Epicutaneous Sensitization and Food Allergy: Preventive Strategies Targeting Skin Barrier Repair—Facts and Challenges
Abstract
:1. Introduction
2. The Dual Allergen Exposure Hypothesis
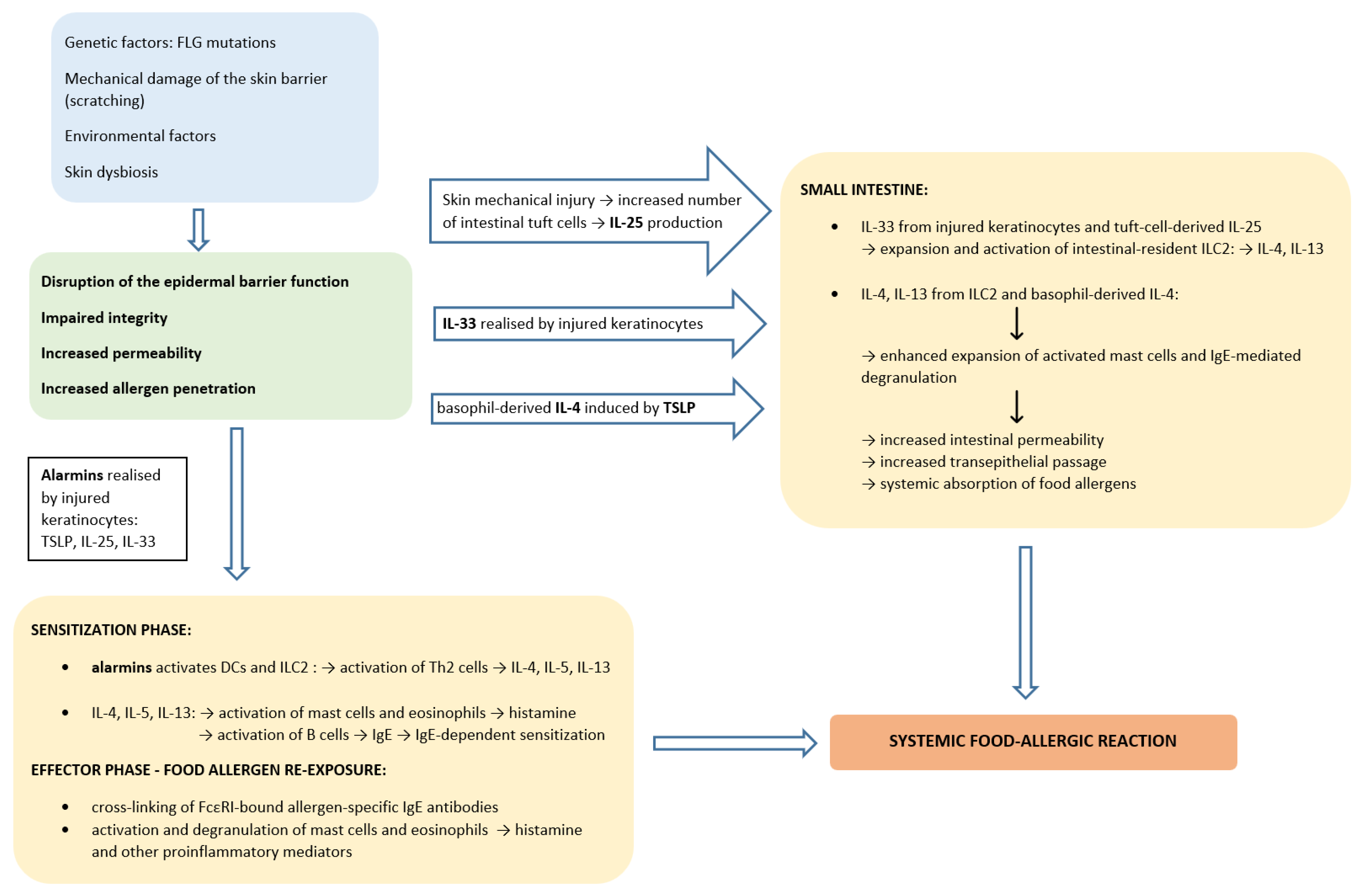
3. The Concept of Epicutaneous Food Allergen Sensitization
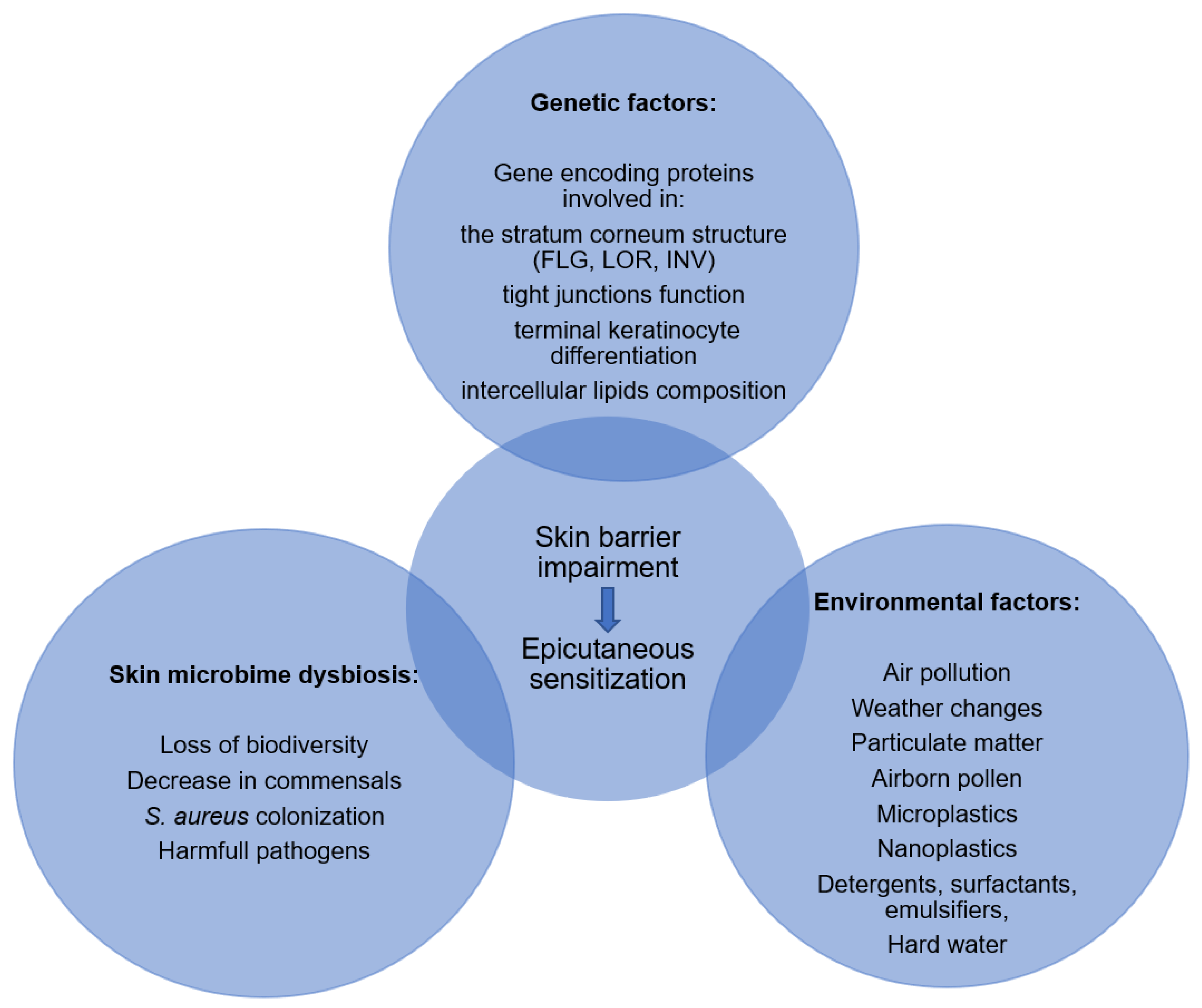
4. Factors Affecting Skin Barrier Function
4.1. Genetic Factors
4.2. Environmental Factors
4.3. Skin Microbiome Dysbiosis
5. Epicutaneous Sensitization in the Pathogenesis of Food Allergy
6. Epicutaneous Sensitization in Unaffected Skin
7. Moisturizers Therapy as Topical Intervention to Improve Skin Barrier Function
7.1. Primary Prevention
7.2. Secondary Prevention
7.3. Controversies and Future Challenges
8. New Topical Interventions Upregulating FLG Expression
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, C.M.; Jiang, J.; Gupta, R.S. Epidemiology and burden of food allergy. Curr. Allergy Asthma. Rep. 2020, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Lyons, S.A.; Clausen, M.; Knulst, A.C.; Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Barreales, L.; Bieli, C.; Dubakiene, R.; Fernandez-Perez, C.; Jedrzejczak-Czechowicz, M.; et al. Prevalence of food sensitization and food allergy in children across Europe. J. Allergy Clin. Immunol. Pract. 2020, 8, 2736–2746.e9. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Warren, C.; Smith, B.; Blumenstock, J.A.; Jiang, J.; Davis, M.M.; Nadeau, K.C. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics 2018, 142, e20181235. [Google Scholar] [CrossRef] [Green Version]
- Upton, J.; Alvaro, M.; Nadeau, K. A perspective on the pediatric death from oral food challenge reported from the Allergy Vigilance Network. Allergy 2019, 74, 1035–1036. [Google Scholar] [CrossRef] [Green Version]
- Shaker, M.S.; Schwartz, J.; Ferguson, M. An update on the impact of food allergy on anxiety and quality of life. Curr. Opin. Pediatr. 2017, 29, 497–502. [Google Scholar] [CrossRef]
- Gupta, R.; Holdford, D.; Bilaver, L.; Dyer, A.; Holl, J.L.; Meltzer, D. The Economic Impact of Childhood Food Allergy in the United States. JAM. A Pediatr. 2013, 167, 1026–1031. [Google Scholar] [CrossRef] [Green Version]
- Scott, L.A.; Jones, B.I.; Berni, T.R.; Berni, E.R.; De Vries, J.; Currie, C.J. Evaluation of the epidemiology of peanut allergy in the United Kingdom. Expert Rev. Clin. Immunol. 2019, 15, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Mersha, T.B. Genetics of Food Allergy. Immunol. Allergy Clin. North Am. 2021, 41, 301–319. [Google Scholar] [CrossRef]
- Davis, E.C.; Jackson, C.M.; Ting, T.; Harizaj, A.; Järvinen, K.M. Predictors and biomarkers of food allergy and sensitization in early childhood. Ann. Allergy Asthma Immunol. 2022, 129, 292–300. [Google Scholar] [CrossRef]
- Peters, R.L.; Mavoa, S.; Koplin, J.J. An Overview of Environmental Risk Factors for Food Allergy. Int. J. Env. Res. Public Health 2022, 19, 722. [Google Scholar] [CrossRef] [PubMed]
- Brough, H.A.; Lanser, B.J.; Sindher, S.B.; Teng, J.M.C.; Leung, D.Y.M.; Venter, C.; Chan, S.M.; Santos, A.F.; Bahnson, H.T.; Guttman-Yassky, E.; et al. Early intervention and prevention of allergic diseases. Allergy 2022, 77, 416–441. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; O’Mahony, L.; Burks, A.W.; Plaut, M.; Lack, G.; Akdis, C.A. Mechanisms of food allergy. J. Allergy Clin. Immunol. 2018, 141, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, R.L.; Neeland, M.R.; Allen, K.J. Primary Prevention of Food Allergy. Curr. Allergy Asthma Rep. 2017, 17, 52. [Google Scholar] [CrossRef]
- Brough, H.A.; Nadeau, K.C.; Sindher, S.B.; Alkotob, S.S.; Chan, S.; Bahnson, H.T.; Leung, D.Y.M.; Lack, G. Epicutaneous sensitization in the development of food allergy: What is the evidence and how can this be prevented? Allergy 2020, 75, 2185–2205. [Google Scholar] [CrossRef]
- Lack, G. Epidemiologic risks for food allergy. J. Allergy Clin. Immunol. 2008, 121, 1331–1336. [Google Scholar] [CrossRef]
- Lack, G. Update on risk factors for food allergy. J. Allergy Clin. Immunol. 2012, 129, 1187–1197. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Szajewska, H.; Lack, G. Food allergy and the gut. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 241–257. [Google Scholar] [CrossRef]
- de Silva, D.; Halken, S.; Singh, C.; Muraro, A.; Angier, E.; Arasi, S.; Arshad, H.; Beyer, K.; Boyle, R.; du Toit, G.; et al. European Academy of Allergy, Clinical Immunology Food Allergy, Anaphylaxis Guidelines Group. Preventing food allergy in infancy and childhood: Systematic review of randomised controlled trials. Pediatr. Allergy Immunol. 2020, 31, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Pabst, O.; Mowat, A.M. Oral tolerance to food protein. Mucosal. Immunol. 2012, 5, 232–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Larsen, V.; Ierodiakonou, D.; Jarrold, K.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Ruparelia, A.; Devani, P.; Trivella, M.; et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: A systematic review and meta-analysis. PLoS Med. 2018, 15, e1002507. [Google Scholar] [CrossRef]
- Perkin, M.R.; Logan, K.; Tseng, A.; Raji, B.; Ayis, S.; Peacock, J.; Brough, H.; Marrs, T.; Radulovic, S.; Craven, J.; et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N. Engl. J. Med. 2016, 374, 1733–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obbagy, J.E.; English, L.K.; Wong, Y.P.; Butte, N.F.; Dewey, K.G.; Fleischer, D.M.; Fox, M.K.; Greer, F.R.; Krebs, N.F.; Scanlon, K.S.; et al. Complementary feeding and food allergy, atopic dermatitis/eczema, asthma, and allergic rhinitis: A systematic review. Am. J. Clin. Nutr 2019, 109, 890S–934S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, J.A.; Dharmage, S.C.; Allen, K.; Koplin, J.; Garcia-Larsen, V.; Boyle, R.; Waidyatillake, N.; Lodge, C.J. Age at introduction to complementary solid food and food allergy and sensitization: A systematic review and meta-analysis. Clin. Exp. Allergy 2019, 49, 754–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trogen, B.; Jacobs, S.; Nowak-Wegrzyn, A. Early Introduction of Allergenic Foods and the Prevention of Food Allergy. Nutrients 2022, 14, 2565. [Google Scholar] [CrossRef]
- Feeney, M.; Du Toit, G.; Roberts, G.; Sayre, P.H.; Lawson, K.; Bahnson, H.T.; Sever, M.L.; Radulovic, S.; Plaut, M.; Lack, G.; et al. Impact of peanut consumption in the LEAP Study: Feasibility, growth, and nutrition. J. Allergy Clin. Immunol. 2016, 138, 1108–1118. [Google Scholar] [CrossRef] [Green Version]
- du Toit, G.; Sayre, P.H.; Roberts, G.; Lawson, K.; Sever, M.L.; Bahnson, H.T.; Fisher, H.R.; Feeney, M.; Radulovic, S.; Basting, M.; et al. Allergen specificity of early peanut consumption and effect on development of allergic disease in the Learning Early About Peanut Allergy study cohort. J. Allergy Clin. Immunol. 2018, 141, 1343–1353. [Google Scholar] [CrossRef] [Green Version]
- Natsume, O.; Kabashima, S.; Nakazato, J.; Yamamoto-Hanada, K.; Narita, M.; Kondo, M.; Saito, M.; Kishino, A.; Takimoto, T.; Inoue, E.; et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): A randomised, double-blind, placebo-controlled trial. Lancet 2017, 389, 276–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, D.J.; Metcalfe, J.; Makrides, M.; Gold, M.S.; Quinn, P.; West, C.E.; Loh, R.; Prescott, S.L. Early regular egg exposure in infants with eczema: A randomized controlled trial. J. Allergy Clin. Immunol. 2013, 132, 387–392.e1. [Google Scholar] [CrossRef] [Green Version]
- Wei-Liang Tan, J.; Valerio, C.; Barnes, E.H.; Turner, P.J.; Van Asperen, P.A.; Kakakios, A.M.; Campbell, D.E.; Beating Egg Allergy Trial (BEAT) Study Group. A randomized trial of egg introduction from 4 months of age in infants at risk for egg allergy. J. Allergy Clin. Immunol. 2017, 139, 1621–1628.e8. [Google Scholar] [CrossRef] [Green Version]
- Bellach, J.; Schwarz, V.; Ahrens, B.; Trendelenburg, V.; Aksünger, Ö.; Kalb, B.; Niggemann, B.; Keil, T.; Beyer, K. Randomized placebo-controlled trial of hen’s egg consumption for primary prevention in infants. J. Allergy Clin. Immunol. 2017, 139, 1591–1599.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, R.L.; Koplin, J.J.; Dharmage, S.C.; Tang, M.L.K.; McWilliam, V.L.; Gurrin, L.C.; Neeland, M.R.; Lowe, A.J.; Ponsonby, A.L.; Allen, K.J. Early Exposure to Cow’s Milk Protein Is Associated with a Reduced Risk of Cow’s Milk Allergic Outcomes. J. Allergy Clin. Immunol. Pract. 2019, 7, 462–470.e1. [Google Scholar] [CrossRef] [PubMed]
- Urashima, M.; Mezawa, H.; Okuyama, M.; Urashima, T.; Hirano, D.; Gocho, N.; Tachimoto, H. Primary Prevention of Cow’s Milk Sensitization and Food Allergy by Avoiding Supplementation With Cow’s Milk Formula at Birth: A Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Fisher, H.R.; Du Toit, G.; Bahnson, H.T.; Lack, G. The challenges of preventing food allergy: Lessons learned from LEAP and EAT. Ann. Allergy Asthma Immunol. 2018, 121, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Voorheis, P.; Bell, S.; Cornelsen, L.; Quaife, M.; Logan, K.; Marrs, T.; Radulovic, S.; Craven, J.; Flohr, C.; Lack, G.; et al. Challenges experienced with early introduction and sustained consumption of allergenic foods in the Enquiring About Tolerance (EAT) study: A qualitative analysis. J. Allergy Clin. Immunol. 2019, 144, 1615–1623. [Google Scholar] [CrossRef] [Green Version]
- Sakihara, T.; Otsuji, K.; Arakaki, Y.; Hamada, K.; Sugiura, S.; Ito, K. Early Discontinuation of Cow’s Milk Protein Ingestion Is Associated with the Development of Cow’s Milk Allergy. J. Allergy Clin. Immunol. Pract. 2022, 10, 172–179. [Google Scholar] [CrossRef]
- Keet, C.; Pistiner, M.; Plesa, M.; Szelag, D.; Shreffler, W.; Wood, R.; Dunlop, J.; Peng, R.; Dantzer, J.; Togias, A. Age and eczema severity, but not family history, are major risk factors for peanut allergy in infancy. J. Allergy Clin. Immunol. 2021, 147, 984–991.e5. [Google Scholar] [CrossRef]
- Walker, M.T.; Green, J.E.; Ferrie, R.P.; Queener, A.M.; Kaplan, M.H.; Cook-Mills, J.M. Mechanism for initiation of food allergy: Dependence on skin barrier mutations and environmental allergen costimulation. J. Allergy Clin. Immunol. 2018, 141, 1711–1725.e9. [Google Scholar] [CrossRef] [Green Version]
- Cook-Mills, J.M.; Emmerson, L.N. Epithelial barrier regulation, antigen sampling, and food allergy. J. Allergy Clin. Immunol. 2022, 150, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Tsakok, T.; Marrs, T.; Mohsin, M.; Baron, S.; du Toit, G.; Till, S.; Flohr, C. Does atopic dermatitis cause food allergy? A systematic review. J. Allergy Clin. Immunol. 2016, 137, 1071–1078. [Google Scholar] [CrossRef] [Green Version]
- Perkin, M.R.; Logan, K.; Bahnson, H.T.; Marrs, T.; Radulovic, S.; Craven, J.; Flohr, C.; Mills, E.N.; Versteeg, S.A.; van Ree, R.; et al. Efficacy of the Enquiring About Tolerance (EAT) study among infants at high risk of developing food allergy. J. Allergy Clin. Immunol. 2019, 144, 1606–1614.e2. [Google Scholar] [CrossRef] [Green Version]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Plaut, M.; Bahnson, H.T.; Mitchell, H.; Radulovic, S.; Chan, S.; Fox, A.; Turcanu, V.; et al. Identifying infants at high risk of peanut allergy: The Learning Early About Peanut Allergy (LEAP) screening study. J. Allergy Clin. Immunol. 2013, 131, 135–143.e1–12. [Google Scholar] [CrossRef] [PubMed]
- Flohr, C.; Perkin, M.; Logan, K.; Marrs, T.; Radulovic, S.; Campbell, L.E.; MacCallum, S.F.; McLean, W.H.I.; Lack, G. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J. Investig. Dermatol. 2014, 134, 345–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, P.E.; Eckert, J.K.; Koplin, J.J.; Lowe, A.J.; Gurrin, L.C.; Dharmage, S.C.; Vuillermin, P.; Tang, M.L.; Ponsonby, A.L.; Matheson, M.; et al. HealthNuts Study Investigators. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clin. Exp. Allergy 2015, 45, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Eller, E.; Kjaer, H.F.; Høst, A.; Andersen, K.E.; Bindslev-Jensen, C. Food allergy and food sensitization in early childhood: Results from the DARC cohort. Allergy 2009, 64, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, D.; Soto-Ramírez, N.; Kurukulaaratchy, R.J.; Holloway, J.W.; Karmaus, W.; Ewart, S.L.; Arshad, S.H.; Erlewyn-Lajeunesse, M. Filaggrin loss-of-function mutations are associated with food allergy in childhood and adolescence. J. Allergy Clin. Immunol. 2014, 134, 876–882.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, A.J.; Abramson, M.J.; Hosking, C.S.; Carlin, J.B.; Bennett, C.M.; Dharmage, S.C.; Hill, D.J. The temporal sequence of allergic sensitization and onset of infantile eczema. Clin. Exp. Allergy 2007, 37, 536–542. [Google Scholar] [CrossRef] [PubMed]
- van Splunter, M.; Liu, L.; van Neerven, R.J.J.; Wichers, H.J.; Hettinga, K.A.; de Jong, N.W. Mechanisms Underlying the Skin-Gut Cross Talk in the Development of IgE-Mediated Food Allergy. Nutrients 2020, 12, 3830. [Google Scholar] [CrossRef]
- Yoshida, T.; Beck, L.A.; De Benedetto, A. Skin barrier defects in atopic dermatitis: From old idea to new opportunity. Allergol. Int. 2022, 71, 3–13. [Google Scholar] [CrossRef]
- Leung, D.Y.M.; Berdyshev, E.; Goleva, E. Cutaneous barrier dysfunction in allergic diseases. J. Allergy Clin. Immunol. 2020, 145, 1485–1497. [Google Scholar] [CrossRef]
- Wickett, R.R.; Visscher, M.O. Structure and function of the epidermal barrier. Am. J. Infect. Control 2009, 34, S98–S110. [Google Scholar] [CrossRef]
- Elias, P.M. Skin barrier function. Curr. Allergy Asthma Rep. 2008, 8, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Darlenski, R.; Kazandjieva, J.; Tsankov, N. Skin barrier function: Morphological basis and regulatory mechanisms. J. Clin. Med. 2011, 4, 36–45. [Google Scholar]
- Lefèvre-Utile, A.; Braun, C.; Haftek, M.; Aubin, F. Five Functional Aspects of the Epidermal Barrier. Int. J. Mol. Sci 2021, 22, 11676. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, S.; Eyerich, K.; Traidl-Hoffmann, C.; Biedermann, T. Cutaneous Barriers and Skin Immunity: Differentiating A Connected Network. Trends Immunol. 2018, 39, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Jeong, S.K.; Ahn, S.K. An update of the defensive barrier function of skin. Yonsei Med. J. 2006, 47, 293–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Werfel, T.; Allam, J.P.; Biedermann, T.; Eyerich, K.; Gilles, S.; Guttman-Yassky, E.; Hoetzenecker, W.; Knol, E.; Simon, H.U.; Wollenberg, A.; et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 336–349. [Google Scholar] [CrossRef] [Green Version]
- Humeniuk, P.; Dubiela, P.; Hoffmann-Sommergruber, K. Dendritic cells and their role in allergy: Uptake, proteolytic processing and presentation of allergens. Int. J. Mol. Sci. 2017, 18, 1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomares, O.; Akdis, M.; Martín-Fontecha, M.; Akdis, C.A. Mechanisms of immune regulation in allergic diseases: The role of regulatory T and B cells. Immunol. Rev. 2017, 278, 219–236. [Google Scholar] [CrossRef]
- Pasha, M.A.; Patel, G.; Hopp, R.; Yang, Q. Role of innate lymphoid cells in allergic diseases. Allergy Asthma Proc. 2019, 40, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, Y.; Pan, J.; Liu, N.; Qin, Y.; Qiu, L.; Liu, M.; Wang, T. The Role of Type 2 Innate Lymphoid Cells in Allergic Diseases. Front. Immunol. 2021, 12, 586078. [Google Scholar] [CrossRef]
- Satitsuksanoa, P.; Daanje, M.; Akdis, M.; Boyd, S.D.; van de Veen, W. Biology and dynamics of B cells in the context of IgE-mediated food allergy. Allergy 2021, 76, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Tordesillas, L.; Berin, M.C.; Sampson, H.A. Immunology of Food Allergy. Immunity 2017, 47, 32–50. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.M.; Turcanu, V.; Stephens, A.C.; Fox, A.T.; Grieve, A.P.; Lack, G. Cutaneous lymphocyte antigen and alpha4beta7 T-lymphocyte responses are associated with peanut allergy and tolerance in children. Allergy 2012, 67, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Weissler, K.A.; Rasooly, M.; DiMaggio, T.; Bolan, H.; Cantave, D.; Martino, D.; Neeland, M.R.; Tang, M.L.K.; Dang, T.D.; Allen, K.J.; et al. Identification and analysis of peanut-specific effector T and regulatory T cells in children allergic and tolerant to peanut. J. Allergy Clin. Immunol. 2018, 141, 1699–1710.e7. [Google Scholar] [CrossRef] [Green Version]
- Strid, J.; Strobel, S. Skin barrier dysfunction and systemic sensitization to allergens through the skin. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Dunkin, D.; Berin, M.C.; Mayer, L. Allergic sensitization can be induced via multiple physiologic routes in an adjuvant-dependent manner. J. Allergy Clin. Immunol. 2011, 128, 1251–1258.e2. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, A.; Ito, N.; Murai, H.; Yasutomi, M.; Naiki, H.; Ohshima, Y. Skin inflammation exacerbates food allergy symptoms in epicutaneously sensitized mice. Allergy 2018, 73, 1313–1321. [Google Scholar] [CrossRef]
- Lack, G.; Fox, D.; Northstone, K.; Golding, J.; Avon Longitudinal Study of Parents and Children Study Team. Factors associated with the development of peanut allergy in childhood. N. Engl. J. Med. 2003, 348, 977–985. [Google Scholar] [CrossRef] [Green Version]
- Fukutomi, Y.; Taniguchi, M.; Nakamura, H.; Akiyama, K. Epidemiological link between wheat allergy and exposure to hydrolyzed wheat protein in facial soap. Allergy 2014, 69, 1405–1411. [Google Scholar] [CrossRef]
- Boussault, P.; Léauté-Labrèze, C.; Saubusse, E.; Maurice-Tison, S.; Perromat, M.; Roul, S.; Sarrat, A.; Taïeb, A.; Boralevi, F. Oat sensitization in children with atopic dermatitis: Prevalence, risks and associated factors. Allergy 2007, 62, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.T.; Sasieni, P.; du Toit, G.; Syed, H.; Lack, G. Household peanut consumption as a risk factor for the development of peanut allergy. J. Allergy Clin. Immunol. 2009, 123, 417–423. [Google Scholar] [CrossRef]
- Brough, H.A.; Santos, A.F.; Makinson, K.; Penagos, M.; Stephens, A.C.; Douiri, A.; Fox, A.T.; Du Toit, G.; Turcanu, V.; Lack, G. Peanut protein in household dust is related to household peanut consumption and is biologically active. J. Allergy Clin. Immunol. 2013, 132, 630–638. [Google Scholar] [CrossRef]
- Brough, H.A.; Kull, I.; Richards, K.; Hallner, E.; Söderhäll, C.; Douiri, A.; Penagos, M.; Melén, E.; Bergström, A.; Turcanu, V.; et al. Environmental peanut exposure increases the risk of peanut sensitization in high-risk children. Clin. Exp. Allergy 2018, 48, 586–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brough, H.A.; Simpson, A.; Makinson, K.; Hankinson, J.; Brown, S.; Douiri, A.; Belgrave, D.C.; Penagos, M.; Stephens, A.C.; McLean, W.H.; et al. Peanut allergy: Effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J. Allergy Clin. Immunol. 2014, 134, 867–875.e1. [Google Scholar] [CrossRef] [PubMed]
- Brough, H.A.; Liu, A.H.; Sicherer, S.; Makinson, K.; Douiri, A.; Brown, S.J.; Stephens, A.C.; Irwin McLean, W.H.; Turcanu, V.; Wood, R.A.; et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J. Allergy Clin. Immunol. 2015, 135, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Horimukai, K.; Morita, K.; Narita, M.; Kondo, M.; Kabashima, S.; Inoue, E.; Sasaki, T.; Niizeki, H.; Saito, H.; Matsumoto, K.; et al. Transepidermal water loss measurement during infancy can predict the subsequent development of atopic dermatitis regardless of filaggrin mutations. Allergol. Int. 2016, 65, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Sherenian, M.G.; Kothari, A.; Biagini, J.M.; Kroner, J.W.; Baatyrbek Kyzy, A.; Johannson, E.; Atluri, G.; He, H.; Martin, L.J.; Khurana Hershey, G.K. Sensitization to peanut, egg or pets is associated with skin barrier dysfunction in children with atopic dermatitis. Clin. Exp. Allergy 2021, 51, 666–673. [Google Scholar] [CrossRef]
- Leung, D.Y.M.; Calatroni, A.; Zaramela, L.S.; LeBeau, P.K.; Dyjack, N.; Brar, K.; David, G.; Johnson, K.; Leung, S.; Ramirez-Gama, M.; et al. The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci. Transl. Med. 2019, 11, eaav2685. [Google Scholar] [CrossRef]
- Wärnberg Gerdin, S.; Lie, A.; Asarnoj, A.; Borres, M.P.; Lødrup Carlsen, K.C.; Färdig, M.; Konradsen, J.R.; Monceyron Jonassen, C.; Olsson Mägi, C.A.; Rehbinder, E.M.; et al. Impaired skin barrier and allergic sensitization in early infancy. Allergy 2022, 77, 1464–1476. [Google Scholar] [CrossRef]
- Elias, P.M. Primary role of barrier dysfunction in the pathogenesis of atopic dermatitis. Exp. Dermatol. 2018, 27, 847–851. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.J.; Estravís, M.; García-Sánchez, A.; Dávila, I.; Isidoro-García, M.; Sanz, C. Genetics and Epigenetics of Atopic Dermatitis: An Updated Systematic Review. Genes 2020, 11, 442. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.A.R.; Vonk, J.M.; Baurecht, H.; Marenholz, I.; Tian, C.; Hoffman, J.D.; Helmer, Q.; Tillander, A.; Ullemar, V.; Lu, Y.; et al. Eleven loci with new reproducible genetic associations with allergic disease risk. J. Allergy Clin. Immunol. 2019, 143, 691–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moosbrugger-Martinz, V.; Leprince, C.; Méchin, M.-C.; Simon, M.; Blunder, S.; Gruber, R.; Dubrac, S. Revisiting the Roles of Filaggrin in Atopic Dermatitis. Int. J. Mol. Sci. 2022, 23, 5318. [Google Scholar] [CrossRef]
- Elias, M.S.; Long, H.A.; Newman, C.F.; Wilson, P.A.; West, A.; McGill, P.J.; Wu, K.C.; Donaldson, M.J.; Reynolds, N.J. Proteomic analysis of filaggrin deficiency identifies molecular signatures characteristic of atopic eczema. J. Allergy Clin. Immunol. 2017, 140, 1299–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drislane, C.; Irvine, A.D. The role of filaggrin in atopic dermatitis and allergic disease. Ann. Allergy Asthma Immunol. 2020, 124, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Kezic, S.; Kemperman, P.M.; Koster, E.S.; de Jongh, C.M.; Thio, H.B.; Campbell, L.E.; Irvine, A.D.; McLean, W.H.; Puppels, G.J.; Caspers, P.J. Loss-of-function mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum. J. Investig. Dermatol. 2008, 128, 2117–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flohr, C.; England, K.; Radulovic, S.; McLean, W.H.; Campbel, L.E.; Barker, J.; Perkin, M.; Lack, G. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. Br. J. Dermatol. 2010, 16, 1333–1336. [Google Scholar] [CrossRef]
- Tenn, M.W.; Ellis, A.K. The clinical relevance of filaggrin mutations: Effect on allergic disease. Ann. Allergy Asthma Immunol. 2016, 117, 483–489. [Google Scholar] [CrossRef]
- Gupta, J.; Margolis, D.J. Filaggrin gene mutations with special reference to atopic dermatitis. Curr. Treat. Options Allergy 2020, 7, 403–413. [Google Scholar] [CrossRef]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Carter, C.A.; Frischmeyer-Guerrerio, P.A. The Genetics of Food Allergy. Curr. Allergy Asthma Rep. 2018, 18, 2. [Google Scholar] [CrossRef]
- Astolfi, A.; Cipriani, F.; Messelodi, D.; De Luca, M.; Indio, V.; Di Chiara, C.; Giannetti, A.; Ricci, L.; Neri, I.; Patrizi, A.; et al. Filaggrin Loss-of-Function Mutations Are Risk Factors for Severe Food Allergy in Children with Atopic Dermatitis. J. Clin. Med. 2021, 10, 233. [Google Scholar] [CrossRef]
- Johansson, E.K.; Bergström, A.; Kull, I.; Lind, T.; Söderhäll, C.; van Hage, M.; Wickman, M.; Ballardini, N.; Wahlgren, C.F. IgE sensitization in relation to preschool eczema and filaggrin mutation. J. Allergy Clin. Immunol. 2017, 140, 1572–1579.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.J.; Asai, Y.; Cordell, H.J.; Campbell, L.E.; Zhao, Y.; Liao, H.; Northstone, K.; Henderson, J.; Alizadehfar, R.; Ben-Shoshan, M.; et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J. Allergy Clin. Immunol. 2011, 127, 661–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ginkel, C.D.; Flokstra-de Blok, B.M.J.; Kollen, B.J.; Kukler, J.; Koppelman, G.H.; Dubois, A.E.J. Loss-of-function variants of the filaggrin gene are associated with clinical reactivity to foods. Allergy 2015, 70, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Kalb, B.; Marenholz, I.; Jeanrenaud, A.C.S.N.; Meixner, L.; Arnau-Soler, A.; Rosillo-Salazar, O.D.; Ghauri, A.; Cibin, P.; Blümchen, K.; Schlags, R.; et al. Filaggrin loss-of-function mutations are associated with persistence of egg and milk allergy. J. Allergy Clin. Immunol. 2022, 150, 1125–1134. [Google Scholar] [CrossRef]
- Liang, Y.; Chang, C.; Lu, Q. The Genetics and Epigenetics of Atopic Dermatitis-Filaggrin and Other Polymorphisms. Clin. Rev. Allergy Immunol. 2016, 51, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Buelow, L.M.; Hoji, A.; Tat, K.; Schroeder-Carter, L.M.; Carroll, D.J.; Cook-Mills, J.M. Mechanisms for Alternaria alternata Function in the Skin During Induction of Peanut Allergy in Neonatal Mice With Skin Barrier Mutations. Front. Allergy 2021, 2, 677019. [Google Scholar] [CrossRef]
- Saunders, S.P.; Goh, C.S.; Brown, S.J.; Palmer, C.N.; Porter, R.M.; Cole, C.; Campbell, L.E.; Gierlinski, M.; Barton, G.J.; Schneider, G.; et al. Tmem79/Matt is the matted mouse gene and is a predisposing gene for atopic dermatitis in human subjects. J. Allergy Clin. Immunol. 2013, 132, 1121–1129. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, S.; von Buenau, B.; Vidal-y-Sy, S.; Hafek, M.; Wladykovski, E.; Houdek, P.; Lezius, S.; Duplan, H.; Bäsler, K.; Dähnhardt-Pfeifer, S.; et al. Claudin-1 decrease impacts epidermal barrier function in atopic dermatitis lesions dose-dependently. Sci. Rep. 2020, 10, 2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brewer, M.G.; Anderson, E.A.; Pandya, R.P.; De Benedetto, A.; Yoshida, T.; Hilimire, T.A.; Martinez-Sobrido, L.; Beck, L.A.; Miller, B.L. Peptides Derived from the Tight Junction Protein CLDN1 Disrupt the Skin Barrier and Promote Responsiveness to an Epicutaneous Vaccine. J. Investig. Dermatol. 2020, 140, 361–369.e3. [Google Scholar] [CrossRef]
- Zaniboni, M.C.; Samorano, L.P.; Orfali, R.L.; Aoki, V. Skin barrier in atopic dermatitis: Beyond filaggrin. Bras Dermatol. 2016, 91, 472–478. [Google Scholar] [CrossRef]
- Brown, S.J. What Have We Learned from GWAS for Atopic Dermatitis? J. Investig. Dermatol. 2021, 141, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Tamari, M.; Hirota, T. Genome-wide association studies of atopic dermatitis. J. Dermatol. 2014, 41, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahara, T.; Kido-Nakahara, M.; Tsuji, G.; Furue, M. Basics and recent advances in the pathophysiology of atopic dermatitis. J. Dermatol. 2021, 48, 130–139. [Google Scholar] [CrossRef]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thyssen, J.P.; Kezic, S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 792–799. [Google Scholar] [CrossRef]
- Celebi Sozener, Z.; Ozdel Ozturk, B.; Cerci, P.; Turk, M.; Gorgulu Akin, B.; Akdis, M.; Altiner, S.; Ozbey, U.; Ogulur, I.; Mitamura, Y.; et al. Epithelial barrier hypothesis: Effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy 2022, 77, 1418–1449. [Google Scholar] [CrossRef]
- To, T.; Zhu, J.; Stieb, D.; Gray, N.; Fong, I.; Pinault, L.; Jerrett, M.; Robichaud, A.; Ménard, R.; van Donkelaar, A.; et al. Early life exposure to air pollution and incidence of childhood asthma, allergic rhinitis and eczema. Eur. Respir. J. 2020, 55, 1900913. [Google Scholar] [CrossRef] [PubMed]
- Patella, V.; Florio, G.; Palmieri, M.; Bousquet, J.; Tonacci, A.; Giuliano, A.; Gangemi, S. Atopic dermatitis severity during exposure to air pollutants and weather changes with an Artificial Neural Network (ANN) analysis. Pediatr. Allergy Immunol. 2020, 31, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.; Lee, J.; Ahn, K.; Kim, J.; Kim, Y.M.; Sun Sim, C.; Kim, Y. Association between particulate matter concentration and symptoms of atopic dermatitis in children living in an industrial urban area of South Korea. Environ. Res. 2018, 160, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kim, J.; Jung, K.; Eo, S.; Ahn, K. The effects of particulate matter on atopic dermatitis symptoms are influenced by weather type: Application of spatial synoptic classification (SSC). Int. J. Hyg. Environ. Health 2018, 221, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Dijkhoff, I.M.; Drasler, B.; Karakocak, B.B.; Petri-Fink, A.; Valacchi, G.; Eeman, M.; Rothen-Rutishauser, B. Impact of airborne particulate matter on skin: A systematic review from epidemiology to in vitro studies. Part. Fibre Toxicol. 2020, 17, 35. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Park, D.; Lee, Y.; Lee, Y.-C. Systematic Review and Meta-Analysis of Human Skin Diseases Due to Particulate Matter. Int. J. Environ. Res. Public Health 2017, 14, 1458. [Google Scholar] [CrossRef] [Green Version]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Ryu, Y.S.; Hyun, Y.J.; Shilnikova, K.; Zhen, A.X.; Jeong, J.W.; Choi, Y.H.; Kang, H.K.; et al. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch. Toxicol. 2018, 92, 2077–2091. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.E.; Kim, J.; Goleva, E.; Berdyshev, E.; Lee, J.; Vang, K.A.; Lee, U.H.; Han, S.; Leung, S.; Hall, C.F.; et al. Particulate matter causes skin barrier dysfunction. JCI Insight 2021, 6, e145185. [Google Scholar] [CrossRef]
- Yee, M.S.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Wong, S.F.; Kok, Y.Y.; Tan, B.K.; Wong, C.Y.; Leong, C.O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Wright, C.; Iyer, A.K.; Wang, L.; Wu, N.; Yakisich, J.S.; Rojanasakul, Y.; Azad, N. Effects of titanium dioxide nanoparticles on human keratinocytes. Drug Chem. Toxicol. 2017, 40, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Cork, M.J.; Danby, S.G.; Vasilopoulos, Y.; Hadgraft, J.; Lane, M.E.; Moustafa, M.; Guy, R.H.; Macgowan, A.L.; Tazi-Ahnini, R.; Ward, S.J. Epidermal barrier dysfunction in atopic dermatitis. J. Investig. Dermatol. 2009, 129, 1892–1908. [Google Scholar] [CrossRef] [PubMed]
- Tanzer, J.; Meng, D.; Ohsaki, A.; Caldwell, J.M.; Mingler, M.K.; Rothenberg, M.E.; Oyoshi, M.K. Laundry detergent promotes allergic skin inflammation and esophageal eosinophilia in mice. PLoS ONE 2022, 17, e0268651. [Google Scholar] [CrossRef] [PubMed]
- Leoty-Okombi, S.; Gillaizeau, F.; Leuillet, S.; Douillard, B.; Le Fresne-Languille, S.; Carton, T.; De Martino, A.; Moussou, P.; Bonnaud-Rosaye, C.; André, V. Effect of Sodium Lauryl Sulfate (SLS) Applied as a Patch on Human Skin Physiology and Its Microbiota. Cosmetics 2021, 8, 6. [Google Scholar] [CrossRef]
- Xian, M.; Wawrzyniak, P.; Rückert, B.; Duan, S.; Meng, Y.; Sokolowska, M.; Globinska, A.; Zhang, L.; Akdis, M.; Akdis, C.A. Anionic surfactants and commercial detergents decrease tight junction barrier integrity in human keratinocytes. J. Allergy Clin. Immunol. 2016, 138, 890–893.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosrowpour, Z.; Ahmad Nasrollahi, S.; Ayatollahi, A.; Samadi, A.; Firooz, A. Effects of four soaps on skin trans-epidermal water loss and erythema index. J. Cosmet. Dermatol. 2019, 18, 857–861. [Google Scholar] [CrossRef]
- Guertler, A.; Moellhoff, N.; Schenck, T.L.; Hagen, C.S.; Kendziora, B.; Giunta, R.E.; French, L.E.; Reinholz, M. Onset of occupational hand eczema among healthcare workers during the SARS-CoV-2 pandemic: Comparing a single surgical site with a COVID-19 intensive care unit. Contact Dermat. 2020, 83, 108–114. [Google Scholar] [CrossRef]
- Kendziora, B.; Guertler, A.; Ständer, L.; Frey, S.; French, L.E.; Wollenberg, A.; Reinholz, M. Evaluation of hand hygiene and onset of hand eczema after the outbreak of SARS-CoV-2 in Munich. Eur. J. Dermatol. 2020, 30, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Jabbar-Lopez, Z.K.; Ung, C.Y.; Alexander, H.; Gurung, N.; Chalmers, J.; Danby, S.; Cork, M.J.; Peacock, J.L.; Flohr, C. The effect of water hardness on atopic eczema, skin barrier function: A systematic review, meta-analysis. Clin. Exp. Allergy 2021, 51, 430–451. [Google Scholar] [CrossRef]
- Perkin, M.R.; Craven, J.; Logan, K.; Strachan, D.; Marrs, T.; Radulovic, S.; Campbell, L.E.; MacCallum, S.F.; McLean, W.H.; Lack, G.; et al. Association between domestic water hardness, chlorine, and atopic dermatitis risk in early life: A population-based cross-sectional study. J. Allergy Clin. Immunol. 2016, 138, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Engebretsen, K.A.; Bager, P.; Wohlfahrt, J.; Skov, L.; Zachariae, C.; Nybo Andersen, A.M.; Melbye, M.; Thyssen, J.P. Prevalence of atopic dermatitis in infants by domestic water hardness and season of birth: Cohort study. J. Allergy Clin. Immunol. 2017, 139, 1568–1574.e1. [Google Scholar] [CrossRef] [Green Version]
- Danby, S.G.; Brown, K.; Wigley, A.M.; Chittock, J.; Pyae, P.K.; Flohr, C.; Cork, M.J. The Effect of Water Hardness on Surfactant Deposition after Washing and Subsequent Skin Irritation in Atopic Dermatitis Patients and Healthy Control Subjects. J. Investig. Dermatol. 2018, 138, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prescott, S.L.; Larcombe, D.L.; Logan, A.C.; West, C.; Burks, W.; Caraballo, L.; Levin, M.; Etten, E.V.; Horwitz, P.; Kozyrskyj, A.; et al. The skin microbiome: Impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organ. J. 2017, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.F.; Ong, R.Y.; Common, J.E. Skin microbiome of atopic dermatitis. Allergol. Int. 2022, 71, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Altunbulakli, C.; Reiger, M.; Neumann, A.U.; Garzorz-Stark, N.; Fleming, M.; Huelpuesch, C.; Castro-Giner, F.; Eyerich, K.; Akdis, C.A.; Traidl-Hoffmann, C. Relations between epidermal barrier dysregulation and Staphylococcus species-dominated microbiome dysbiosis in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2018, 142, 1643–1647.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakatsuji, T.; Gallo, R.L. The role of the skin microbiome in atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 122, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Tauber, M.; Balica, S.; Hsu, C.Y.; Jean-Decoster, C.; Lauze, C.; Redoules, D.; Viodé, C.; Schmitt, A.M.; Serre, G.; Simon, M.; et al. Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 1272–1274.e3. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, B.E.; Ahn, K.; Leung, D.Y.M. Interactions Between Atopic Dermatitis and Staphylococcus aureus Infection: Clinical Implications. Allergy Asthma Immunol. Res. 2019, 11, 593–603. [Google Scholar] [CrossRef]
- Simpson, E.L.; Villarreal, M.; Jepson, B.; Rafaels, N.; David, G.; Hanifin, J.; Taylor, P.; Boguniewicz, M.; Yoshida, T.; De Benedetto, A.; et al. Patients with Atopic Dermatitis Colonized with Staphylococcus aureus Have a Distinct Phenotype and Endotype. J. Investig. Dermatol. 2018, 138, 2224–2233. [Google Scholar] [CrossRef] [Green Version]
- Czarnowicki, T.; Krueger, J.G.; Guttman-Yassky, E. Skin barrier and immune dysregulation in atopic dermatitis: An evolving story with important clinical implications. J. Allergy Clin. Immunol. Pract. 2014, 2, 371–379, quiz 380-1. [Google Scholar] [CrossRef]
- Clausen, M.L.; Edslev, S.M.; Andersen, P.S.; Clemmensen, K.; Krogfelt, K.A.; Agner, T. Staphylococcus aureus colonization in atopic eczema and its association with filaggrin gene mutations. Br. J. Dermatol. 2017, 177, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, J.A.; Irvine, A.D.; Foster, T.J. Staphylococcus aureus and Atopic Dermatitis: A Complex and Evolving Relationship. Trends Microbiol. 2018, 26, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Blom, E.; Camberis, M.; Prout, M.; Tang, S.C.; Le Gros, G. Staphylococcal-derived superantigen enhances peanut induced Th2 responses in the skin. Clin. Exp. Allergy 2012, 42, 305–314. [Google Scholar] [CrossRef]
- Jones, A.L.; Curran-Everett, D.; Leung, D.Y.M. Food allergy is associated with Staphylococcus aureus colonization in children with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 1247–1248.e3. [Google Scholar] [CrossRef] [PubMed]
- Tsilochristou, O.; du Toit, G.; Sayre, P.H.; Roberts, G.; Lawson, K.; Sever, M.L.; Bahnson, H.T.; Radulovic, S.; Basting, M.; Plaut, M.; et al. Association of Staphylococcus aureus colonization with food allergy occurs independently of eczema severity. J. Allergy Clin. Immunol. 2019, 144, 494–503. [Google Scholar] [CrossRef]
- Galand, C.; Leyva-Castillo, J.M.; Yoon, J.; Han, A.; Lee, M.S.; McKenzie, A.N.J.; Stassen, M.; Oyoshi, M.K.; Finkelman, F.D.; Geha, R.S. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J. Allergy Clin. Immunol. 2016, 138, 1356–1366. [Google Scholar] [CrossRef] [Green Version]
- Bartnikas, L.M.; Gurish, M.F.; Burton, O.T.; Leisten, S.; Janssen, E.; Oettgen, H.C.; Beaupré, J.; Lewis, C.N.; Austen, K.F.; Schulte, S.; et al. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 451–460.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyva-Castillo, J.M.; Hener, P.; Jiang, H.; Li, M. TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J. Investig. Dermatol. 2013, 133, 154–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodoun, M.V.; Tomar, S.; Tocker, J.E.; Wang, Y.H.; Finkelman, F.D. Prevention of food allergy development and suppression of established food allergy by neutralization of thymic stromal lymphopoietin, IL-25, and IL-33. J. Allergy Clin. Immunol. 2018, 141, 171–179.e1. [Google Scholar] [CrossRef] [Green Version]
- Tamagawa-Mineoka, R.; Okuzawa, Y.; Masuda, K.; Katoh, N. Increased serum levels of interleukin 33 in patients with atopic dermatitis. J. Am. Acad Dermatol. 2014, 70, 882–888. [Google Scholar] [CrossRef]
- Imai, Y. Interleukin-33 in atopic dermatitis. J. Dermatol. Sci. 2019, 96, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Savinko, T.; Matikainen, S.; Saarialho-Kere, U.; Lehto, M.; Wang, G.; Lehtimäki, S.; Karisola, P.; Reunala, T.; Wolff, H.; Lauerma, A.; et al. IL-33 and ST2 in atopic dermatitis: Expression profiles and modulation by triggering factors. J. Investig. Dermatol. 2012, 132, 1392–1400. [Google Scholar] [CrossRef] [Green Version]
- Chinthrajah, S.; Cao, S.; Liu, C.; Lyu, S.C.; Sindher, S.B.; Long, A.; Sampath, V.; Petroni, D.; Londei, M.; Nadeau, K.C. Phase 2a randomized, placebo-controlled study of anti-IL-33 in peanut allergy. JCI Insight 2019, 4, e131347. [Google Scholar] [CrossRef] [Green Version]
- Muto, T.; Fukuoka, A.; Kabashima, K.; Ziegler, S.F.; Nakanishi, K.; Matsushita, K.; Yoshimoto, T. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. Int. Immunol. 2014, 26, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Borowczyk, J.; Shutova, M.; Brembilla, N.C.; Boehncke, W.H. IL-25 (IL-17E) in epithelial immunology and pathophysiology. J. Allergy Clin. Immunol. 2021, 148, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Cherrier, M.; Cerf-Bensussan, N. Scratching Beneath the Surface: Linking Skin Pathology with Food Allergy. Immunity 2019, 50, 1124–1126. [Google Scholar] [CrossRef] [PubMed]
- von Moltke, J.; Ji, M.; Liang, H.E.; Locksley, R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.B.; Chen, C.Y.; Liu, B.; Mugge, L.; Angkasekwinai, P.; Facchinetti, V.; Dong, C.; Liu, Y.J.; Rothenberg, M.E.; Hogan, S.P.; et al. IL-25 and CD4(+) TH2 cells enhance type 2 innate lymphoid cell-derived IL-13 production, which promotes IgE-mediated experimental food allergy. J. Allergy Clin. Immunol. 2016, 137, 1216–1225.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Benedetto, A.; Kubo, A.; Beck, L.A. Skin barrier disruption: A requirement for allergen sensitization? J. Investig. Dermatol. 2012, 132, 949–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyoshi, M.K.; Larson, R.P.; Ziegler, S.F.; Geha, R.S. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J. Allergy Clin. Immunol. 2010, 126, 976–984.e5. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, B.E.; Lee, J.; Han, Y.; Jun, H.Y.; Kim, H.; Choi, J.; Leung, D.Y.M.; Ahn, K. Epidermal thymic stromal lymphopoietin predicts the development of atopic dermatitis during infancy. J. Allergy Clin. Immunol. 2016, 137, 1282–1285.e4. [Google Scholar] [CrossRef] [Green Version]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, T.H.; Zhu, T.R.; Tran, K.A.; Sivamani, R.K.; Shi, V.Y. Epithelial barrier dysfunctions in atopic dermatitis: A skin-gut-lung model linking microbiome alteration and immune dysregulation. Br. J. Dermatol. 2018, 179, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Segaud, J.; Yao, W.; Marschall, P.; Daubeuf, F.; Lehalle, C.; German, B.; Meyer, P.; Hener, P.; Hugel, C.; Flatter, E.; et al. Context-dependent function of TSLP and IL-1β in skin allergic sensitization and atopic march. Nat. Commun. 2022, 13, 4703. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Borcard, L.; Walsh, K.P.; Pena Rodriguez, M.; Mueller, C.; Kim, B.S.; Kubo, M.; Artis, D.; Noti, M. Basophil-derived IL-4 promotes epicutaneous antigen sensitization concomitant with the development of food allergy. J. Allergy Clin. Immunol. 2018, 141, 223–234.e5. [Google Scholar] [CrossRef] [Green Version]
- Noti, M.; Kim, B.S.; Siracusa, M.C.; Rak, G.D.; Kubo, M.; Moghaddam, A.E.; Sattentau, Q.A.; Comeau, M.R.; Spergel, J.M.; Artis, D. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. J. Allergy Clin. Immunol. 2014, 133, 1390–1399.e6. [Google Scholar] [CrossRef] [Green Version]
- Smeekens, J.M.; Kulis, M.D. Mouse Models of Food Allergy in the Pursuit of Novel Treatment Modalities. Front. Allergy 2021, 2, 810067. [Google Scholar] [CrossRef]
- Tham, E.H.; Leung, D.Y. Mechanisms by Which Atopic Dermatitis Predisposes to Food Allergy and the Atopic March. Allergy Asthma Immunol. Res. 2019, 11, 4–15. [Google Scholar] [CrossRef]
- Tordesillas, L.; Goswami, R.; Benedé, S.; Grishina, G.; Dunkin, D.; Järvinen, K.M.; Maleki, S.J.; Sampson, H.A.; Berin, M.C. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J. Clin. Investig. 2014, 124, 4965–4975. [Google Scholar] [CrossRef] [Green Version]
- Yagami, A.; Aihara, M.; Ikezawa, Z.; Hide, M.; Kishikawa, R.; Morita, E.; Chinuki, Y.; Fukutomi, Y.; Urisu, A.; Fukushima, A.; et al. Outbreak of immediate-type hydrolyzed wheat protein allergy due to a facial soap in Japan. J. Allergy Clin. Immunol. 2017, 140, 879–881.e7. [Google Scholar] [CrossRef] [Green Version]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Prim. 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Del Duca, E.; Diaz, A.; Kim, H.J.; Gay-Mimbrera, J.; Zhang, N.; Wu, J.; Beaziz, J.; Estrada, Y.; Krueger, J.G.; et al. Mild atopic dermatitis lacks systemic inflammation and shows reduced nonlesional skin abnormalities. J. Allergy Clin. Immunol. 2021, 147, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Kinberger, M.; Arents, B.; Aszodi, N.; Avila Valle, G.; Barbarot, S.; Bieber, T.; Brough, H.A.; Calzavara Pinton, P.; Christen-Zäch, S.; et al. European guideline (EuroGuiDerm) on atopic eczema-part II: Non-systemic treatments and treatment recommendations for special AE patient populations. J. Eur. Acad Dermatol. Venereol. 2022, 36, 1904–1926. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-Y.; Um, J.-Y.; Chung, B.-Y.; Lee, S.-Y.; Park, J.-S.; Kim, J.-C.; Park, C.-W.; Kim, H.-O. Moisturizer in Patients with Inflammatory Skin Diseases. Medicina 2022, 58, 888. [Google Scholar] [CrossRef]
- Schachner, L.A.; Hebert, A.A.; Anneke Andriessen, A.; Benjamin, L.T.; Ana, M.; Duarte, A.M.; Goldberg, N.; Kwong, P.C.; Rico, T.S.; Eichenfield, L.F. A Global Review on the Risk Factors and Management of Early Atopic Dermatitis in Children Ages 0 to 2 Years Old. J. Drugs Dermatol. 2019, 18, 1020–1027. [Google Scholar]
- Elias, P.M. Optimizing emollient therapy for skin barrier repair in atopic dermatitis. Ann. Allergy Asthma Immunol. 2022, 128, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, J.W.; Darlenski, R.; Lachmann, N.; Baudouin, C.; Msika, P.; De Belilovsky, C.; Hachem, J.P. Infant epidermal skin physiology: Adaptation after birth. Br. J. Dermatol. 2012, 166, 483–490. [Google Scholar] [CrossRef]
- Simpson, E.L.; Chalmers, J.R.; Hanifin, J.M.; Thomas, K.S.; Cork, M.J.; McLean, W.H.; Brown, S.J.; Chen, Z.; Chen, Y.; Williams, H.C. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J. Allergy Clin. Immunol. 2014, 134, 818–823. [Google Scholar] [CrossRef] [Green Version]
- Horimukai, K.; Morita, K.; Narita, M.; Kondo, M.; Kitazawa, H.; Nozaki, M.; Shigematsu, Y.; Yoshida, K.; Niizeki, H.; Motomura, K.; et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 824–830.e6. [Google Scholar] [CrossRef] [Green Version]
- Glatz, M.; Jo, J.H.; Kennedy, E.A.; Polley, E.C.; Segre, J.A.; Simpson, E.L.; Kong, H.H. Emollient use alters skin barrier and microbes in infants at risk for developing atopic dermatitis. PLoS ONE 2018, 13, e0192443. [Google Scholar] [CrossRef] [Green Version]
- Yonezawa, K.; Haruna, M.; Matsuzaki, M.; Shiraishi, M.; Kojima, R. Effects of moisturizing skincare on skin barrier function and the prevention of skin problems in 3-month-old infants: A randomized controlled trial. J. Dermatol. 2018, 45, 24–30. [Google Scholar] [CrossRef] [PubMed]
- McClanahan, D.; Wong, A.; Kezic, S.; Samrao, A.; Hajar, T.; Hill, E.; Simpson, E.L. A randomized controlled trial of an emollient with ceramide and filaggrin-associated amino acids for the primary prevention of atopic dermatitis in high-risk infants. J. Eur. Acad Dermatol. Venereol 2019, 33, 2087–2094. [Google Scholar] [CrossRef]
- Dissanayake, E.; Tani, Y.; Nagai, K.; Sahara, M.; Mitsuishi, C.; Togawa, Y.; Suzuki, Y.; Nakano, T.; Yamaide, F.; Ohno, H.; et al. Skin Care and Synbiotics for Prevention of Atopic Dermatitis or Food Allergy in Newborn Infants: A 2 × 2 Factorial, Randomized, Non-Treatment Controlled Trial. Int. Arch. Allergy Immunol. 2019, 180, 202–211. [Google Scholar] [CrossRef]
- Skjerven, H.O.; Rehbinder, E.M.; Vettukattil, R.; LeBlanc, M.; Granum, B.; Haugen, G.; Hedlin, G.; Landrø, L.; Marsland, B.J.; Rudi, K.; et al. Skin emollient and early complementary feeding to prevent infant atopic dermatitis (PreventADALL): A factorial, multicentre, cluster-randomised trial. Lancet 2020, 395, 951–961. [Google Scholar] [CrossRef]
- Chalmers, J.R.; Haines, R.H.; Bradshaw, L.E.; Montgomery, A.A.; Thomas, K.S.; Brown, S.J.; Ridd, M.J.; Lawton, S.; Simpson, E.L.; Cork, M.J.; et al. Daily emollient during infancy for prevention of eczema: The BEEP randomised controlled trial. Lancet 2020, 395, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, L.E.; Wyatt, L.A.; Brown, S.J.; Haines, R.H.; Montgomery, A.A.; Perkin, M.R.; Lawton, S.; Sach, T.H.; Chalmers, J.R.; Ridd, M.J.; et al. Emollients for prevention of atopic dermatitis: 5-year findings from the BEEP randomized trial. [published online ahead of print, 2022 Oct 19]. Allergy, 2022; Epub ahead of print. [Google Scholar] [CrossRef]
- Skjerven, H.O.; Lie, A.; Vettukattil, R.; Rehbinder, E.M.; LeBlanc, M.; Asarnoj, A.; Carlsen, K.H.; Despriee, A.W.; Färdig, M.; Wärnberg Gerdin, S.; et al. Early food intervention and skin emollients to prevent food allergy in young children (PreventADALL): A factorial, multicentre, cluster-randomised trial. Lancet 2022, 399, 2398–2411. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.J.; Su, J.C.; Allen, K.J.; Abramson, M.J.; Cranswick, N.; Robertson, C.F.; Forster, D.; Varigos, G.; Hamilton, S.; Kennedy, R.; et al. A randomized trial of a barrier lipid replacement strategy for the prevention of atopic dermatitis and allergic sensitization: The PEBBLES pilot study. Br. J. Dermatol. 2018, 178, e19–e21. [Google Scholar] [CrossRef]
- Lowe, A.; Su, J.; Tang, M.; Lodge, C.J.; Matheson, M.; Allen, K.J.; Varigos, G.; Sasi, A.; Cranswick, N.; Hamilton, S.; et al. PEBBLES study protocol: A randomised controlled trial to prevent atopic dermatitis, food allergy and sensitisationin infants with a family history of allergic disease using a skin barrier improvement strategy. BMJ. Open 2019, 9, e024594. [Google Scholar] [CrossRef] [Green Version]
- Eichner, B.; Michaels, L.A.C.; Branca, K.; Ramsey, K.; Mitchell, J.; Morris, C.D.; Fagnan, L.J.; Dolor, R.J.; Elder, N.; Hahn, D.L.; et al. A Community-based Assessment of Skin Care, Allergies, and Eczema (CASCADE): An atopic dermatitis primary prevention study using emollients-protocol for a randomized controlled trial. Trials 2020, 21, 243. [Google Scholar] [CrossRef] [Green Version]
- Sindher, S.; Alkotob, S.S.; Shojinaga, M.N.; Brough, H.A.; Bahnson, H.T.; Chan, S.; Lack, G.; Leung, D.Y.M.; Nadeau, K.C. Pilot study measuring transepidermal water loss (TEWL) in children suggests trilipid cream is more effective than a paraffin-based emollient. Allergy 2020, 75, 2662–2664. [Google Scholar] [CrossRef]
- Sindher, S.; Alkotob, S.S.; Shojinaga, M.N.; Hamilton, R.; Chan, S.; Cao, S.; Bahnson, H.T.; Brough, H.A.; Lack, G.; Leung, D.Y.M.; et al. Increases in plasma IgG4/IgE with trilipid vs paraffin/petrolatum-based emollients for dry skin/eczema. Pediatr. Allergy Immunol. 2020, 31, 699–703. [Google Scholar] [CrossRef]
- Kelleher, M.M.; Cro, S.; Van Vogt, E.; Cornelius, V.; Lodrup Carlsen, K.C.; Ove Skjerven, H.; Rehbinder, E.M.; Lowe, A.; Dissanayake, E.; Shimojo, N.; et al. Skincare interventions in infants for preventing eczema and food allergy: A cochrane systematic review and individual participant data meta-analysis. Clin. Exp. Allergy 2021, 51, 402–418. [Google Scholar] [CrossRef]
- Marrs, T.; Perkin, M.R.; Logan, K.; Craven, J.; Radulovic, S.; McLean, W.H.I.; Versteeg, S.A.; van Ree, R.; Lack, G.; Flohr, C.; et al. Bathing frequency is associated with skin barrier dysfunction and atopic dermatitis at three months of age. J. Allergy Clin. Immunol. Pract. 2020, 8, 2820–2822. [Google Scholar] [CrossRef]
- Perkin, M.R.; Logan, K.; Marrs, T.; Radulovic, S.; Craven, J.; Boyle, R.J.; Chalmers, J.R.; Williams, H.C.; Versteeg, S.A.; van Ree, R.; et al. Association of frequent moisturizer use in early infancy with the development of food allergy. J. Allergy Clin. Immunol. 2021, 147, 967–976.e1. [Google Scholar] [CrossRef]
- Danby, S.G.; Chalmers, J.; Brown, K.; Williams, H.C.; Cork, M.J. A functional mechanistic study of the effect of emollients on the structure and function of the skin barrier. Br. J. Dermatol. 2016, 175, 1011–1019. [Google Scholar] [CrossRef]
- Cooke, A.; Cork, M.J.; Victor, S.; Campbell, M.; Danby, S.; Chittock, J.; Lavender, T. Olive Oil, Sunflower Oil or no Oil for Baby Dry Skin or Massage: A Pilot, Assessor-blinded, Randomized Controlled Trial (the Oil in Baby SkincaRE [OBSeRvE] Study). Acta Dermatol. Venereol. 2016, 96, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Katibi, O.S.; Cork, M.J.; Flohr, C.; Danby, S.G. Moisturizer therapy in prevention of atopic dermatitis and food allergy: To use or disuse? Ann. Allergy Asthma Immunol. 2022, 128, 512–525. [Google Scholar] [CrossRef]
- Zhong, Y.; Samuel, M.; van Bever, H.; Tham, E.H. Emollients in infancy to prevent atopic dermatitis: A systematic review and meta-analysis. Allergy 2022, 77, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, M.; Balachander, B.; Gupta, S.; Sankar, M.J. Topical emollient application in term healthy newborns: A systematic review. J. Glob. Health 2022, 12, 12002. [Google Scholar] [CrossRef] [PubMed]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Impli-cations in Atopic Dermatitis. Int. J. Mol. Sci 2020, 21, 5382. [Google Scholar] [CrossRef] [PubMed]
- Fukuie, T.; Nomura, I.; Horimukai, K.; Manki, A.; Masuko, I.; Futamura, M.; Narita, M.; Ohzeki, T.; Matsumoto, K.; Saito, H.; et al. Proactive treatment appears to decrease serum immunoglobulin-E levels in patients with severe atopic dermatitis. Br. J. Dermatol. 2010, 163, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, Y.; Yang, L.; Yamamoto-Hanada, K.; Narita, M.; Saito, H.; Ohya, Y. Earlier aggressive treatment to shorten the duration of eczema in infants resulted in fewer food allergies at 2 years of age. J. Allergy Clin. Immunol. Pract. 2020, 8, 1721–1724.e6. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Hanada, K.; Kobayashi, T.; Williams, H.C.; Mikami, M.; Saito-Abe, M.; Morita, K.; Natsume, O.; Sato, M.; Iwama, M.; Miyaji, Y.; et al. Early aggressive intervention for infantile atopic dermatitis to prevent development of food allergy: A multicenter, investigator-blinded, randomized, parallel group controlled trial (PACI Study)-protocol for a randomized controlled trial. Clin. Transl. Allergy 2018, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Rowley, G.G.; MacNeill, S.J.; Ridd, M.J. Emollient satisfaction questionnaire: Validation study in children with eczema. Clin. Exp. Dermatol. 2022, 47, 1337–1345. [Google Scholar] [CrossRef]
- Al-Zuhairy, S.A.S.; Kadhum, W.R.; Alhijjaj, M.; Kadhim, M.M.; Al-Janabi, A.S.; Salman, A.W.; Al-Sharifi, H.K.R.; Khadom, A.A. Development and Evaluation of Biocompatible Topical Petrolatum-liquid Crystal Formulations with Enhanced Skin Permeation Properties. J. Oleo Sci. 2022, 71, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Danby, S.G.; Andrew, P.V.; Kay, L.J.; Pinnock, A.; Chittock, J.; Brown, K.; Williams, S.F.; Cork, M.J. Enhancement of stratum corneum lipid structure improves skin barrier function and protects against irritation in adults with dry, eczema-prone, skin. Br. J. Dermatol. 2022, 186, 875–886. [Google Scholar] [CrossRef]
- van Zuuren, E.J.; Fedorowicz, Z.; Christensen, R.; Lavrijsen, A.; Arents, B.W.M. Emollients and moisturisers for eczema. Cochrane Database Syst. Rev. 2017, 2, CD012119. [Google Scholar] [CrossRef]
- Danby, S.G.; Draelos, Z.D.; Gold, L.F.S.; Cha, A.; Vlahos, B.; Aikman, L.; Sanders, P.; Wu-Linhares, D.; Cork, M.J. Vehicles for atopic dermatitis therapies: More than just a placebo. J. Dermatol. Treat. 2022, 33, 685–698. [Google Scholar] [CrossRef]
- Celleno, L. Topical urea in skincare: A review. Dermatol. Ther. 2018, 31, e12690. [Google Scholar] [CrossRef]
- Karagounis, T.K.; Gittler, J.K.; Rotemberg, V.; Morel, K.D. Use of "natural" oils for moisturization: Review of olive, coconut, and sunflower seed oil. Pediatr. Dermatol. 2019, 36, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Vanessa, V.V.; Wan Ahmad Kammal, W.S.L.; Lai, Z.W.; How, K.N. A Review of Moisturizing Additives for Atopic Dermatitis. Cosmetics 2022, 9, 75. [Google Scholar] [CrossRef]
- Ryczaj, K.; Dumycz, K.; Spiewak, R.; Feleszko, W. Contact allergens in moisturizers in preventative emollient therapy–A systematic review. Clin. Transl. Allergy 2022, 12, e12150. [Google Scholar] [CrossRef] [PubMed]
- Łoś-Rycharska, E.; Gołębiewski, M.; Grzybowski, T.; Rogalla-Ładniak, U.; Krogulska, A. The microbiome and its impact on food allergy and atopic dermatitis in children. Postep. Dermatol. Alergol. 2020, 37, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Lin, G.; Ferenczi, K. The skin microbiome and the gut-skin axis. Clin. Dermatol. 2021, 39, 829–839. [Google Scholar] [CrossRef]
- Bunyavanich, S. Food allergy: Could the gut microbiota hold the key? Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 201–202. [Google Scholar] [CrossRef]
- Gołębiewski, M.; Łoś-Rycharska, E.; Sikora, M.; Grzybowski, T.; Gorzkiewicz, M.; Krogulska, A. Mother’s milk microbiome shaping fecal and skin microbiota in infants with food allergy and atopic dermatitis: A pilot analysis. Nutrients 2021, 13, 3600. [Google Scholar] [CrossRef]
- van den Elsen, L.W.J.; Garssen, J.; Burcelin, R.; Verhasselt, V. Shaping the Gut Microbiota by Breastfeeding: The Gateway to Allergy Prevention? Front. Pediatr. 2019, 7, 47. [Google Scholar] [CrossRef]
- Dzidic, M.; Mira, A.; Artacho, A.; Abrahamsson, T.R.; Jenmalm, M.C.; Collado, M.C. Allergy development is associated with consumption of breastmilk with a reduced microbial richness in the first month of life. Pediatr. Allergy Immunol. 2020, 31, 250–257. [Google Scholar] [CrossRef]
- Ho, N.T.; Li, F.; Lee-Sarwar, K.A.; Tun, H.M.; Brown, B.P.; Pannaraj, P.S.; Bender, J.M.; Azad, M.B.; Thompson, A.L.; Weiss, S.T.; et al. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat. Commun. 2018, 9, 4169. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Sitarik, A.R.; Woodcroft, K.; Johnson, C.C.; Zoratti, E. Birth Mode, Breastfeeding, Pet Exposure, and Antibiotic Use: Associations With the Gut Microbiome and Sensitization in Children. Curr. Allergy Asthma Rep. 2019, 19, 22. [Google Scholar] [CrossRef]
- Sitarik, A.R.; Bobbitt, K.R.; Havstad, S.L.; Fujimura, K.E.; Levin, A.M.; Zoratti, E.M.; Kim, H.; Woodcroft, K.J.; Wegienka, G.; Ownby, D.R.; et al. Breast Milk Transforming Growth Factor β Is Associated With Neonatal Gut Microbial Composition. J. Pediatr. Gastroenterol. Nutr. 2017, 65, e60–e67. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.C.; Ownby, D.R. The infant gut bacterial microbiota and risk of pediatric asthma and allergic diseases. Transl. Res. 2017, 179, 60–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajani, P.S.; Seppo, A.E.; Järvinen, K.M. Immunologically Active Components in Human Milk and Development of Atopic Disease, With Emphasis on Food Allergy, in the Pediatric Population. Front. Pediatr. 2018, 6, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Home–ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 14 February 2023).
- Nagel-Wolfrum, K.; Möller, F.; Penner, I.; Baasov, T.; Wolfrum, U. Targeting Nonsense Mutations in Diseases with Translational Read-Through-Inducing Drugs (TRIDs). BioDrugs 2016, 30, 49–74. [Google Scholar] [CrossRef]
- Campofelice, A.; Lentini, L.; Di Leonardo, A.; Melfi, R.; Tutone, M.; Pace, A.; Pibiri, I. Strategies against Nonsense: Oxadiazoles as Translational Readthrough-Inducing Drugs (TRIDs). Int. J. Mol. Sci. 2019, 20, 3329. [Google Scholar] [CrossRef] [Green Version]
- Stout, T.E.; McFarland, T.; Mitchell, J.C.; Appukuttan, B.; Timothy Stout, J. Recombinant filaggrin is internalized and processed to correct filaggrin deficiency. J. Investig. Dermatol. 2014, 134, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Lim, K.M. Skin barrier dysfunction and filaggrin. Arch. Pharm. Res. 2021, 44, 36–48. [Google Scholar] [CrossRef]
- Peltonen, J.M.; Pylkkänen, L.; Jansén, C.T.; Volanen, I.; Lehtinen, T.; Laihia, J.K.; Leino, L. Three randomised phase I/IIa trials of 5% cis-urocanic acid emulsion cream in healthy adult subjects and in patients with atopic dermatitis. Acta Dermatol. Venereol. 2014, 94, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Papp, K.; Szepietowski, J.C.; Kircik, L.; Toth, D.; Kuligowski, M.E.; Venturanza, M.E.; Sun, K.; Simpson, E.L. Efficacy and Safety of Ruxolitinib Cream for the Treatment of Atopic Dermatitis: Results from Two Phase 3, Randomized, Double-Blind Studies. SKIN J. Cutan. Med. 2020, 4, s95. [Google Scholar] [CrossRef]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Kaino, H.; Nagata, T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J. Am. Acad. Dermatol. 2020, 82, 823–831. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S.; Sun, K.; Papp, K.; Venturanza, M.; Nasir, A.; Kuligowski, M.E. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: Results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study. J. Am. Acad Dermatol. 2020, 82, 1305–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLornan, D.P.; Pope, J.E.; Gotlib, J.; Harrison, C.N. Current and future status of JAK inhibitors. Lancet 2021, 398, 803–816. [Google Scholar] [CrossRef]
- Sideris, N.; Paschou, E.; Bakirtzi, K.; Kiritsi, D.; Papadimitriou, I.; Tsentemeidou, A.; Sotiriou, E.; Vakirlis, E. New and Upcoming Topical Treatments for Atopic Dermatitis: A Review of the Literature. J. Clin. Med. 2022, 11, 4974. [Google Scholar] [CrossRef] [PubMed]
- Peppers, J.; Paller, A.S.; Maeda-Chubachi, T.; Wu, S.; Robbins, K.; Gallagher, K.; Kraus, J.E. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J. Am. Acad Dermatol. 2019, 80, 89–98.e3. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Stein Gold, L.; Soung, J.; Tallman, A.M.; Rubenstein, D.S.; Gooderham, M. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J. Am. Acad. Dermatol. 2021, 84, 632–638. [Google Scholar] [CrossRef]
- Furue, M.; Uchi, H.; Mitoma, C.; Hashimoto-Hachiya, A.; Tanaka, Y.; Ito, T.; Tsuji, G. Implications of tryptophan photoproduct FICZ in oxidative stress and terminal differentiation of keratinocytes. G Ital. Dermatol. Venereol. 2019, 154, 37–41. [Google Scholar] [CrossRef]
- Kiyomatsu-Oda, M.; Uchi, H.; Morino-Koga, S.; Furue, M. Protective role of 6-formylindolo[3,2-b]carbazole (FICZ), an endogenous ligand for arylhydrocarbon receptor, in chronic mite-induced dermatitis. J. Dermatol. Sci. 2018, 90, 284–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Song, K.M.; Jung, C.H. Diosmin restores the skin barrier by targeting the aryl hydrocarbon receptor in atopic dermatitis. Phytomedicine 2021, 81, 153418. [Google Scholar] [CrossRef]
- Jia, T.; Qiao, W.; Yao, Q.; Wu, W.; Kaku, K. Treatment with Docosahexaenoic Acid Improves Epidermal Keratinocyte Differentiation and Ameliorates Inflammation in Human Keratinocytes and Reconstructed Human Epidermis Models. Molecules 2019, 24, 3156. [Google Scholar] [CrossRef] [Green Version]
- Czarnowicki, T.; Dohlman, A.B.; Malik, K.; Antonini, D.; Bissonnette, R.; Chan, T.C.; Zhou, L.; Wen, H.C.; Estrada, Y.; Xu, H.; et al. Effect of short-term liver X receptor activation on epidermal barrier features in mild to moderate atopic dermatitis: A randomized controlled trial. Ann. Allergy Asthma Immunol. 2018, 120, 631–640.e11. [Google Scholar] [CrossRef]
- Bielach-Bazyluk, A.; Zbroch, E.; Mysliwiec, H.; Rydzewska-Rosolowska, A.; Kakareko, K.; Flisiak, I.; Hryszko, T. Sirtuin 1 and Skin: Implications in Intrinsic and Extrinsic Aging–A Systematic Review. Cells 2021, 10, 813. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Park, K.Y.; Seo, S.J. Adiponectin Upregulates Filaggrin Expression via SIRT1-Mediated Signaling in Human Normal Keratinocytes. Ann. Dermatol. 2017, 29, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Che, D.N.; Cho, B.O.; Shin, J.Y.; Kang, H.J.; Kim, J.; Choi, J.; Jang, S.I. Anti-atopic dermatitis effects of hydrolyzed celery extract in mice. J. Food Biochem. 2020, 44, e13198. [Google Scholar] [CrossRef]
- Che, D.N.; Cho, B.O.; Shin, J.Y.; Kang, H.J.; Kim, J.S.; Oh, H.; Kim, Y.S.; Jang, S.I. Apigenin Inhibits IL-31 Cytokine in Human Mast Cell and Mouse Skin Tissues. Molecules 2019, 24, 1290. [Google Scholar] [CrossRef] [Green Version]
- Egawa, G.; Kabashima, K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J. Allergy Clin. Immunol. 2016, 138, 350–358.e1. [Google Scholar] [CrossRef] [Green Version]
- Furue, M. Regulation of Skin Barrier Function via Competition between AHR Axis versus IL-13/IL-4-JAK-STAT6/STAT3 Axis: Pathogenic and Therapeutic Implications in Atopic Dermatitis. J. Clin. Med. 2020, 9, 3741. [Google Scholar] [CrossRef]
- Renert-Yuval, Y.; Guttman-Yassky, E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann. Allergy Asthma Immunol. 2020, 124, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Moyle, M.; Cevikbas, F.; Harden, J.L.; Guttman-Yassky, E. Understanding the immune landscape in atopic dermatitis: The era of biologics and emerging therapeutic approaches. Exp. Dermatol. 2019, 28, 756–768. [Google Scholar] [CrossRef] [Green Version]
- Szalus, K.; Trzeciak, M.; Nowicki, R.J. JAK-STAT Inhibitors in Atopic Dermatitis from Pathogenesis to Clinical Trials Results. Microorganisms 2020, 8, 1743. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Guttman-Yassky, E. JAK Inhibitors for Atopic Dermatitis: An Update. Am. J. Clin. Dermatol. 2019, 20, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, Y.; Shinozaki, Y.; Yamamoto, Y.; Katsuda, E.; Taniai-Riya, K.; Toyoda, K.; Kakimoto, K.; Kimoto, Y.; Amano, W.; Konishi, N.; et al. A novel JAK inhibitor JTE-052 reduces skin inflammation and ameliorates chronic dermatitis in rodent models: Comparison with conventional therapeutic agents. Exp. Dermatol. 2018, 27, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Heron, C.E.; Ghamrawi, R.I.; Strowd, L.C.; Feldman, S.R. Emerging Role of Janus Kinase Inhibitors for the Treatment of Atopic Dermatitis. Immunotargets 2020, 9, 255–272. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Anderson, K.R.; Tollefson, M.M. New and emerging therapies for pediatric atopic dermatitis. Paediatr. Drugs 2019, 21, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Clarysse, K.; Pfaff, C.; Marquardt, Y.; Huth, L.; Kortekaas, K.; Kluwig, D.; Lüscher, B.; Gutermuth, J.; Baron, J. JAK1/3 inhibition preserves epidermal morphology in full-thickness 3D skin models of atopic dermatitis and psoriasis. J. Eur. Acad Dermatol. Venereol 2019, 33, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Nakahara, T. Revival of AHR Agonist for the Treatment of Atopic Dermatitis: Tapinarof. Curr. Treat. Options Allergy 2020, 7, 414–421. [Google Scholar] [CrossRef]
- Bissonnette, R.; Stein Gold, L.; Rubenstein, D.S.; Tallman, A.M.; Armstrong, A. Tapinarof in the treatment of psoriasis: A review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J. Am. Acad Dermatol. 2021, 84, 1059–1067. [Google Scholar] [CrossRef] [PubMed]

| Intervention | Area of Focus | References |
|---|---|---|
| Primary prevention Topical moisturizers | ||
| Preventive therapy with petrolatum-based moisturizers for atopic dermatitis Preventive therapy with moisturizers containing ceramide and amino acids for atopic dermatitis Preventive therapy with moisturizers and synbiotics for atopic dermatitis and food allergy Preventive therapy with petrolatum-based moisturizers for atopic dermatitis and food allergy Preventive therapy with trilipid ceramide-dominant moisturizers for atopic dermatitis and food allergy Link between moisturizers and food allergy | [178,179,180,181] [182] [183] [184,185,186,187] [188,189,190] [194] | |
| Secondary prevention | ||
| Proactive atopic dermatitis therapy with TCS and moisturizers and the prevention of food allergy | [202,203,204] | |
| Topical interventions upregulating FLG expression | ||
| Gene-based approach Direct replacement of FLG Indirect replacement therapy JAK inhibitors Enhancement FLG expression | “Read-through” drugs Topical application of recombinant FLG monomer Topical application of FLG metabolites: trans-urocanic acid and pyrrolidine carboxylic acid Inhibition of cytokine-mediated FLG downregulation: delgocitinib (JTE-052) and ruxolitinib AHR agonists: tapinarof, tryptophan photoproduct (FICH), diosmin Peroxisome proliferator-activated receptors (PPARs) agonists Liver X receptor (LXR) agonists Sirtuin 1 (SIRT1) Bioflavonoids: apigenin, hesperidin, apigetrin | [226,227] [228] [229,230] [231,232,233,234,235] [236,237,238,239,240] [241] [242] [243,244] [245,246] |
| Microbiome interventions | ||
| Microbial transplantation of commensal bacteria | [225] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębińska, A.; Sozańska, B. Epicutaneous Sensitization and Food Allergy: Preventive Strategies Targeting Skin Barrier Repair—Facts and Challenges. Nutrients 2023, 15, 1070. https://doi.org/10.3390/nu15051070
Dębińska A, Sozańska B. Epicutaneous Sensitization and Food Allergy: Preventive Strategies Targeting Skin Barrier Repair—Facts and Challenges. Nutrients. 2023; 15(5):1070. https://doi.org/10.3390/nu15051070
Chicago/Turabian StyleDębińska, Anna, and Barbara Sozańska. 2023. "Epicutaneous Sensitization and Food Allergy: Preventive Strategies Targeting Skin Barrier Repair—Facts and Challenges" Nutrients 15, no. 5: 1070. https://doi.org/10.3390/nu15051070
APA StyleDębińska, A., & Sozańska, B. (2023). Epicutaneous Sensitization and Food Allergy: Preventive Strategies Targeting Skin Barrier Repair—Facts and Challenges. Nutrients, 15(5), 1070. https://doi.org/10.3390/nu15051070






