Prenatal Factors Associated with Maternal Cardiometabolic Risk Markers during Pregnancy: The ECLIPSES Study
Abstract
:1. Introduction
2. Materials and Methods
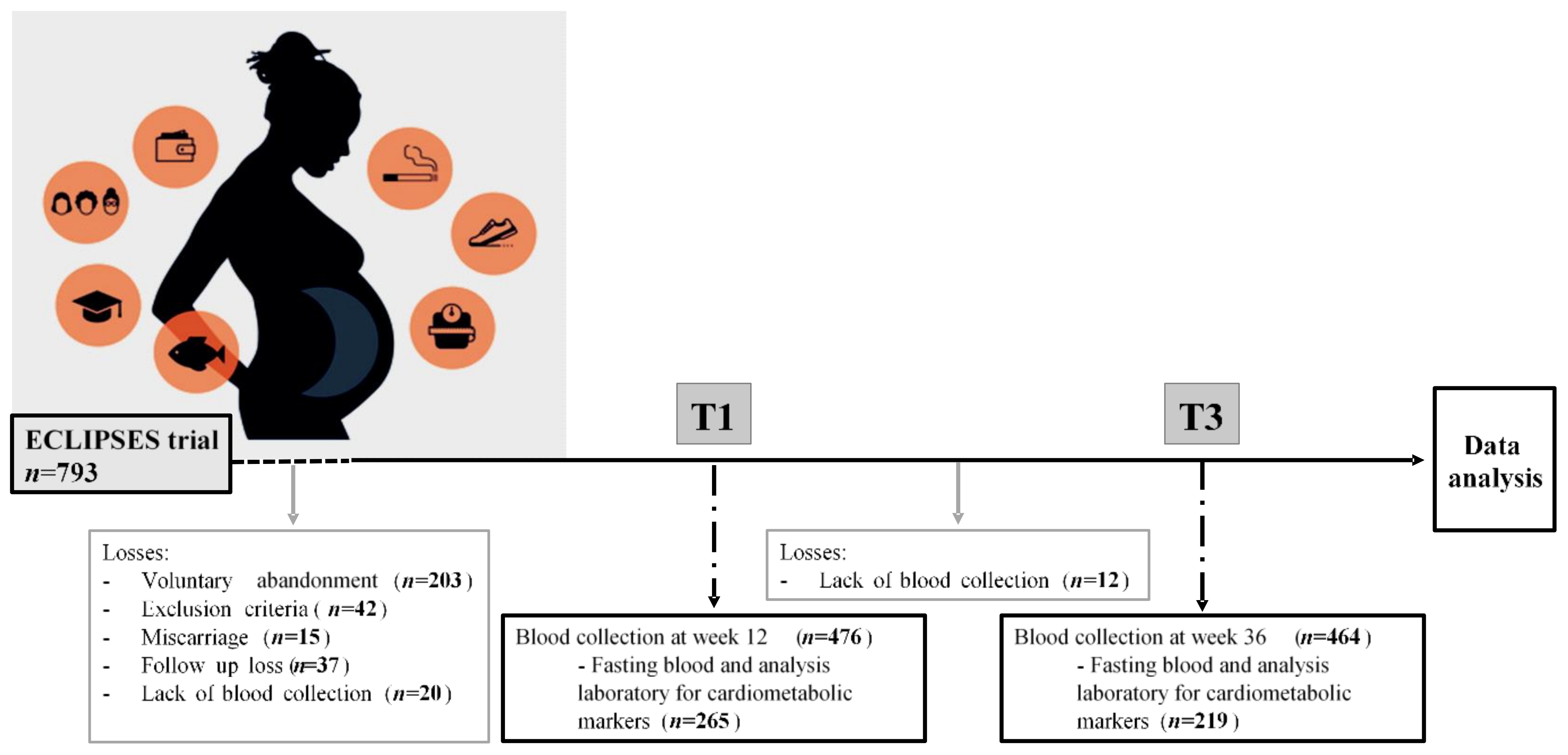
2.1. Study Design
2.2. Data Collection
2.3. Cardiometabolic Risk Markers
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagraj, S.; Kennedy, S.H.; Norton, R.; Jha, V.; Praveen, D.; Hinton, L.; Hirst, J.E. Cardiometabolic Risk Factors in Pregnancy and Implications for Long-Term Health: Identifying the Research Priorities for Low-Resource Settings. Front. Cardiovasc. Med. 2020, 7, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bener, A.; Saleh, N.M. The Impact of Socio-Economic, Lifestyle Habits, and Obesity in Developing of Pregnancy-Induced Hypertension in Fast-Growing Country: Global Comparisons. Clin. Exp. Obs. Gynecol. 2013, 40, 52–57. [Google Scholar]
- Sampson, L.; Dasgupta, K.; Ross, N.A. The Association between Socio-Demographic Marginalization and Plasma Glucose Levels at Diagnosis of Gestational Diabetes. Diabet. Med. 2014, 31, 1563–1567. [Google Scholar] [CrossRef] [PubMed]
- Assibey-Mensah, V.; Fabio, A.; Mendez, D.D.; Lee, P.C.; Roberts, J.M.; Catov, J.M. Neighbourhood Assets and Early Pregnancy Cardiometabolic Risk Factors. Paediatr. Perinat. Epidemiol. 2019, 33, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Flor-Alemany, M.; Acosta, P.; Marín-Jiménez, N.; Baena-García, L.; Aranda, P.; Aparicio, V.A. Influence of the Degree of Adherence to the Mediterranean Diet and Its Components on Cardiometabolic Risk during Pregnancy. The GESTAFIT Project. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2311–2318. [Google Scholar] [CrossRef]
- Martin, C.L.; Siega-Riz, A.M.; Sotres-Alvarez, D.; Robinson, W.R.; Daniels, J.L.; Perrin, E.M.; Stuebe, A.M. Maternal Dietary Patterns Are Associated with Lower Levels of Cardiometabolic Markers during Pregnancy. Paediatr. Perinat. Epidemiol. 2016, 30, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Al Wattar, B.H.; Dodds, J.; Placzek, A.; Beresford, L.; Spyreli, E.; Moore, A.; Gonzalez Carreras, F.J.; Austin, F.; Murugesu, N.; Roseboom, T.J.; et al. Mediterranean-Style Diet in Pregnant Women with Metabolic Risk Factors (ESTEEM): A Pragmatic Multicentre Randomised Trial. PLoS Med. 2019, 16, e1002857. [Google Scholar] [CrossRef]
- Asemi, Z.; Tabassi, Z.; Samimi, M.; Fahiminejad, T.; Esmaillzadeh, A. Favourable Effects of the Dietary Approaches to Stop Hypertension Diet on Glucose Tolerance and Lipid Profiles in Gestational Diabetes: A Randomised Clinical Trial. Br. J. Nutr. 2013, 109, 2024–2030. [Google Scholar] [CrossRef]
- Timmermans, S.; Steegers-Theunissen, R.P.M.; Vujkovic, M.; Bakker, R.; den Breeijen, H.; Raat, H.; Russcher, H.; Lindemans, J.; Hofman, A.; Jaddoe, V.W.V.; et al. Major Dietary Patterns and Blood Pressure Patterns during Pregnancy: The Generation R Study. Am. J. Obs. Gynecol. 2011, 205, 337.e1–337.e12. [Google Scholar] [CrossRef] [Green Version]
- Mottola, M.F.; Davenport, M.H.; Ruchat, S.M.; Davies, G.A.; Poitras, V.J.; Gray, C.E.; Jaramillo Garcia, A.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. 2019 Canadian Guideline for Physical Activity throughout Pregnancy. Br. J. Sport. Med. 2018, 52, 1339–1346. [Google Scholar] [CrossRef] [Green Version]
- Rublevskaya, A.; Bichan, N.A. Arterial Hypertension in Pregnant Women: The Effect of Body Mass Index and Smoking. Eur. Heart J. 2021, 42, 2893. [Google Scholar] [CrossRef]
- Aagaard-Tillery, K.M.; Porter, T.F.; Lane, R.H.; Varner, M.W.; Lacoursiere, D.Y. In Utero Tobacco Exposure Is Associated with Modified Effects of Maternal Factors on Fetal Growth. Am. J. Obs. Gynecol. 2008, 198, 66.e1–66.e6. [Google Scholar] [CrossRef] [PubMed]
- Haskins, A.E.; Bertone-Johnson, E.R.; Pekow, P.; Carbone, E.; Fortner, R.T.; Chasan-Taber, L. Smoking during Pregnancy and Risk of Abnormal Glucose Tolerance: A Prospective Cohort Study. BMC Pregnancy Childbirth 2010, 10, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iscan, A.; Yigitoglu, M.R.; Ece, A.; Ari, Z.; Akyildiz, M. The Effect of Cigarette Smoking during Pregnancy on Cord Blood Lipid, Lipoprotein and Apolipoprotein Levels. Jpn Heart J. 1997, 38, 497–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bizoń, A.; Milnerowicz, H. The Effect of Passive and Active Exposure to Tobacco Smoke on Lipid Profile Parameters and the Activity of Certain Membrane Enzymes in the Blood of Women in the First Trimester of Pregnancy. Env. Toxicol. Pharm. 2017, 53, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Iwama, N.; Metoki, H.; Nishigori, H.; Mizuno, S.; Takahashi, F.; Tanaka, K.; Watanabe, Z.; Saito, M.; Sakurai, K.; Ishikuro, M.; et al. Association between Alcohol Consumption during Pregnancy and Hypertensive Disorders of Pregnancy in Japan: The Japan Environment and Children’s Study. Hypertens. Res. 2019, 42, 85–94. [Google Scholar] [CrossRef]
- Välimäki, M.; Halmesmäki, E.; Keso, L.; Ylikorkala, O.; Ylikahri, R. Serum Lipids and Lipoproteins in Alcoholic Women during Pregnancy. Metabolism 1990, 39, 486–493. [Google Scholar] [CrossRef]
- Yen, I.W.; Lee, C.N.; Lin, M.W.; Fan, K.C.; Wei, J.N.; Chen, K.Y.; Chen, S.C.; Tai, Y.Y.; Kuo, C.H.; Lin, C.H.; et al. Overweight and Obesity Are Associated with Clustering of Metabolic Risk Factors in Early Pregnancy and the Risk of GDM. PLoS ONE 2019, 14, e0225978. [Google Scholar] [CrossRef]
- Roland, M.C.P.; Lekva, T.; Godang, K.; Bollerslev, J.; Henriksen, T. Changes in Maternal Blood Glucose and Lipid Concentrations during Pregnancy Differ by Maternal Body Mass Index and Are Related to Birthweight: A Prospective, Longitudinal Study of Healthy Pregnancies. PLoS ONE 2020, 15, e0232749. [Google Scholar] [CrossRef]
- Geraghty, A.A.; Alberdi, G.; O’Sullivan, E.J.; O’Brien, E.C.; Crosbie, B.; Twomey, P.J.; McAuliffe, F.M. Maternal and Fetal Blood Lipid Concentrations during Pregnancy Differ by Maternal Body Mass Index: Findings from the ROLO Study. BMC Pregnancy Childbirth 2017, 17, 4–10. [Google Scholar] [CrossRef]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-Analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef] [PubMed]
- Voerman, E.; Santos, S.; Inskip, H.; Amiano, P.; Barros, H.; Charles, M.A.; Chatzi, L.; Chrousos, G.P.; Corpeleijn, E.; Crozier, S.; et al. Association of Gestational Weight Gain With Adverse Maternal and Infant Outcomes. JAMA 2019, 321, 1702–1715. [Google Scholar] [PubMed] [Green Version]
- Acosta-Manzano, P.; Acosta, F.M.; Flor-Alemany, M.; Gavilan-Carrera, B.; Delgado-Fernandez, M.; Baena-Garcia, L.; Segura-Jimenez, V.; Aparicio, V.A. The Protective Role of Physical Fitness on Cardiometabolic Risk During Pregnancy: The GESTAtion and FITness Project. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Niu, J.; Lv, L.; Duan, D.; Wen, J.; Lin, X.; Mai, C.; Zhou, Y. Clustering of Metabolic Risk Factors and Adverse Pregnancy Outcomes: A Prospective Cohort Study. Diabetes Metab. Res. Rev. 2016, 32, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Arija, V.; Fargas, F.; March, G.; Abajo, S.; Basora, J.; Canals, J.; Ribot, B.; Aparicio, E.; Serrat, N.; Hernández-Martínez, C.; et al. Adapting Iron Dose Supplementation in Pregnancy for Greater Effectiveness on Mother and Child Health: Protocol of the ECLIPSES Randomized Clinical Trial. BMC Pregnancy Childbirth 2014, 14, 33. [Google Scholar] [CrossRef] [Green Version]
- Institut d’Estadística de Catalunya. Classificació Catalana d’ocupacions 2011 (CCO-2011). Adaptació de La CNO-2011; Generalitat de Catalunya: Barcelona, Spain, 2013; Available online: https://www.idescat.cat/serveis/biblioteca/docs/cat/cco2011.pdf (accessed on 24 December 2022).
- Hinkle, S.N.; Zhang, C.; Grantz, K.L.; Sciscione, A.; Wing, D.A.; Grobman, W.A.; Newman, R.B.; D’Alton, M.E.; Skupski, D.; Nageotte, M.P.; et al. Nutrition during Pregnancy: Findings from the National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies–Singleton Cohort. Curr. Dev. Nutr. 2021, 5, nzaa182. [Google Scholar] [CrossRef]
- Fagerström, K.O. Measuring Degree of Physical Dependence to Tobacco Smoking with Reference to Individualization of Treatment. Addict. Behav. 1978, 3, 235–241. [Google Scholar] [CrossRef]
- Rodríguez, I.T.; Ballart, J.F.; Pastor, G.C.; Jordà, E.B.; Val, V.A. Validation of a short questionnaire on frequency of dietary intake: Reproducibility and validity. Nutr. Hosp. 2008, 23, 242–252. [Google Scholar]
- Fernández-Barrés, S.; Romaguera, D.; Valvi, D.; Martínez, D.; Vioque, J.; Navarrete-Muñoz, E.M.; Amiano, P.; Gonzalez-Palacios, S.; Guxens, M.; Pereda, E.; et al. Mediterranean Dietary Pattern in Pregnant Women and Offspring Risk of Overweight and Abdominal Obesity in Early Childhood: The INMA Birth Cohort Study. Pediatr. Obes. 2016, 11, 491–499. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Global Dabatase on Body Mass Index; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Kathleen, M.; Rasmussen, K.; Yaktine, A. Institute of Medicine and National Research Council. In Weight Gain during Pregnancy: Reexamining the Guidelines; National Academy of Science; The National Academies Press: Washington, DC, USA, 2009; Volume 1, p. 2. [Google Scholar]
- Strevens, H.; Kristensen, K.; Langhoff-Roos, J.; Wide-Swensson, D. Blood Pressure Patterns through Consecutive Pregnancies Are Influenced by Body Mass Index. Am. J. Obs. Gynecol. 2002, 187, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Strevens, H.; Wide-Swensson, D.; Ingemarsson, I. Blood Pressure during Pregnancy in a Swedish Population; Impact of Parity. Acta Obs. Gynecol. Scand. 2001, 80, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Li, H.; Cai, W.; Niu, X.; Ji, W.; Zhang, Z.; Niu, J.; Zhou, X.; Li, Y. Excessive Gestational Weight Gain in Accordance with the IOM Criteria and the Risk of Hypertensive Disorders of Pregnancy: A Meta-Analysis. BMC Pregnancy Childbirth 2018, 18, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genova, M.P.; Todorova-Ananieva, K.; Tzatchev, K. Impact of Body Mass Index on Insulin Sensitivity/Resistance in Pregnant Women with and without Gestational Diabetes Mellitus. Acta Med. Bulg. 2013, 40, 60–67. [Google Scholar]
- Alvarado, F.L.; O’Tierney-Ginn, P.; Catalano, P. Contribution of Gestational Weight Gain on Maternal Glucose Metabolism in Women with GDM and Normal Glucose Tolerance. J. Endocr. Soc. 2021, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Omaña-Guzmán, L.I.; Ortiz-Hernández, L.; Ancira-Moreno, M.; Morales-Hernández, V.; O’Neill, M.S.; Vadillo-Ortega, F. Association of Pre-Pregnancy Body Mass Index and Rate of Weight Gain during Pregnancy with Maternal Indicators of Cardiometabolic Risk. Nutr. Diabetes 2021, 11, bvaa195. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, K.L.; Gyllenhammer, L.E.; Entringer, S.; Wadhwa, P.D. Rate of Gestational Weight Gain and Glucose-Insulin Metabolism Among Hispanic Pregnant Women With Overweight and Obesity. J. Clin. Endocrinol. Metab. 2022, 107, e734–e744. [Google Scholar] [CrossRef]
- Reyes, L.M.; Khurana, R.; Usselman, C.W.; Busch, S.A.; Skow, R.J.; Boulé, N.G.; Davenport, M.H.; Steinback, C.D. Sympathetic Nervous System Activity and Reactivity in Women with Gestational Diabetes Mellitus. Physiol. Rep. 2020, 8, e14504. [Google Scholar] [CrossRef]
- Canale, M.P.; Manca Di Villahermosa, S.; Martino, G.; Rovella, V.; Noce, A.; de Lorenzo, A.; di Daniele, N. Obesity-Related Metabolic Syndrome: Mechanisms of Sympathetic Overactivity. Int. J. Endocrinol. 2013, 2013, 865965. [Google Scholar] [CrossRef] [Green Version]
- Mauri, M.; Calmarza, P.; Ibarretxe, D. Dyslipemias and Pregnancy, an Update. Clínica E Investig. En Arterioscler. 2021, 33, 41–52. [Google Scholar] [CrossRef]
- Diareme, M.; Karkalousos, P.; Theodoropoulos, G.; Strouzas, S.; Lazanas, N. Lipid Profile of Healthy Women During Normal Pregnancy. J. Med. Biochem. 2009, 28, 152–160. [Google Scholar] [CrossRef] [Green Version]
- Nelson, S.M.; Matthews, P.; Poston, L. Maternal Metabolism and Obesity: Modifiable Determinants of Pregnancy Outcome. Hum. Reprod. Update 2009, 16, 255–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahratian, A.; Misra, V.K.; Trudeau, S.; Misra, D.P. Prepregnancy Body Mass Index and Gestational Age-Dependent Changes in Lipid Levels during Pregnancy. Obstet. Gynecol. 2010, 116, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Scifres, C.M.; Catov, J.M. The Impact of Maternal Obesity and Gestational Weight Gain on Early and Mid-Pregnancy Lipid Profiles. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Chen, H.; Xi, F.; Sagnelli, M.; Zhao, B.; Chen, Y.; Yang, M.; Xu, D.; Jiang, Y.; Chen, G.; et al. Association between Maternal Blood Lipids Levels during Pregnancy and Risk of Small-for-Gestational-Age Infants. Sci. Rep. 2020, 10, 19865. [Google Scholar] [CrossRef]
- Brown, S.D.; Hedderson, M.M.; Ehrlich, S.F.; Galarce, M.N.; Tsai, A.L.; Quesenberry, C.P.; Ferrara, A. Gestational Weight Gain and Optimal Wellness (GLOW): Rationale and Methods for a Randomized Controlled Trial of a Lifestyle Intervention among Pregnant Women with Overweight or Obesity. BMC Pregnancy Childbirth 2019, 19, 145. [Google Scholar] [CrossRef]
- Catalano, P. Maternal Pre-Pregnancy BMI: Harbinger of Late-Pregnancy Maternal Lipid Profile. BJOG 2016, 123, 579. [Google Scholar] [CrossRef] [Green Version]
- Amati, F.; Hassounah, S.; Swaka, A. The Impact of Mediterranean Dietary Patterns during Pregnancy on Maternal and Offspring Health. Nutrients 2019, 11, 1098. [Google Scholar] [CrossRef] [Green Version]
- Romero-Rodríguez, E.; Cuevas, L.; Simón, L.; Bermejo-Sánchez, E.; Galán, I. Changes in Alcohol Intake during Pregnancy in Spain, 1980 to 2014. Alcohol. Clin. Exp. Res. 2019, 43, 2367–2373. [Google Scholar] [CrossRef]
- Cluette-Brown, J.; Mulligan, J.; Doyle, K.; Hagan, S.; Osmolski, T.; Hojnacki, J. Oral Nicotine Induces an Atherogenic Lipoprotein Profile. Proc. Soc. Exp. Biol. Med. 1986, 182, 409–413. [Google Scholar] [CrossRef]
- Hojnacki, J.; Mulligan, J.; Cluette-Brown, J.; Igoe, F.; Osmolski, T. Oral Nicotine Impairs Clearance of Plasma Low Density Lipoproteins. Proc. Soc. Exp. Biol. Med. 1986, 182, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Mjøs, O.D. Lipid Effects of Smoking. Am. Heart J. 1988, 115, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Lobelo, F.; Aguilar-de Plata, A.C.; Izquierdo, M.; García-Hermoso, A. Exercise during Pregnancy on Maternal Lipids: A Secondary Analysis of Randomized Controlled Trial. BMC Pregnancy Childbirth 2017, 17, 396. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhang, C.J.P.; Fang, X.; Tan, Z.; Yan, N.; Ming, W.-K.; Wan, Z. Relationship of Objectively Measuring Physical Activity and Sitting Time on Plasma Lipid Metabolism During Pregnancy. Res. Sq. 2021, preprint. [Google Scholar]
- Burton, G.J.; Jauniaux, E. Development of the Human Placenta and Fetal Heart: Synergic or Independent? Front. Physiol. 2018, 9, 373. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.M.; Coolman, M.; Steegers, E.A.P.; Jaddoe, V.W.V.; Moll, H.A.; Hofman, A.; Mackenbach, J.P.; Raat, H. Maternal Educational Level and Risk of Gestational Hypertension: The Generation R Study. J. Hum. Hypertens 2008, 22, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, R.; Goodarzi-Khoigani, M.; Allameh, Z.; Mazloomy Mahmoodabad, S.; Baghiani Moghadam, M.; Nadjarzadeh, A.; Mardanian, F. Association between Socioeconomic Status and Homeostasis Model Assessment-Insulin Resistance Index and Mediating Variables at the First Trimester of Pregnancy. Iran J. Nurs. Midwifery Res. 2022, 27, 166–168. [Google Scholar]

| General Characteristics | Summary Statistics |
|---|---|
| Age (years), mean ± SD | 29.6 ± 4.7 |
| Age categories (years), n (%) | |
| <25 | 40 (15) |
| 25–29 | 73 (28) |
| ≥30 | 152 (57) |
| Weight (kg), mean ± SD | 63.3 ± 9.6 |
| BMI (kg/m2), mean ± SD | 24.1 ± 3.5 |
| BMI categories, n (%) | |
| 18.5–24.9 (normal weight) | 169 (64) |
| 25.0–29.9 (overweight) | 82 (31) |
| ≥30 (obesity) | 14 (5) |
| GWG (kg), mean ± SD | 10.4 ± 3.6 |
| IOM GWG recommendations, n (%) † | |
| Insufficient | 119 (45) |
| Adequate | 99 (37) |
| Excessive | 47 (18) |
| Educational level, n (%) | |
| Low (primary or below) | 83 (31) |
| Medium (secondary) | 97 (37) |
| High (university or above) | 84 (32) |
| Social class, n (%) | |
| Low | 35 (13) |
| Medium | 180 (68) |
| High | 49 (19) |
| Smoking status, n (%) | |
| Never smoker | 185 (70) |
| Former smoker | 42 (16) |
| Current smoker | 37 (14) |
| Alcohol consumption | |
| No | 222 (87) |
| Yes | 33 (13) |
| Physical Activity (METs-min/week) | |
| T1 (<1070) | 87 (33) |
| T2 (1070–3336) | 117 (44) |
| T3 (≥3336) | 60 (23) |
| rMedDiet score (point) | |
| T1 (<9) | 92 (36) |
| T2 (9–12) | 107 (42) |
| T3 (≥12) | 56 (22) |
| Cardiometabolic Risk Markers in the First Trimester | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | SBP (mm Hg) | DBP (mm Hg) | Glucose (mg/dL) | Insulin (mU/L) ‡ | HOMA-IR ‡ | Triglycerides (mg/dL) ‡ | HDL-c (mg/dL) | LDL-c (mg/dL) | ||||||||||
| Characteristics | β | p | β | p | β | p | β | p | β | p | β | p | β | p | β | p | β | p |
| Age categories (years) | ||||||||||||||||||
| <25 vs. 25–29 | −0.09 | 0.843 | 1.62 | 0.491 | 1.73 | 0.269 | 2.97 | 0.188 | −0.20 | 0.085 | −0.16 | 0.208 | −0.13 | 0.095 | 4.88 | 0.068 | −1.90 | 0.714 |
| <25 vs. ≥30 | 0.04 | 0.920 | 2.02 | 0.371 | 1.93 | 0.199 | 3.34 | 0.123 | −0.08 | 0.442 | −0.04 | 0.731 | −0.05 | 0.508 | 6.93 | 0.007 ** | 2.24 | 0.652 |
| BMI categories | ||||||||||||||||||
| Normal weight vs. | 5.83 | <0.001 ** | 5.42 | 0.001 ** | 3.64 | <0.001 ** | 1.95 | 0.188 | 0.30 | <0.001 ** | 0.33 | <0.001 ** | 0.09 | 0.067 | −2.56 | 0.144 | 6.80 | 0.045 * |
| overweight/obesity | ||||||||||||||||||
| Educational level | ||||||||||||||||||
| Low/medium vs. high | −0.68 | 0.030 * | −4.12 | 0.017 * | −1.61 | 0.158 | −1.46 | 0.373 | 0.01 | 0.923 | −0.01 | 0.937 | −0.08 | 0.162 | 2.92 | 0.132 | 0.31 | 0.934 |
| Social class | ||||||||||||||||||
| Low vs. medium/high | −0.05 | 0.889 | 1.96 | 0.383 | 0.06 | 0.969 | 0.23 | 0.916 | −0.18 | 0.107 | −0.16 | 0.186 | −0.00 | 0.998 | 0.17 | 0.947 | 1.18 | 0.811 |
| Smoking status | ||||||||||||||||||
| Never smoker vs. | −0.20 | 0.489 | −2.62 | 0.103 | −0.99 | 0.353 | 2.33 | 0.130 | −0.05 | 0.492 | −0.01 | 0.893 | −0.09 | 0.081 | −1.54 | 0.395 | −3.63 | 0.303 |
| current/former smoker | ||||||||||||||||||
| Alcohol consumption | ||||||||||||||||||
| No vs. yes | 0.02 | 0.970 | 3.21 | 0.144 | 1.70 | 0.243 | −0.44 | 0.834 | −0.08 | 0.473 | −0.07 | 0.529 | −0.04 | 0.547 | 0.48 | 0.845 | 0.97 | 0.840 |
| PA (METs-min/week) | ||||||||||||||||||
| T1 vs. T2 | 0.05 | 0.875 | −0.32 | 0.851 | −1.50 | 0.181 | 0.46 | 0.775 | 0.017 | 0.839 | 0.01 | 0.871 | −0.06 | 0.307 | 0.11 | 0.954 | −2.98 | 0.420 |
| T1 vs. T3 | −0.57 | 0.113 | −0.94 | 0.632 | −1.45 | 0.265 | 0.85 | 0.648 | −0.08 | 0.421 | −0.07 | 0.497 | −0.12 | 0.068 | 2.38 | 0.282 | −9.83 | 0.023 * |
| rMedDiet score (point) | ||||||||||||||||||
| T1 vs. T2 | −0.31 | 0.308 | −1.33 | 0.422 | −0.32 | 0.770 | 0.74 | 0.643 | −0.01 | 0.968 | 0.00 | 0.993 | 0.09 | 0.087 | −0.63 | 0.738 | 3.02 | 0.407 |
| T1 vs. T3 | −0.45 | 0.216 | −0.20 | 0.919 | −0.30 | 0.823 | 1.32 | 0.487 | −0.09 | 0.357 | −0.07 | 0.517 | 0.11 | 0.077 | 0.48 | 0.829 | −3.78 | 0.387 |
| Cardiometabolic Risk Markers in the Third Trimester | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | SBP (mm Hg) | DBP (mm Hg) | Glucose (mg/dL) | Insulin (mU/L) ‡ | HOMA-IR ‡ | Triglycerides (mg/dL) ‡ | HDL-c (mg/dL) | LDL-c (mg/dL) | ||||||||||
| Characteristics | β | p | β | p | β | p | β | p | β | p | β | p | β | p | β | p | β | p |
| Age categories (years) | ||||||||||||||||||
| <25 vs. 25–29 | 0.50 | 0.225 | −0.92 | 0.705 | −0.41 | 0.822 | 2.48 | 0.276 | −0.15 | 0.264 | −0.11 | 0.444 | −0.02 | 0.844 | 3.49 | 0.278 | 6.05 | 0.456 |
| <25 vs. ≥30 | 0.50 | 0.200 | 0.25 | 0.913 | −0.69 | 0.696 | 3.03 | 0.164 | −0.19 | 0.140 | −0.15 | 0.283 | −0.04 | 0.712 | 3.07 | 0.317 | 8.81 | 0.256 |
| BMI categories | ||||||||||||||||||
| Normal weight vs. over | 4.14 | <0.001 ** | −0.79 | 0.669 | 2.33 | 0.097 | −1.99 | 0.252 | 0.16 | 0.120 | 0.11 | 0.305 | 0.11 | 0.180 | −5.10 | 0.039 * | −1.21 | 0.845 |
| weight/obesity | ||||||||||||||||||
| IOM GWG recommendations | ||||||||||||||||||
| Adequate vs. insufficient | −1.32 | <0.001 ** | -4.07 | 0.018 * | -3.45 | 0.008 * | -3.38 | 0.036 * | -0.05 | 0.584 | -0.10 | 0.340 | 0.09 | 0.229 | 0.90 | 0.690 | −0.27 | 0.962 |
| Adequate vs. excessive | 2.15 | <0.001 ** | 5.76 | 0.017 * | 1.01 | 0.580 | -0.58 | 0.795 | 0.10 | 0.448 | 0.11 | 0.441 | 0.04 | 0.730 | 8.02 | 0.012 * | 4.46 | 0.576 |
| Educational level | ||||||||||||||||||
| Low/medium vs. high | −0.58 | 0.053 | 0.16 | 0.929 | −0.74 | 0.577 | −1.28 | 0.436 | −0.09 | 0.323 | −0.11 | 0.297 | −0.08 | 0.330 | 2.23 | 0.336 | 1.12 | 0.060 |
| Social class | ||||||||||||||||||
| Low vs. medium/high | −0.38 | 0.342 | −1.78 | 0.447 | −0.64 | 0.719 | −5.87 | 0.008 * | −0.40 | 0.002 * | −0.49 | <0.001 ** | −0.15 | 0.153 | 2.32 | 0.454 | −7.57 | 0.335 |
| Smoking status | ||||||||||||||||||
| Never smoker vs. | 0.29 | 0.311 | −0.43 | 0.799 | 1.27 | 0.321 | −0.77 | 0.625 | 0.09 | 0.304 | 0.08 | 0.395 | 0.18 | 0.016 * | 3.07 | 0.168 | 1.19 | 0.034 * |
| current/former smoker | ||||||||||||||||||
| Alcohol consumption | ||||||||||||||||||
| No vs. yes | −0.20 | 0.586 | 4.74 | 0.032 * | 3.57 | 0.034 * | 1.41 | 0.493 | −0.02 | 0.859 | 0.01 | 0.959 | −0.00 | 1.000 | −1.94 | 0.503 | 1.57 | 0.032 * |
| PA (METs-min/week) | ||||||||||||||||||
| T1 vs. T2 | 0.19 | 0.523 | 1.99 | 0.257 | −1.15 | 0.388 | 1.33 | 0.414 | 0.00 | 0.960 | 0.03 | 0.789 | −0.09 | 0.230 | 1.54 | 0.504 | −2.35 | 0.685 |
| T1 vs. T3 | −0.52 | 0.130 | 0.62 | 0.760 | −1.72 | 0.263 | −1.75 | 0.356 | 0.13 | 0.237 | 0.09 | 0.465 | −0.01 | 0.877 | 0.67 | 0.801 | −4.47 | 0.508 |
| rMedDiet score (point) | ||||||||||||||||||
| T1 vs. T2 | 0.21 | 0.491 | −1.27 | 0.470 | 0.66 | 0.624 | −1.99 | 0.226 | −0.02 | 0.819 | −0.06 | 0.536 | 0.05 | 0.562 | 3.66 | 0.117 | −7.02 | 0.233 |
| T1 vs. T3 | −0.19 | 0.587 | 1.42 | 0.494 | 0.14 | 0.931 | −0.84 | 0.665 | −0.11 | 0.339 | −0.12 | 0.333 | 0.06 | 0.527 | 2.12 | 0.438 | −2.89 | 0.675 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motevalizadeh, E.; Díaz-López, A.; Martín-Luján, F.; Basora, J.; Arija, V. Prenatal Factors Associated with Maternal Cardiometabolic Risk Markers during Pregnancy: The ECLIPSES Study. Nutrients 2023, 15, 1135. https://doi.org/10.3390/nu15051135
Motevalizadeh E, Díaz-López A, Martín-Luján F, Basora J, Arija V. Prenatal Factors Associated with Maternal Cardiometabolic Risk Markers during Pregnancy: The ECLIPSES Study. Nutrients. 2023; 15(5):1135. https://doi.org/10.3390/nu15051135
Chicago/Turabian StyleMotevalizadeh, Ehsan, Andrés Díaz-López, Francisco Martín-Luján, Josep Basora, and Victoria Arija. 2023. "Prenatal Factors Associated with Maternal Cardiometabolic Risk Markers during Pregnancy: The ECLIPSES Study" Nutrients 15, no. 5: 1135. https://doi.org/10.3390/nu15051135
APA StyleMotevalizadeh, E., Díaz-López, A., Martín-Luján, F., Basora, J., & Arija, V. (2023). Prenatal Factors Associated with Maternal Cardiometabolic Risk Markers during Pregnancy: The ECLIPSES Study. Nutrients, 15(5), 1135. https://doi.org/10.3390/nu15051135










