diabetic cognitive impairment. BioMed Res. Int. 2013, 2013, 924327. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, M.; Yin, H. Signaling pathways involved in endoplasmic reticulum stress-induced neuronal apoptosis. Int. J. Neurosci. 2013, 123, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, E.; Oliveira, C.R.; Pereira, C.M. The release of calcium from the endoplasmic reticulum induced by amyloid-beta and prion peptides activates the mitochondrial apoptotic pathway. Neurobiol. Dis. 2008, 30, 331–342. [Google Scholar] [CrossRef]
- Qi, H.; Xu, G.; Peng, X.-L.; Li, X.; Shuai, J.; Xu, R. Roles of four feedback loops in mitochondrial permeability transition pore opening induced by Ca2+ and reactive oxygen species. Phys. Rev. E 2020, 102, 62422. [Google Scholar] [CrossRef]
- Zhu, J.; Jin, M.; Wang, J.; Zhang, H.; Wu, Y.; Li, D.; Ji, X.; Yang, H.; Yin, C.; Ren, T. TNFα induces Ca2+ influx to accelerate extrinsic apoptosis in hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. 2018, 37, 43. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; Bacskai, B.J. Mitochondria and calcium in Alzheimer’s disease: From cell signaling to neuronal cell death. Trends Neurosci. 2021, 44, 136–151. [Google Scholar] [CrossRef]
- Cheng, J.; Dong, Y.; Ma, J.; Pan, R.; Liao, Y.; Kong, X.; Li, X.; Li, S.; Chen, P.; Wang, L. Microglial Calhm2 regulates neuroinflammation and contributes to Alzheimer’s disease pathology. Sci. Adv. 2021, 7, eabe3600. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-P.; Ye, J.-W.; Wang, X.; Zhu, L.-P.; Hu, Q.-H.; Wang, Q.; Ke, D.; Tian, Q.; Wang, J.-Z. Tau-induced Ca2+/calmodulin-dependent protein kinase-IV activation aggravates nuclear tau hyperphosphorylation. Neurosci. Bull. 2018, 34, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-T.; Yeh, Y.-T.; Lin, C.-C.; Yang, L.-Y.; Chiang, C.-P. Akkermansia muciniphila is negatively correlated with hemoglobin A1c in refractory diabetes. Microorganisms 2020, 8, 1360. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, L.; Sun, L.; Ye, X.; Ma, M.; Dou, M.; Shi, L. Association Between Intestinal Prevotella copri Abundance and Glycemic Fluctuation in Patients with Brittle Diabetes. Diabetes Metab. Syndr. Obes. 2023, 16, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, B.; Yu, D.; Zhu, C. Gut microbiota: An important player in type 2 diabetes mellitus. Front. Cell. Infect. Microbiol. 2022, 12, 112. [Google Scholar] [CrossRef]
- Pajic, P.; Pavlidis, P.; Dean, K.; Neznanova, L.; Romano, R.-A.; Garneau, D.; Daugherity, E.; Globig, A.; Ruhl, S.; Gokcumen, O. Independent amylase gene copy number bursts correlate with dietary preferences in mammals. Elife 2019, 8, e44628. [Google Scholar] [CrossRef]
- Ross, A.B.; Shertukde, S.P.; Staffier, K.L.; Chung, M.; Jacques, P.F.; McKeown, N.M. The relationship between whole-grain intake and measures of cognitive decline, mood, and anxiety—A systematic review. Adv. Nutr. 2023, 14, 652–670. [Google Scholar] [CrossRef]
- Sanders, L.M.; Zhu, Y.; Wilcox, M.L.; Koecher, K.; Maki, K.C. Whole grain intake, compared to refined grain, improves postprandial glycemia and insulinemia: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 63, 5339–5357. [Google Scholar] [CrossRef]
- Ginieis, R.; Franz, E.A.; Oey, I.; Peng, M. The “sweet” effect: Comparative assessments of dietary sugars on cognitive performance. Physiol. Behav. 2018, 184, 242–247. [Google Scholar] [CrossRef]
- Kuroda, Y.; MATSUZAkI, K.; WAkATSUkI, H.; Shido, O.; Harauma, A.; Moriguchi, T.; Sugimoto, H.; Yamaguchi, S.; Yoshino, K.; Hashimoto, M. Influence of ultra-high hydrostatic pressurizing brown rice on cognitive functions and mental health of elderly Japanese individuals: A 2-year randomized and controlled trial. J. Nutr. Sci. Vitaminol. 2019, 65, S80–S87. [Google Scholar] [CrossRef]
- Shi, H.; Yu, Y.; Lin, D.; Zheng, P.; Zhang, P.; Hu, M.; Wang, Q.; Pan, W.; Yang, X.; Hu, T. β-glucan attenuates cognitive impairment via the gut-brain axis in diet-induced obese mice. Microbiome 2020, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Okahata, C.; Onozato, M.; Minami, T.; Maeshima, M.; Ogihara, K.; Yamazaki, S.; Takahashi, Y.; Nakamura, S. Buckwheat Flour and Its Starch Prevent Age-Related Cognitive Decline by Increasing Hippocampal BDNF Production in Senescence-Accelerated Mouse Prone 8 Mice. Nutrients 2022, 14, 2708. [Google Scholar] [CrossRef] [PubMed]
- Kendig, M.D.; Boakes, R.A.; Rooney, K.B.; Corbit, L.H. Chronic restricted access to 10% sucrose solution in adolescent and young adult rats impairs spatial memory and alters sensitivity to outcome devaluation. Physiol. Behav. 2013, 120, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Beilharz, J.E.; Maniam, J.; Morris, M.J. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain Behav. Immun. 2014, 37, 134–141. [Google Scholar] [CrossRef]
- Reichelt, A.C.; Killcross, S.; Hambly, L.D.; Morris, M.J.; Westbrook, R.F. Impact of adolescent sucrose access on cognitive control, recognition memory, and parvalbumin immunoreactivity. Learn. Mem. 2015, 22, 215–224. [Google Scholar] [CrossRef]
- Wu, H.-W.; Ren, L.-F.; Zhou, X.; Han, D.-W. A high-fructose diet induces hippocampal insulin resistance and exacerbates memory deficits in male Sprague-Dawley rats. Nutr. Neurosci. 2015, 18, 323–328. [Google Scholar] [CrossRef]
- Sachdeva, A.K.; Dharavath, R.N.; Chopra, K. Time-response studies on development of cognitive deficits in an experimental model of insulin resistance. Clin. Nutr. 2019, 38, 1447–1456. [Google Scholar] [CrossRef]
- Sangüesa, G.; Cascales, M.; Griñán, C.; Sánchez, R.M.; Roglans, N.; Pallàs, M.; Laguna, J.C.; Alegret, M. Impairment of novel object recognition memory and brain insulin signaling in fructose-but not glucose-drinking female rats. Mol. Neurobiol. 2018, 55, 6984–6999. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, Q.; Chang, Y.; Jia, L.; Zhai, L.; Bai, Y.; Sun, Q.; Wei, W. Learning and memory impairment and transcriptomic profile in hippocampus of offspring after maternal fructose exposure during gestation and lactation. Food Chem. Toxicol. 2022, 169, 113394. [Google Scholar] [CrossRef]
- Ediga, M.G.; Annapureddy, S.; Salikineedy, K.; Nimgampalle, M. Aspartame consumption causes cognitive impairment in streptozotocin-induced diabetic Wistar rats. Biologia 2023, 78, 2393–2407. [Google Scholar] [CrossRef]
- Tsan, L.; Chometton, S.; Hayes, A.M.; Klug, M.E.; Zuo, Y.; Sun, S.; Bridi, L.; Lan, R.; Fodor, A.A.; Noble, E.E. Early-life low-calorie sweetener consumption disrupts glucose regulation, sugar-motivated behavior, and memory function in rats. JCI Insight 2022, 7, e157714. [Google Scholar] [CrossRef] [PubMed]
- Kendig, M.D.; Fu, M.X.; Rehn, S.; Martire, S.I.; Boakes, R.A.; Rooney, K.B. Metabolic and cognitive improvement from switching to saccharin or water following chronic consumption by female rats of 10% sucrose solution. Physiol. Behav. 2018, 188, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Keenan, M.J.; Zhou, J.; Hegsted, M.; Pelkman, C.; Durham, H.A.; Coulon, D.B.; Martin, R.J. Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv. Nutr. 2015, 6, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Raatz, S.K.; Idso, L.; Johnson, L.K.; Jackson, M.I.; Combs, G.F., Jr. Resistant starch analysis of commonly consumed potatoes: Content varies by cooking method and service temperature but not by variety. Food Chem. 2016, 208, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Xu, Q.; Kong, Q.; Li, F.; Lu, L.; Xu, Y.; Wei, Y. Synthesis and Functions of Resistant Starch. Adv. Nutr. 2023, 14, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hamaker, B.R. Slowly digestible starch: Concept, mechanism, and proposed extended glycemic index. Crit. Rev. Food Sci. Nutr. 2009, 49, 852–867. [Google Scholar] [CrossRef] [PubMed]
- Fortune, N.C.; Harville, E.W.; Guralnik, J.M.; Gustat, J.; Chen, W.; Qi, L.; Bazzano, L.A. Dietary intake and cognitive function: Evidence from the Bogalusa Heart Study. Am. J. Clin. Nutr. 2019, 109, 1656–1663. [Google Scholar] [CrossRef]
- Ozawa, M.; Shipley, M.; Kivimaki, M.; Singh-Manoux, A.; Brunner, E.J. Dietary pattern, inflammation and cognitive decline: The Whitehall II prospective cohort study. Clin. Nutr. 2017, 36, 506–512. [Google Scholar] [CrossRef]
- Khan, N.A.; Raine, L.B.; Drollette, E.S.; Scudder, M.R.; Kramer, A.F.; Hillman, C.H. Dietary fiber is positively associated with cognitive control among prepubertal children. J. Nutr. 2015, 145, 143–149. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E. Microbial degradation of whole-grain complex carbohydrates and impact on short-chain fatty acids and health. Adv. Nutr. 2015, 6, 206–213. [Google Scholar] [CrossRef]
- Yu, L.; Gao, Y.; Ye, Z.; Duan, H.; Zhao, J.; Zhang, H.; Narbad, A.; Tian, F.; Zhai, Q.; Chen, W. Interaction of beta-glucans with gut microbiota: Dietary origins, structures, degradation, metabolism, and beneficial function. Crit. Rev. Food Sci. Nutr. 2023. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Wang, Z.; Hao, Y.; Bai, W.; Wang, Z.; Wang, J. 5-Heptadecylresorcinol, a Biomarker for Whole Grain Rye Consumption, Ameliorates Cognitive Impairments and Neuroinflammation in APP/PS1 Transgenic Mice. Mol. Nutr. Food Res. 2020, 64, 1901218. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Chu, Y. Whole grain oats, more than just a fiber: Role of unique phytochemicals. Mol. Nutr. Food Res. 2017, 61, 1600715. [Google Scholar] [CrossRef] [PubMed]
- Uenobe, M.; Saika, T.; Waku, N.; Ohno, M.; Inagawa, H. Effect of continuous dewaxed brown rice ingestion on the cognitive function of elderly individuals. J. Nutr. Sci. Vitaminol. 2019, 65, S122–S124. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, S.; Percaccio, E.; Gullì, M.; Romano, A.; Vitalone, A.; Mazzanti, G.; Gaetani, S.; Di Sotto, A. Recent Advances in the Neuroprotective Properties of Ferulic Acid in Alzheimer’s Disease: A Narrative Review. Nutrients 2022, 14, 3709. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.V.; Small, D.M. Physiological mechanisms by which non-nutritive sweeteners may impact body weight and metabolism. Physiol. Behav. 2015, 152, 381–388. [Google Scholar] [CrossRef]
- Hamelin, H.; Poizat, G.; Florian, C.; Kursa, M.B.; Pittaras, E.; Callebert, J.; Rampon, C.; Taouis, M.; Hamed, A.; Granon, S. Prolonged consumption of sweetened beverages lastingly deteriorates cognitive functions and reward processing in mice. Cereb. Cortex 2022, 32, 1365–1378. [Google Scholar] [CrossRef]
- Crézé, C.; Candal, L.; Cros, J.; Knebel, J.-F.; Seyssel, K.; Stefanoni, N.; Schneiter, P.; Murray, M.M.; Tappy, L.; Toepel, U. The impact of caloric and non-caloric sweeteners on food intake and brain responses to food: A randomized crossover controlled trial in healthy humans. Nutrients 2018, 10, 615. [Google Scholar] [CrossRef]
- Lohner, S.; Toews, I.; Meerpohl, J.J. Health outcomes of non-nutritive sweeteners: Analysis of the research landscape. Nutr. J. 2017, 16, 55. [Google Scholar] [CrossRef]
- Rutters, F.; Nieuwenhuizen, A.G.; Lemmens, S.G.; Born, J.M.; Westerterp-Plantenga, M.S. Acute stress-related changes in eating in the absence of hunger. Obesity 2009, 17, 72–77. [Google Scholar] [CrossRef]
- Zuker; Charles, S. Food for the brain. Cell 2015, 161, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.J.; Campbell, I.C.; Troop, N. Increases in Weight during Chronic Stress are Partially Associated with a Switch in Food Choice towards Increased Carbohydrate and Saturated Fat Intake. Eur. Eat. Disord. Rev. 2013, 22, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.P.; Mota, M.; Martins, F.O.; Nogueira, C.; Gonçalves, T.; Carneiro, T.; Pinto, J.; Duarte, D.; Barros, A.S.; Jones, J.G.; et al. Intestinal Microbial and Metabolic Profiling of Mice Fed with High-Glucose and High-Fructose Diets. J. Proteome Res. 2018, 17, 2880–2891. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Lee, E.; Oh, M.-J.; Kim, Y.; Park, H.-Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Araki, Y.; Hanzawa, F.; Umeki, M.; Kojima, T.; Nishimura, N.; Ikeda, S.; Mochizuki, S.; Oda, H. High sucrose diet-induced dysbiosis of gut microbiota promotes fatty liver and hyperlipidemia in rats. J. Nutr. Biochem. 2021, 93, 108621. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, K.; Hauck, L.; Jeffrey, B.; Elias, V.; Humphrey, A.; Nath, R.; Perrone, A.; Bermudez, L. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience 2015, 300, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Laffin, M.; Fedorak, R.; Zalasky, A.; Park, H.; Gill, A.; Agrawal, A.; Keshteli, A.; Hotte, N.; Madsen, K.L. A high-sugar diet rapidly enhances susceptibility to colitis via depletion of luminal short-chain fatty acids in mice. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- E Noble, E.; Hsu, T.M.; Jones, R.B.; A Fodor, A.; I Goran, M.; E Kanoski, S. Early-Life Sugar Consumption Affects the Rat Microbiome Independently of Obesity. J. Nutr. 2017, 147, 20–28. [Google Scholar] [CrossRef]
- Nickerson, K.P.; Homer, C.R.; Kessler, S.P.; Dixon, L.J.; Kabi, A.; Gordon, I.O.; Johnson, E.E.; de la Motte, C.A.; McDonald, C. The Dietary Polysaccharide Maltodextrin Promotes Salmonella Survival and Mucosal Colonization in Mice. PLoS ONE 2014, 9, e101789. [Google Scholar] [CrossRef]
- Nickerson, K.P.; McDonald, C. Crohn’s Disease-Associated Adherent-Invasive Escherichia coli Adhesion Is Enhanced by Exposure to the Ubiquitous Dietary Polysaccharide Maltodextrin. PLoS ONE 2012, 7, e52132. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Yu, L.; Gu, Z.; Li, C.; Ban, X.; Cheng, L.; Hong, Y.; Li, Z. Novel Short-Clustered Maltodextrin as a Dietary Starch Substitute Attenuates Metabolic Dysregulation and Restructures Gut Microbiota in db/db Mice. J. Agric. Food Chem. 2020, 68, 12400–12412. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Chen, T.; Green, S.J.; Mutlu, E.; Martin, B.R.; Rumpagaporn, P.; Patterson, J.A.; Keshavarzian, A.; Hamaker, B.R. Physical Inaccessibility of a Resistant Starch Shifts Mouse Gut Microbiota to Butyrogenic Firmicutes. Mol. Nutr. Food Res. 2019, 63, e1801012. [Google Scholar] [CrossRef] [PubMed]
- Sorndech, W.; Rodtong, S.; Blennow, A.; Tongta, S. Impact of Resistant Maltodextrins and Resistant Starch on Human Gut Microbiota and Organic Acids Production. Starch 2018, 71, 1800231. [Google Scholar] [CrossRef]
- He, B.; Nohara, K.; Ajami, N.J.; Michalek, R.D.; Tian, X.; Wong, M.; Losee-Olson, S.H.; Petrosino, J.F.; Yoo, S.-H.; Shimomura, K.; et al. Transmissible microbial and metabolomic remodeling by soluble dietary fiber improves metabolic homeostasis. Sci. Rep. 2015, 5, srep10604. [Google Scholar] [CrossRef] [PubMed]
- Guan, N.; He, X.; Wang, S.; Liu, F.; Huang, Q.; Fu, X.; Chen, T.; Zhang, B. Cell Wall Integrity of Pulse Modulates the in Vitro Fecal Fermentation Rate and Microbiota Composition. J. Agric. Food Chem. 2020, 68, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Rovalino-Córdova, A.M.; Fogliano, V.; Capuano, E. Effect of bean structure on microbiota utilization of plant nutrients: An in-vitro study using the simulator of the human intestinal microbial ecosystem (SHIME®). J. Funct. Foods 2020, 73, 104087. [Google Scholar] [CrossRef]
- Tachon, S.; Zhou, J.; Keenan, M.; Martin, R.; Marco, M.L. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol. Ecol. 2012, 83, 299–309. [Google Scholar] [CrossRef]
- Li, Z.-T.; Hu, G.-A.; Zhu, L.; Zhao, Z.-C.; Jiang, Y.; Gao, M.-J.; Zhan, X.-B. In vitro digestion and fecal fermentation of highly resistant starch rice and its effect on the gut microbiota. Food Chem. 2021, 361, 130095. [Google Scholar] [CrossRef]
- Martínez, I.; Kim, J.; Duffy, P.R.; Schlegel, V.L.; Walter, J. Resistant Starches Types 2 and 4 Have Differential Effects on the Composition of the Fecal Microbiota in Human Subjects. PLoS ONE 2010, 5, e15046. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Y.; Yan, B.; Zhao, S.; Zhou, X. Regulation of tartary buckwheat-resistant starch on intestinal microflora in mice fed with high-fat diet. Food Sci. Nutr. 2020, 8, 3243–3251. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Huang, C.; Lin, S.; Zheng, M.; Chen, C.; Zheng, B.; Zhang, Y. Lotus Seed Resistant Starch Regulates Gut Microbiota and Increases Short-Chain Fatty Acids Production and Mineral Absorption in Mice. J. Agric. Food Chem. 2017, 65, 9217–9225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, S.; Jiang, Y.; Wei, Y.; Zhou, X. Regulatory Function of Buckwheat-Resistant Starch Supplementation on Lipid Profile and Gut Microbiota in Mice Fed with a High-Fat Diet. J. Food Sci. 2019, 84, 2674–2681. [Google Scholar] [CrossRef]
- Umu, Ö.C.; A Frank, J.; Fangel, J.U.; Oostindjer, M.; da Silva, C.S.; Bolhuis, E.J.; Bosch, G.; Willats, W.G.T.; Pope, P.B.; Diep, D.B. Resistant starch diet induces change in the swine microbiome and a predominance of beneficial bacterial populations. Microbiome 2015, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, B.; Chen, T.; Li, C.; Fu, X.; Huang, Q. Chemical Cross-Linking Controls in Vitro Fecal Fermentation Rate of High-Amylose Maize Starches and Regulates Gut Microbiota Composition. J. Agric. Food Chem. 2019, 67, 13728–13736. [Google Scholar] [CrossRef]
- Upadhyaya, B.; McCormack, L.; Fardin-Kia, A.R.; Juenemann, R.; Nichenametla, S.; Clapper, J.; Specker, B.; Dey, M. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci. Rep. 2016, 6, 28797. [Google Scholar] [CrossRef]
- Qin, R.; Wang, J.; Chao, C.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. RS5 Produced More Butyric Acid through Regulating the Microbial Community of Human Gut Microbiota. J. Agric. Food Chem. 2021, 69, 3209–3218. [Google Scholar] [CrossRef]
- Zhou, Q.; Fu, X.; Dhital, S.; Zhai, H.; Huang, Q.; Zhang, B. In vitro fecal fermentation outcomes of starch-lipid complexes depend on starch assembles more than lipid type. Food Hydrocoll. 2021, 120, 106941. [Google Scholar] [CrossRef]

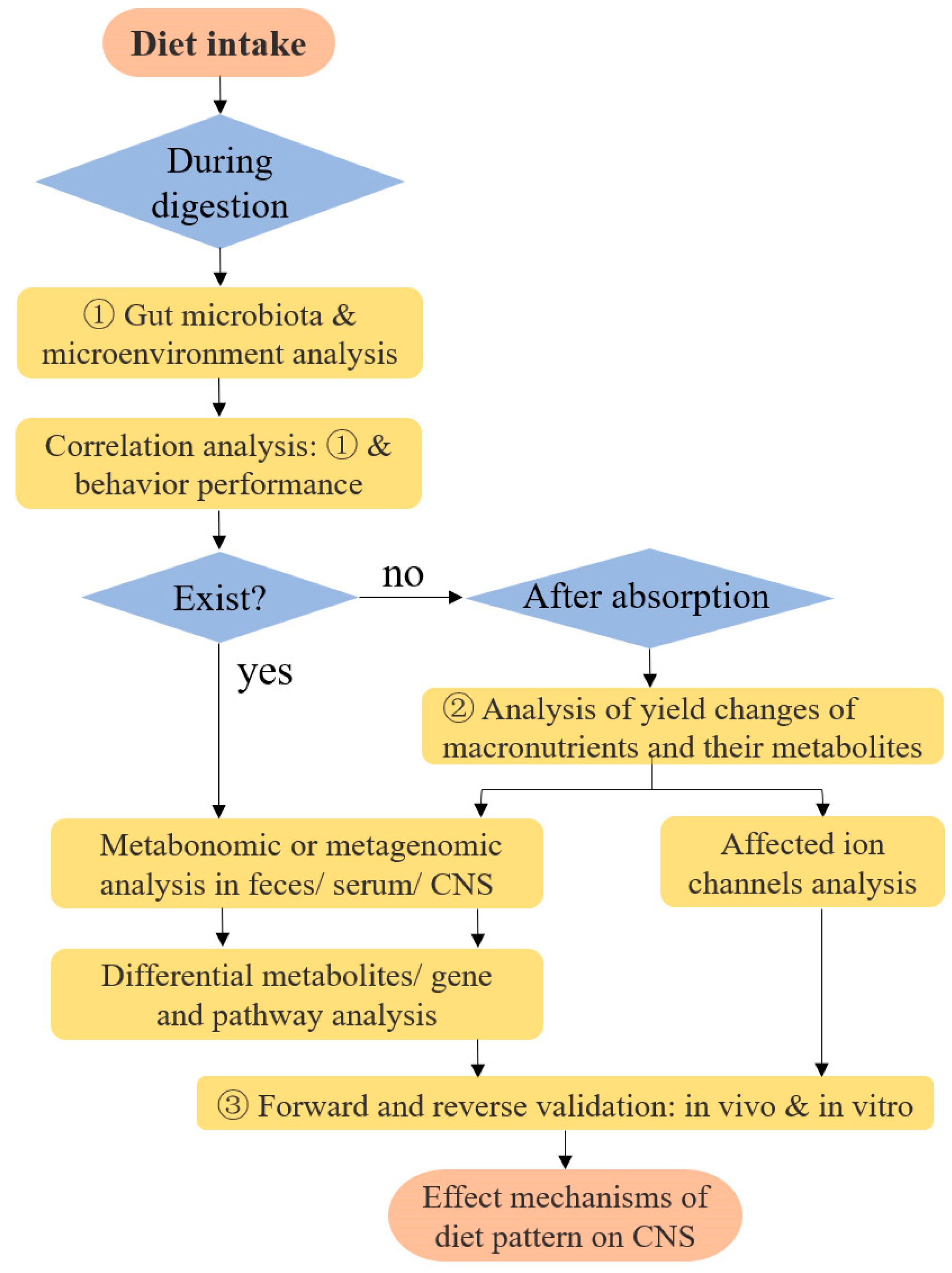

| Research Model | Diet-Induced Diseases | Control | Behavioral Tests | Outcomes and Conclusions | Ref. | |
|---|---|---|---|---|---|---|
| HUMANS | Diabetics: 40–75 years old | T2DM | People with normal glucose metabolism | Tests in 3 cognitive domains: memory/attention/information processing speed | Diabetics performed worse in all cognitive domains. It can be largely explained by hyperglycemia. | [8] |
| Diabetics: 70.9 years old | T2DM with sever glucose variability | T2DM with relatively stable blood glucose | Annually observational follow-up for 4.8 years | Cognitive functions can be influenced by glucose variability independently of mean blood glucose. | [7] | |
| Young adults: 20 years old | Obesity, prediabetes | Persons with normal glucose | Inhibitory control/sustained attention/working memory | Higher glucose levels were associated with poorer cognitive performance, especially for prediabetes. | [12] | |
| MICE | 8 weeks old | T2DM induced by STZ and 20% Fr solution | Healthy mice with standard chow and water | After 38 days intervention | There is a strong association between hyperglycemia, hyperinsulinemia, neuroinflammation, and cognitive dysfunction in T2DM mice model. | [51] |
| 4 months of age | T2DM (db/db mice): ad libitum to standard chow | db/db mice: Intermittent fasting (IF) | After 28 days of exposure | db/db: cognitive decline; db/db-IF:cognitive improved; mitochondrial biogenesis and energy metabolism gene expression in hippocampus increased; microbial metabolites re-structured. | [44] | |
| Juvenile: 5 weeks old; elderly: 1 year old | Obesity induced by HFD | Healthy mice with standard chow | After 11/24 weeks of exposure | Obesity causes a dysmetabolic phenotype in both age groups. Older age exacerbates neuroinflammatory response and cognitive decline. | [36] | |
| 8 weeks old | Obesity induced by HFD and 34% Su solution | Healthy mice with standard chow and water | After 10 weeks feeding of high-calorie diet (HFD/HSD) | HFD/HSD intervention produces obesity and cognitive decline, which is accompanied by increased microglial activation and reduced numbers of dendritic spines. | [49] | |
| 8 weeks old | Hyperglycemia induced by chronic social defeat (CSD) | Healthy mice with normal blood glucose | 3- and 5 weeks post-CSD | Hyperglycemia threatens long-term glucose homeostasis and causes spatial memory dysfunction. | [52] | |
| RATS | 5 weeks old | Hyperglycemia induced by STZ (STZ group) | Blood glucose controlled with insulin injection (STZ + insulin group) | 60 days after blood glucose control | Chronic hyperglycemia can compromise cognition by reducing hippocampal ERK signaling and inducing neurotoxicity. | [53] |
| Rat pups | Hyperglycemia induced by STZ /glucose injection | Treated with equal citrate buffer | After 10 days of glucose injection/5 days of STZ treatment | Hyperglycemia alters substrate transport, lactate homeostasis, dendritogenesis, and glutamate—glutamine cycling in the developing hippocampus. | [54] | |
| Research Model | Dietary Patterns | Control | Exposure | Behavioral Tests | Outcomes and Conclusions | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| HUMANS | Women: 50–79 years old | HSD | Usual diet (higher dietary sugar intake) | Behavioral modification training | 8.1 years | Annually observational follow-up for 15 years | An estimated increase of 10 g/day in total sugar intake was associated with an increased AD risk by 1.3–1.4%. | [39] |
| Young adults: about 23 years of age | NNS | Fr/Gl/Su solution | Sucralose solution | Instant testing | After 250 mL solution drink | Gl and Su led to poorer performance on the assessed tasks as opposed to Fr and placebo, especially under the fasting condition. | [160] | |
| (equal sweetness intensity) | ||||||||
| Healthy elderly (72.9 years old) participants | Whole grains | High hydrostatic pressurizing brown rice (UHHPBR) | Polished white rice (WR) | 24 months | After 24 months of exposure | Long-term consumption of UHHPBR increases information processing speed in the elderly, suggesting a protective effect of UHHPBR administration against age-related cognitive decline. | [161] | |
| MICE | Juvenile: 4 weeks old | Fr | High Fr diet (30% calories) | Standard chow | 12 weeks | — | High Fr feeding leads to damaged IEB, elevated serum endotoxin levels, hippocampal neuroinflammatory response, and neuronal loss. | [76] |
| 11 weeks old | Whole grains | Oat β-glucan added in HFD | HFD; Standard chow | 15 weeks | After 15 weeks of exposure | β-glucan intake can improve gut barrier function, reduce endotoxemia, and enhance cognitive function via more optimized synaptic and signaling pathways in critical brain areas. | [162] | |
| 18 weeks old | BWF | Standard chow | 15 weeks | After 26 weeks of exposure | BWF intake can suppresses cognitive decline by increasing hippocampal BDNF production in SAMP8 mice. | [163] | ||
| RATS | Adolescents: PN 21; young adults: PN 56 | Su | 10% Su solution | 0.1% sodium saccharin solution (standard chow) | 4 weeks | Adolescents: PN 55; young adults: PN 91 | Sucrose intervention can disrupt spatial cognition and reward-related behavior in the absence of obesity. | [164] |
| Adults: age not specified | 10% Su solution | Water (standard chow) | 3 weeks | After 21 days of exposure | Sucrose intervention can disrupt hippocampal-dependent place recognition memory; neuroinflammation and OS play a role in this impairment. | [165] | ||
| Adolescents: PN 28 | 10% Su solution | Water (standard chow) | 5 weeks | PN 62 | The expression of parvalbumin-immunoreactive GABAergic interneurons has decreased; both prefrontal and hippocampal functions have declined. | [166] | ||
| 8 weeks old | Fr | 10% Fr solution | Water (standard chow) | 8 months | After 8 months of exposure | High Fr diet induced peripheral IR and an abnormal insulin-signaling pathway in the hippocampus, which exacerbated memory deficits. | [167] | |
| 6 weeks old | 15% Fr solution | Water (standard chow) | 24 weeks | 7, 10, 14, 16, 18, 20, 22, and 24 weeks | IR/cognitive dysfunction appeared from 7th/20th week. Fr-induced neuroinflammation and OS impaired neuronal signaling and synaptic plasticity. | [168] | ||
| 8 weeks old | 10% Fr solution | Water (standard chow) | 7 months | After 7 months of exposure | The induced cognitive deficits are related to increased OS, hypertriglyceridemia, impaired insulin signaling, and altered mitochondrial dynamics. | [169] | ||
| Mother rats: from GD0 (gestational day) | 13%/40% Fr solution | Water (standard chow) | GD0-PN 21 (offspring) | Postnatal day 60 offspring | Maternal Fr exposure during gestation and lactation can impair cognition in offspring and affect brain function at the transcriptome level. | [170] | ||
| Adolescents: PN 30 /Adults: PN 60 | HFCS | 11% Su solution /11% HFCS-55 | Water (Low-fat chow) | 4 weeks | PN 60 /PN 90 | Adolescents: both learning and memory functions have declined; adults: no significant impact. | [34] | |
| Juvenile /Adolescent rats: PN 26 | HFCS-55 | Water (standard chow) | 4 weeks | PN 175 | HSD in early life may confer long-lasting impairments in memory function, which are not reversible by simply removing sugars from the diet. | [31] | ||
| Juvenile/ adolescents: PN 26–28 | 65% Fr + 35% Gl soluton | Water (standard chow) | 6 weeks | PN 67 | The abundance of P. distasonis and P. johnsonii has elevated; hippocampal function has declined. | [33] | ||
| Healthy/T2DM adult rats | NNS | Aspartame (ASP) solution | 0.9% NaCl | 30 days | After 30 days of oral gavage | ASP administration to healthy/diabetic rats has shown adverse effects linked to cognitive dysfunction. | [171] | |
| Adolescents: PN 25 | Acesulfame potassium/saccharin/stevia (LCS) solution | Water (standard chow) | 30 days | After 30 days of exposure | Habitual-life LCS consumption has long-lasting implications for hippocampal-dependent memory in rats. | [172] | ||
| 6–7 weeks old | Su/saccharin solution | Water (standard chow) | 10% Su: 4 weeks; Su/water/saccharin: 4 weeks | After 4–8 weeks of exposure | 4 weeks of Su exposure results in cognitive decline. Switching from Su to water or saccharin produces similar improvements on cognitive measures. | [173] | ||
| 8–11 weeks old | Maltodex -trin | 10.4% Su/maltodextrin solution | Water (standard chow) | 17 days | After 17 days of exposure | Impaired performance on a location recognition task was found in both groups. | [112] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Cheng, L.; Hong, Y.; Li, Z.; Li, C.; Ban, X.; Zhu, L.; Gu, Z. A Comprehensive Review of the Effects of Glycemic Carbohydrates on the Neurocognitive Functions Based on Gut Microenvironment Regulation and Glycemic Fluctuation Control. Nutrients 2023, 15, 5080. https://doi.org/10.3390/nu15245080
Yin J, Cheng L, Hong Y, Li Z, Li C, Ban X, Zhu L, Gu Z. A Comprehensive Review of the Effects of Glycemic Carbohydrates on the Neurocognitive Functions Based on Gut Microenvironment Regulation and Glycemic Fluctuation Control. Nutrients. 2023; 15(24):5080. https://doi.org/10.3390/nu15245080
Chicago/Turabian StyleYin, Jian, Li Cheng, Yan Hong, Zhaofeng Li, Caiming Li, Xiaofeng Ban, Ling Zhu, and Zhengbiao Gu. 2023. "A Comprehensive Review of the Effects of Glycemic Carbohydrates on the Neurocognitive Functions Based on Gut Microenvironment Regulation and Glycemic Fluctuation Control" Nutrients 15, no. 24: 5080. https://doi.org/10.3390/nu15245080
APA StyleYin, J., Cheng, L., Hong, Y., Li, Z., Li, C., Ban, X., Zhu, L., & Gu, Z. (2023). A Comprehensive Review of the Effects of Glycemic Carbohydrates on the Neurocognitive Functions Based on Gut Microenvironment Regulation and Glycemic Fluctuation Control. Nutrients, 15(24), 5080. https://doi.org/10.3390/nu15245080






