Dietary Sources of Anthocyanins and Their Association with Metabolome Biomarkers and Cardiometabolic Risk Factors in an Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Anthropometric Measurements
2.3. Dietary Data
Dietary Intake of Anthocyanins
2.4. Blood Sampling, Analysis of Cardiometabolic Risk Factors and Metabolomics
2.4.1. Metabolomics Analysis of Plasma Samples
2.4.2. Metabolomics Data Pre-Processing
2.5. Statistical Analyses
3. Results
3.1. Sociodemographic, Clinical, and Dietary Characteristics
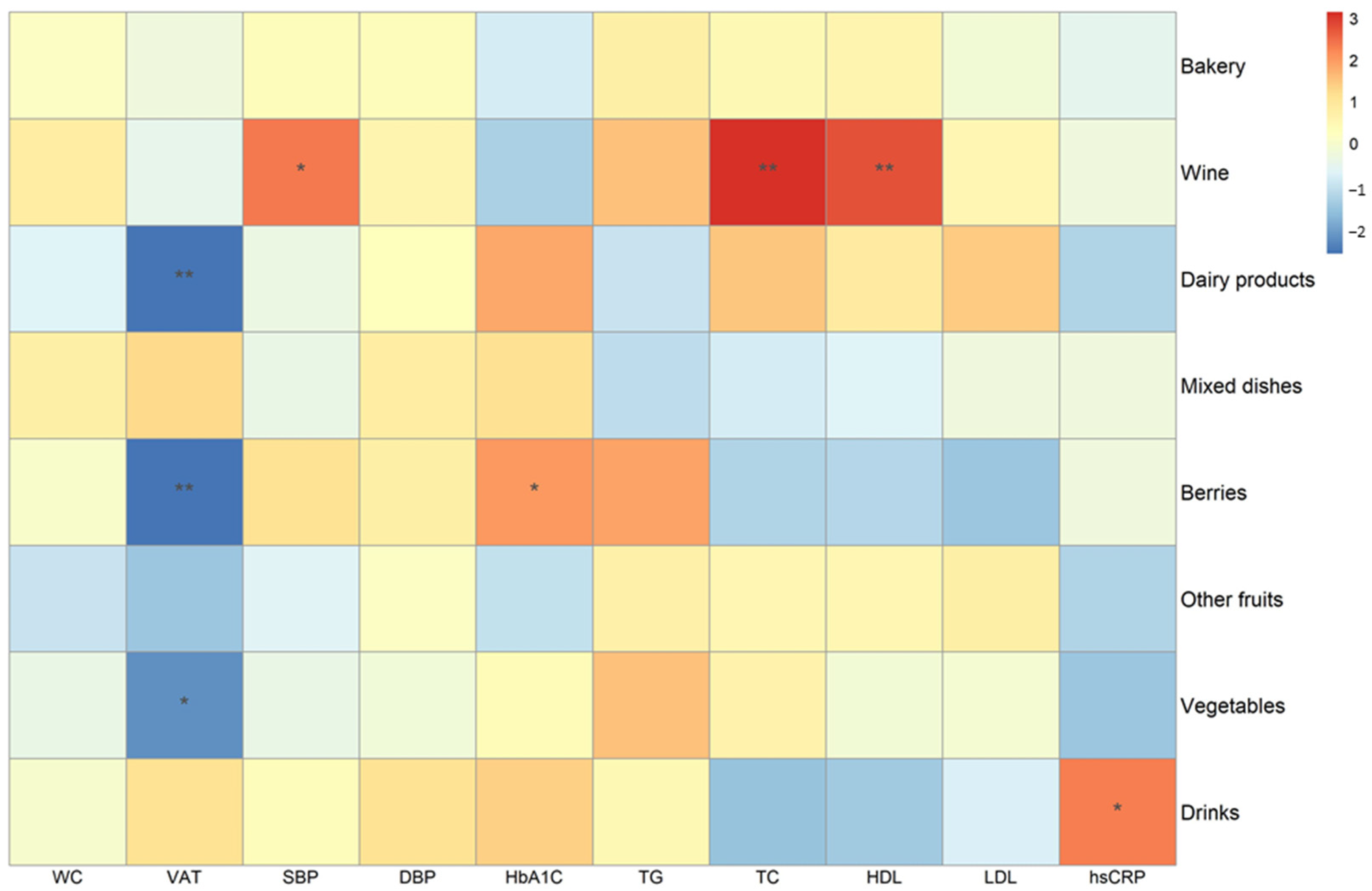
3.2. Association between Intake of ACN Dietary Sources and Cardiometabolic Risk Factors
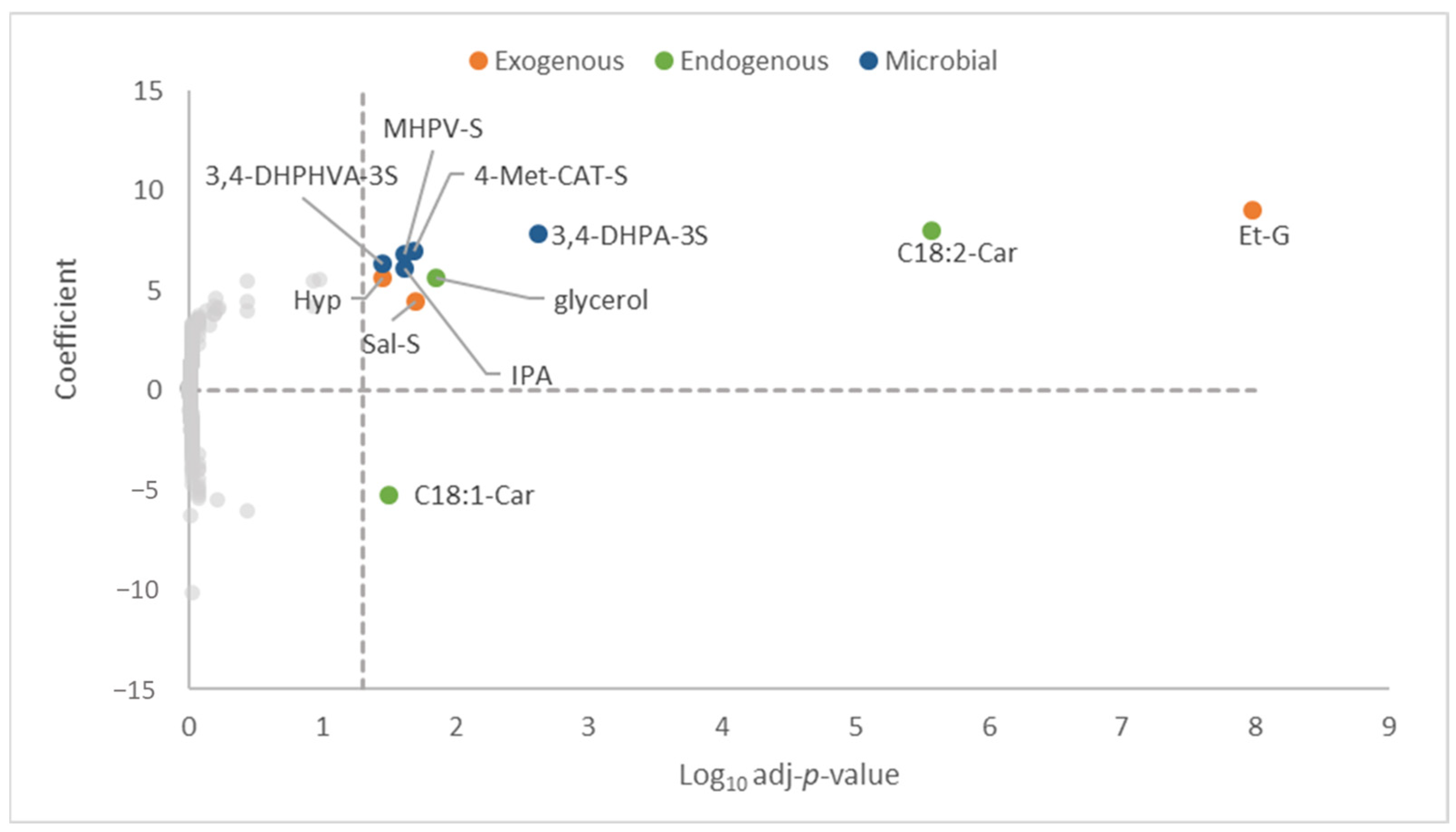
3.3. Metabolome Biomarkers Associated with Total ACN Intake
3.4. Metabolome Biomarkers Associated with Intake of ACNs Related to Different ACN Dietary Sources
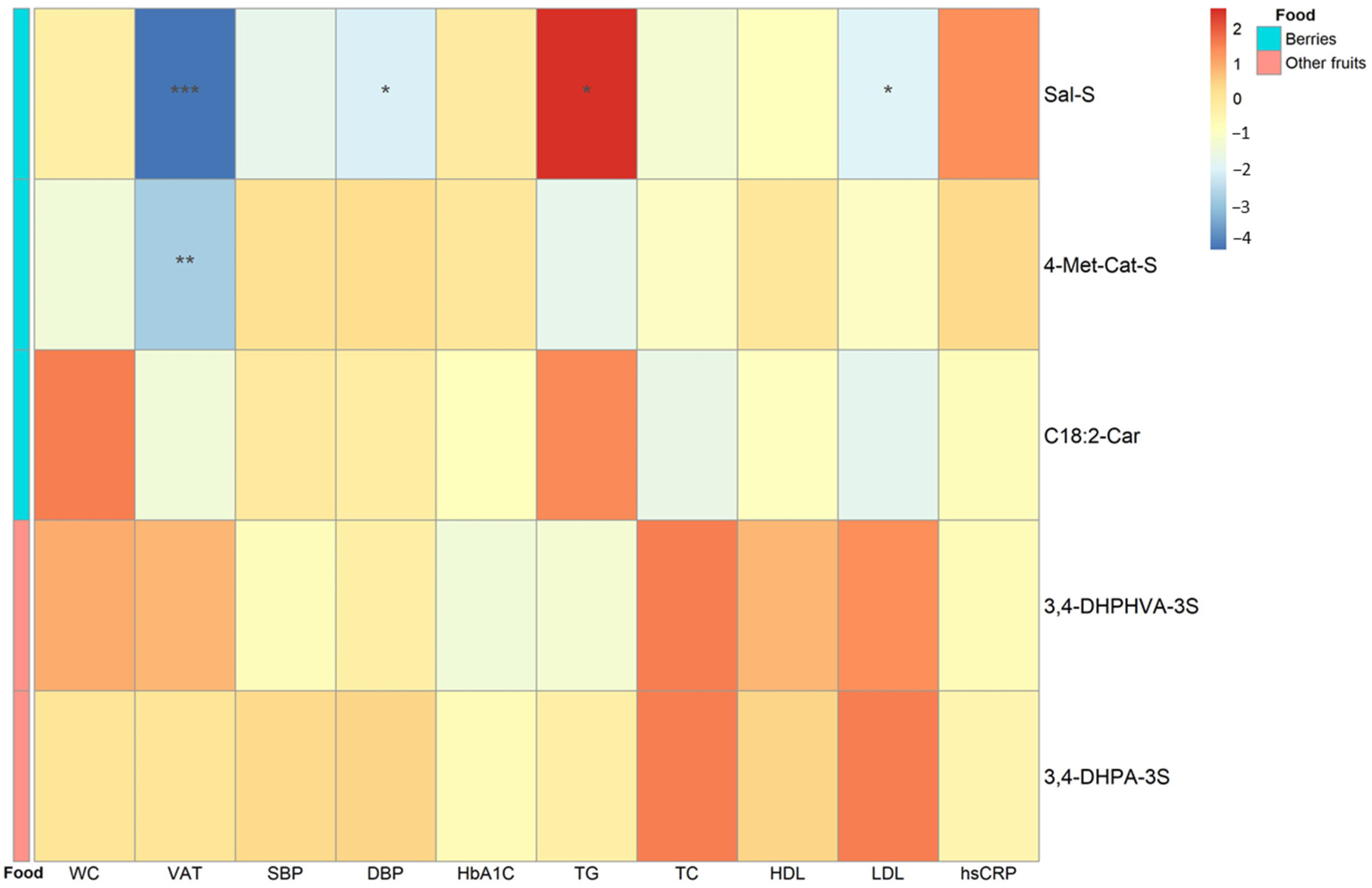
3.5. Associations between Selected ACN-Related Metabolome Biomarkers and Cardiometabolic Risk Factors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Celli, G.B.; Tan, C.; Selig, M.J.; States, U. Anthocyanidins and Anthocyanins. Encycl. Food Chem. 2017, 218–223. [Google Scholar] [CrossRef]
- Fang, J. Bioavailability of Anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-Week Consumption of a Wild Blueberry Powder Drink Increases Bifidobacteria in the Human Gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F.; Group, F. Health Benefits of Anthocyanins and Molecular Mechanisms: Update from Recent Decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Lee, S.; Keirsey, K.I.; Kirkland, R.; Grunewald, Z.I.; Fischer, J.G.; de La Serre, C.B. Blueberry Supplementation Influences the Gut Microbiota, Inflammation, and Insulin Resistance in High-Fat-Diet-Fed Rats. J. Nutr. 2018, 148, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A. Berry Anthocyanin Intake and Cardiovascular Health. Mol. Aspects Med. 2018, 61, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Stalmach, A.; Edwards, C.A.; Wightman, J.D.; Crozier, A. Gastrointestinal Stability and Bioavailability of (Poly) Phenolic Compounds Following Ingestion of Concord Grape Juice by Humans. Mol. Nutr. Food Res. 2012, 56, 497–509. [Google Scholar] [CrossRef]
- Azzini, E.; Vitaglione, P.; Intorre, F.; Napolitano, A.; Durazzo, A.; Foddai, M.S.; Fumagalli, A.; Catasta, G.; Rossi, L.; Venneria, E.; et al. Bioavailability of Strawberry Antioxidants in Human Subjects. Br. J. Nutr. 2010, 104, 1165–1173. [Google Scholar] [CrossRef] [Green Version]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A. The Pharmacokinetics of Anthocyanins and Their Metabolites in Humans. Br. J. Clin. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [Green Version]
- Tjønneland, A.; Olsen, A.; Boll, K.; Stripp, C.; Christensen, J.; Engholm, G.; Overvad, K. Study Design, Exposure Variables, and Socioeconomic Determinants of Participation in Diet, Cancer and Health: A Population-Based Prospective Cohort Study of 57,053 Men and Women in Denmark. Scand. J. Public Health 2007, 35, 432–441. [Google Scholar] [CrossRef]
- Petersen, K.E.N.; Halkjær, J.; Loft, S.; Tjønneland, A.; Olsen, A. Cohort Profile and Representativeness of Participants in the Diet, Cancer and Health-Next Generations Cohort Study. Eur. J. Epidemiol. 2022, 37, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Wark, P.A.; Hardie, L.J.; Frost, G.S.; Alwan, N.A.; Carter, M.; Elliott, P.; Ford, H.E.; Hancock, N.; Morris, M.A.; Mulla, U.Z.; et al. Validity of an Online 24-h Recall Tool (Myfood24) for Dietary Assessment in Population Studies: Comparison with Biomarkers and Standard Interviews. BMC Med. 2018, 16, 136. [Google Scholar] [CrossRef] [PubMed]
- Finglas, P.M.; Roe, M.; Pinchen, H.M.; Berry, R.; Church, S.; Dodhia, S.K.; Farron-Wilson, M.; Swan, G. McCance and Widdowson’s The Composition of Foods, Seventh Summary Edition; Royal Society of Chemistry: Cambridg, UK, 2015. [Google Scholar]
- Tjønneland, A.; Overvad, K.; Haraldsdóttir, J.; Bang, S.; Ewertz, M.; Jensen, O.M. Validation of a Semiquantitative Food Frequency Questionnaire Developed in Denmark. Int. J. Epidemiol. 1991, 20, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Knaze, V.; Rothwell, J.A.; Zamora-Ros, R.; Moskal, A.; Kyrø, C.; Jakszyn, P.; Skeie, G.; Weiderpass, E.; De Magistris, M.S.; Agnoli, C.; et al. A New Food-Composition Database for 437 Polyphenols in 19,899 Raw and Prepared Foods Used to Estimate Polyphenol Intakes in Adults from 10 European Countries. Am. J. Clin. Nutr. 2018, 108, 517–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanuza, F.; Zamora-Ros, R.; Rostgaard-Hansen, A.L.; Tjønneland, A.; Landberg, R.; Halkjær, J.; Andres-Lacueva, C. Descriptive Analysis of Dietary (Poly)Phenol Intake in the Subcohort MAX from DCH-NG: “Diet, Cancer and Health—Next Generations Cohort”. Eur. J. Nutr. 2022, 62, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, S.; Bernardi, S.; Del Bo’, C.; Cherubini, A.; Porrini, M.; Gargari, G.; Hidalgo-Liberona, N.; Gonzalez-Dominguez, R.; Peron, G.; Zamora-Ros, R.; et al. Effect of a Polyphenol-Rich Dietary Pattern on Intestinal Permeability and Gut and Blood Microbiomics in Older Subjects: Study Protocol of the MaPLE Randomised Controlled Trial. BMC Geriatr. 2020, 20, 77. [Google Scholar] [CrossRef] [Green Version]
- González-Domínguez, R.; Jáuregui, O.; Queipo-Ortuño, M.I.; Andrés-Lacueva, C. Characterization of the Human Exposome by a Comprehensive and Quantitative Large-Scale Multianalyte Metabolomics Platform. Anal. Chem. 2020, 92, 13767–13775. [Google Scholar] [CrossRef]
- Andres-Lacueva, C.; Shukitt-Hale, B.; Galli, R.L.; Jauregui, O.; Lamuela-Raventos, R.M.; Joseph, J.A. Anthocyanins in Aged Blueberry-Fed Rats Are Found Centrally and May Enhance Memory. Nutr. Neurosci. 2005, 8, 111–120. [Google Scholar] [CrossRef]
- Castellano-Escuder, P.; Gonzalez-Domnguez, R.; Carmona-Pontaque, F.; Andrés-Lacueva, C.; Sanchez-Pla, A. POMAShiny: A User-Friendly Web-Based Workflow for Metabolomics and Proteomics Data Analysis. PLoS Comput. Biol. 2021, 17, e1009148. [Google Scholar] [CrossRef]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef] [Green Version]
- Croissant, Y.; Millo, G. Panel Data Econometrics in R: The Plm Package. J. Stat. Softw. 2008, 27, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Haslbeck, J.M.B.; Waldorp, L.J. MGM: Estimating Time-Varying Mixed Graphical Models in High-Dimensional Data. J. Stat. Softw. 2020, 93. [Google Scholar] [CrossRef]
- Roura, E.; Andrés-Lacueva, C.; Estruch, R.; Bilbao, M.L.M.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. The Effects of Milk as a Food Matrix for Polyphenols on the Excretion Profile of Cocoa (-)-Epicatechin Metabolites in Healthy Human Subjects. Br. J. Nutr. 2008, 100, 846–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-González, I.; Jiménez-Girón, A.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. Profiling of Microbial-Derived Phenolic Metabolites in Human Feces after Moderate Red Wine Intake. J. Agric. Food Chem. 2013, 61, 9470–9479. [Google Scholar] [CrossRef]
- Ulaszewska, M.; Garcia-Aloy, M.; Vázquez-Manjarrez, N.; Soria-Florido, M.T.; Llorach, R.; Mattivi, F.; Manach, C. Food Intake Biomarkers for Berries and Grapes. Genes Nutr. 2020, 15, 17. [Google Scholar] [CrossRef]
- Faraj, B.A.; Camp, V.M.; Davis, D.C.; Lenton, J.D.; Kutner, M. Elevation of Plasma Salsolinol Sulfate in Chronic Alcoholics as Compared to Nonalcoholics. Alcohol. Clin. Exp. Res. 1989, 13, 155–163. [Google Scholar] [CrossRef]
- Rojkovicova, T.; Mechref, Y.; Starkey, J.A.; Wu, G.; Bell, R.L.; McBride, W.J.; Novotny, M.V. Quantitative Chiral Analysis of Salsolinol in Different Brain Regions of Rats Genetically Predisposed to Alcoholism. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 863, 206–214. [Google Scholar] [CrossRef]
- Chen, P.S.; Yang, Y.K.; Yeh, T.L.; Lee, I.H.; Yao, W.J.; Chiu, N.T.; Lu, R.B. Correlation between Body Mass Index and Striatal Dopamine Transporter Availability in Healthy Volunteers-A SPECT Study. Neuroimage 2008, 40, 275–279. [Google Scholar] [CrossRef]
- Meireles, M.; Rodríguez-Alcalá, L.M.; Marques, C.; Norberto, S.; Freitas, J.; Fernandes, I.; Mateus, N.; Gomes, A.; Faria, A.; Calhau, C. Effect of Chronic Consumption of Blackberry Extract on High-Fat Induced Obesity in Rats and Its Correlation with Metabolic and Brain Outcomes. Food Funct. 2016, 7, 127–139. [Google Scholar] [CrossRef]
- Zhang, X.; Sandhu, A.; Edirisinghe, I.; Burton-Freeman, B.M. Plasma and Urinary (Poly)Phenolic Profiles after 4-Week Red Raspberry (Rubus Idaeus L.) Intake with or without Fructo-Oligosaccharide Supplementation. Molecules 2020, 25, 4777. [Google Scholar] [CrossRef]
- Baron, G.; Altomare, A.; Regazzoni, L.; Fumagalli, L.; Artasensi, A.; Borghi, E.; Ottaviano, E.; Del Bo, C.; Riso, P.; Allegrini, P.; et al. Profiling Vaccinium Macrocarpon Components and Metabolites in Human Urine and the Urine Ex-Vivo Effect on Candida Albicans Adhesion and Biofilm-Formation. Biochem. Pharmacol. 2020, 173, 113726. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; Berends, L.; van der Velpen, V.; Jennings, A.; Haag, L.; Chandra, P.; Kay, C.D.; Rimm, E.B.; Cassidy, A. Blueberry Anthocyanin Intake Attenuates the Postprandial Cardiometabolic Effect of an Energy-Dense Food Challenge: Results from a Double Blind, Randomized Controlled Trial in Metabolic Syndrome Participants. Clin. Nutr. 2022, 41, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Straßmann, S.; Passon, M.; Schieber, A. Chemical Hemisynthesis of Sulfated Cyanidin-3-o-Glucoside and Cyanidin Metabolites. Molecules 2021, 26, 2146. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xiao, D.; Zhang, X.; Sandhu, A.K.; Chandra, P.; Kay, C.; Edirisinghe, I.; Burton-Freeman, B. Strawberry Consumption, Cardiometabolic Risk Factors, and Vascular Function: A Randomized Controlled Trial in Adults with Moderate Hypercholesterolemia. J. Nutr. 2021, 151, 1517–1526. [Google Scholar] [CrossRef]
- Heiss, C.; Istas, G.; Feliciano, R.P.; Weber, T.; Wang, B.; Favari, C.; Mena, P.; Del Rio, D.; Rodriguez-Mateos, A. Daily Consumption of Cranberry Improves Endothelial Function in Healthy Adults: A Double Blind Randomized Controlled Trial. Food Funct. 2022, 13, 3812–3824. [Google Scholar] [CrossRef]
- Behrendt, I.; Röder, I.; Will, F.; Mostafa, H.; Gonzalez-Dominguez, R.; Meroño, T.; Andres-Lacueva, C.; Fasshauer, M.; Rudloff, S.; Kuntz, S. Influence of Plasma-Isolated Anthocyanins and Their Metabolites on Cancer Cell Migration (HT-29 and Caco-2) In Vitro: Results of the ATTACH Study. Antioxidants 2022, 11, 1341. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Slimani, N.; Romieu, I.; Touillaud, M.; Kaaks, R.; Teucher, B.; Mattiello, A.; Grioni, S.; et al. Estimation of the Intake of Anthocyanidins and Their Food Sources in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Br. J. Nutr. 2011, 106, 1090–1099. [Google Scholar] [CrossRef] [Green Version]
- Lagiou, P.; Rossi, M.; Lagiou, A.; Tzonou, A.; La Vecchia, C.; Trichopoulos, D. Flavonoid Intake and Liver Cancer: A Case-Control Study in Greece. Cancer Causes Control 2008, 19, 813–818. [Google Scholar] [CrossRef]
- Lagiou, P.; Samoli, E.; Lagiou, A.; Tzonou, A.; Kalandidi, A.; Peterson, J.; Dwyer, J.; Trichpoulos, D. Intake of Specific Flavonoid Classes and Coronary Heart Disease—A Case-Control Study in Greece. Eur. J. Clin. Nutr. 2004, 58, 1643–1648. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; Garavello, W.; Talamini, R.; Negri, E.; Bosetti, C.; Dal Maso, L.; Lagiou, P.; Tavani, A.; Polesel, J.; Barzan, L.; et al. Flavonoids and the Risk of Oral and Pharyngeal Cancer: A Case-Control Study from Italy. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1621–1625. [Google Scholar] [CrossRef] [Green Version]

| All n = 624 k = 1351 | Tertile 1 <0.3 mg ACN/Day k = 453 | Tertile 2 0.3–8.9 mg ACN/Day k = 448 | Tertile 3 >8.9 mg ACN/Day k = 450 | |
|---|---|---|---|---|
| Age (years) | 44.7 ± 12.3 | 43.7 ± 12.6 | 44.2 ±12.5 | 46.1 ± 11.8 |
| Gender, female (n, %) | 745 (55) | 236 (52) | 250 (55) | 256 (57) |
| BMI (kg/m2) | 25 ± 4 | 25 ± 4 | 24 ± 3 | 25 ± 4 |
| WC (cm) | 87.5 ± 12.1 | 88.7 ± 12.4 | 86.0 ± 11.6 | 87.7 ± 12 |
| VAT (L) | 1.3 (0.7–2.5) | 1.5 (0.8–2.6) | 1.2 (0.6–2.1) | 1.4 (0.8–2.5) |
| Physical activity (n, %) | ||||
| Not regular | 114 (17) | 41 (19) | 25 (12) | 37 (18) |
| Once/month last 6 months | 52 (89 | 15 (79 | 22 (11) | 12 (6) |
| Once/month last 12 months | 510 (75) | 162 (74) | 159 (77) | 152 (76) |
| Smoking status (n, %) | ||||
| Never | 353 (52.2) | 114 (52.3) | 117 (56.89 | 97 (48.3) |
| Former | 186 (27.5) | 53 (24.3) | 53 (25.79) | 69 (34.39) |
| Current | 137 (20.3) | 51 (23.4) | 36 (17.59) | 35 (17.4) |
| SBP (mmHg) | 117 ± 16 | 117 ± 15 | 116 ± 15 | 116 ± 16 |
| DBP (mmHg) | 81 ± 11 | 80 ± 10 | 79 ± 10 | 80 ± 11 |
| HbA1c (mmol/mol) | 34.5 ± 6 | 34.6 ± 7 | 33.8 ± 5 | 34.6 ± 6 |
| TG (mmol/L) | 1.1 (0.8–1.6) | 1.1 (0.8–1.7) | 1.0 (0.8–1.4) | 1.1 (0.8–1.6) |
| TC (mmol/L) | 4.9 ± 1.0 | 4.9 ± 0.9 | 4.9 ± 0.9 | 5.1 ± 1.0 |
| HDL (mmol/L) | 1.6 ± 0.4 | 1.5 ± 0.4 | 1.6 ± 0.4 | 1.6 ± 0.5 |
| LDL (mmol/L) | 3.0 ± 0.9 | 3.0 ± 0.9 | 2.9 ± 0.9 | 3.1 ± 0.9 |
| hsCRP (mg/L) | 0.7 (0.3–1.6) | 0.8 (0.3–1.6) | 0.7 (0.3–1.5) | 0.7 (0.3–1.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, H.; Meroño, T.; Miñarro, A.; Sánchez-Pla, A.; Lanuza, F.; Zamora-Ros, R.; Rostgaard-Hansen, A.L.; Estanyol-Torres, N.; Cubedo-Culleré, M.; Tjønneland, A.; et al. Dietary Sources of Anthocyanins and Their Association with Metabolome Biomarkers and Cardiometabolic Risk Factors in an Observational Study. Nutrients 2023, 15, 1208. https://doi.org/10.3390/nu15051208
Mostafa H, Meroño T, Miñarro A, Sánchez-Pla A, Lanuza F, Zamora-Ros R, Rostgaard-Hansen AL, Estanyol-Torres N, Cubedo-Culleré M, Tjønneland A, et al. Dietary Sources of Anthocyanins and Their Association with Metabolome Biomarkers and Cardiometabolic Risk Factors in an Observational Study. Nutrients. 2023; 15(5):1208. https://doi.org/10.3390/nu15051208
Chicago/Turabian StyleMostafa, Hamza, Tomás Meroño, Antonio Miñarro, Alex Sánchez-Pla, Fabián Lanuza, Raul Zamora-Ros, Agnetha Linn Rostgaard-Hansen, Núria Estanyol-Torres, Marta Cubedo-Culleré, Anne Tjønneland, and et al. 2023. "Dietary Sources of Anthocyanins and Their Association with Metabolome Biomarkers and Cardiometabolic Risk Factors in an Observational Study" Nutrients 15, no. 5: 1208. https://doi.org/10.3390/nu15051208
APA StyleMostafa, H., Meroño, T., Miñarro, A., Sánchez-Pla, A., Lanuza, F., Zamora-Ros, R., Rostgaard-Hansen, A. L., Estanyol-Torres, N., Cubedo-Culleré, M., Tjønneland, A., Landberg, R., Halkjær, J., & Andres-Lacueva, C. (2023). Dietary Sources of Anthocyanins and Their Association with Metabolome Biomarkers and Cardiometabolic Risk Factors in an Observational Study. Nutrients, 15(5), 1208. https://doi.org/10.3390/nu15051208









