Dietary Intake and Circulating Amino Acid Concentrations in Relation with Bone Metabolism Markers in Children Following Vegetarian and Omnivorous Diets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Dietary Assessment
2.3. Biochemical Analyses
2.4. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ewy, M.W.; Patel, A.; Abdelmagid, M.G.; Elfadil, O.M.; Bonnes, S.L.; Salonen, B.R.; Hurt, R.T.; Mundi, M.S. Plant-based diet: Is it as good as an animal-based diet when it comes to protein? Curr. Nutr. Rep. 2022, 11, 337–346. [Google Scholar] [CrossRef]
- Darling, A.L.; Millward, D.J.; Torgerson, D.J.; Hewitt, C.E.; Lanham-New, S.A. Dietary protein and bone health: A systemic review and meta-analysis. Am. J. Clin. Nutr. 2009, 90, 1674–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsh, K.A.; Munn, E.A.; Baines, S.K. Protein and vegetarian diets. Med. J. Aust. 2013, 199, S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Kersting, M.; Alexy, U.; Schurmann, S. Critical dietary habits in early childhood: Principles and practice. World Rev. Nutr. Diet 2016, 115, 24–35. [Google Scholar] [PubMed]
- Baroni, L.; Goggi, S.; Battino, M. Planning well-balanced vegetarian diets in infants, children, and adolescents: The VegPlate Junior. J. Acad. Nutr. Diet 2019, 119, 1067–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Devignes, C.S.; Carmeliet, G.; Stegen, S. Amino acid metabolism in skeletal cells. Bone Rep. 2022, 17, 101620. [Google Scholar] [CrossRef]
- Ding, K.H.; Cain, M.; Davis, M.; Bergson, C.; McGee-Lawrence, M.; Perkins, C.; Hardigan, T.; Shi, X.; Zhong, Q.; Xu, J.; et al. Amino acids as signaling molecules modulating bone turnover. Bone 2018, 115, 15–24. [Google Scholar] [CrossRef]
- Cui, Z.; Feng, H.; He, B.; He, J.; Tian, Y. Relationship between serum amino acid levels and bone mineral density: A Mendelian Randomization Study. Front. Endocrinol. 2021, 12, 763538. [Google Scholar] [CrossRef]
- Lv, Z.; Shi, W.; Zhang, Q. Role of essential amino acids in age-induced bone loss. Int. J. Mol. Sci. 2022, 23, 11281. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Wang, W.; Liu, J. Insulin stimulates osteoblast proliferation and differentiation through ERK and PI3K in MG-63 cells. Cell Biochem. Funct. 2010, 28, 334–341. [Google Scholar] [CrossRef]
- Wolfe, R.R. Branched-chain amino acids and muscle protein synthesis in humans: Myth or reality? J. Int. Soc. Sports Nutr. 2017, 14, e30. [Google Scholar] [CrossRef] [Green Version]
- Gojda, J.; Rossmeislova, L.; Strakova, R.; Turmova, J.; Elkalaf, M.; Jacek, M.; Tuma, P.; Potockova, J.; Krauzova, E.; Waldauf, P.; et al. Chronic dietary exposure to branched chain amino acids impairs glucose disposal in vegans but not in omnivores. Eur. J. Clin. Nutr. 2017, 71, 594–601. [Google Scholar] [CrossRef]
- Peretti, N.; Darmaun, D.; Chouraqui, J.P.; Bocquet, A.; Briend, A.; Feillet, F.; Frelut, M.L.; Guimber, D.; Hankard, R.; Lapillonne, A.; et al. Vegetarian diet in children and adolescents: A health benefit? Arch. Pediatr. 2020, 27, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, M.E.; Panza, R.; Farella, I.; Posa, D.; Capozza, M.; di Mauro, A.; Laforgia, N. Vegetarian and vegan weaning of the infant: How common and how evidence-based? A population-based survey and narrative review. Int. J. Environ. Res. Public Health 2020, 17, 4835. [Google Scholar] [CrossRef] [PubMed]
- Melina, V.; Craig, W.; Lewin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Craig, W.J.; Mangels, A.R. Position of the American Dietetic Association: Vegetarian diets. J. Am. Diet. Assoc. 2009, 109, 1266–1282. [Google Scholar]
- Appleby, P.N.; Key, T. The long-term health of vegetarians and vegans. Proc. Nutr. Soc. 2016, 75, 287–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-metabolic benefits of plant-based diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef] [Green Version]
- Van Winckel, M.; Vande Velde, S.; De Bruyne, R.; Van Biervliet, S. Clinical practice: Vegetarian infant and child nutrition. Eur. J. Pediatr. 2011, 170, 1489–1494. [Google Scholar] [CrossRef]
- Schurmann, S.; Kersting, M.; Alexy, U. Vegetarian diets in children: A systematic review. Eur. J. Nutr. 2017, 56, 1797–1817. [Google Scholar] [CrossRef] [PubMed]
- Cofnas, N. Is vegetarianism healthy for children? Crit. Rev. Food Sci. Nutr. 2019, 59, 2052–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, F.L.; Lloren, J.I.C.; Haddad, E.; Jaceldo-Siegl, K.; Knutsen, S.; Sabate, J.; Fraser, G.E. Plasma, urine and adipose tissue biomarkers of dietary intake differ between vegetarian and non-vegetarian diet groups in the Adventist Health Study-2. J. Nutr. 2019, 149, 667–675. [Google Scholar] [CrossRef]
- Miles, F.L.; Orlich, M.J.; Mashchak, A.; Chandler, P.D.; Lampe, J.W.; Duerksen-Hughes, P.; Fraser, G.E. The biology of veganism: Plasma metabolomics analysis reveals distinct profiles of vegans and non-vegetarians in the Adventist Health Study-2 Cohort. Nutrients 2022, 14, 709. [Google Scholar] [CrossRef]
- Hernández-Alonso, P.; Becerra-Tomás, N.; Papandreou, C.; Bulló, M.; Guasch-Ferré, M.; Toledo, E.; Ruiz-Canela, M.; Clish, C.B.; Corella, D.; Dennis, C.; et al. Plasma metabolomics profiles are associated with the amount and source of protein intake: A metabolomics approach within the PREDIMED Study. Mol. Nutr. Food Res. 2020, 64, e2000178. [Google Scholar] [CrossRef]
- Neufingerl, N.; Eilander, A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: A systemic review. Nutrients 2022, 14, 29. [Google Scholar] [CrossRef]
- Tong, T.Y.N.; Appleby, P.N.; Armstrong, M.E.G.; Fensom, G.K.; Knuppel, A.; Papier, K.; Perez-Cornago, A.; Travis, R.C.; Key, T.J. Vegetarian and vegan diets and risks of total and site-specific fractures: Results from the prospective EPIC-Oxford study. BMC Med. 2020, 18, 353. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.I. Vegetarian diets and bone status. Am. J. Clin. Nutr. 2014, 100, 329S–335S. [Google Scholar] [CrossRef] [Green Version]
- Iguacel, I.; Miguel-Berges, M.L.; Gomez-Bruton, A.; Moreno, L.; Julian, C. Veganism, vegetarianism, bone mineral density, and fracture risk: A systemic review and meta-analysis. Nutr. Rev. 2019, 77, 1–18. [Google Scholar] [CrossRef]
- Schini, M.; Vilaca, T.; Gossiel, F.; Salam, S.; Eastell, R. Bone turnover markers: Basic biology to clinical applications. Endocr. Rev. 2022, bnac031. [Google Scholar] [CrossRef]
- Vasikaran, S.D.; Miura, M.; Pikner, R.; Bhattoa, H.P.; Cavalier, E.; IOF-IFCC Joint C-BM. Practical considerations for the clinical application of bone turnover markers in osteoporosis. Calcif. Tissue Int. 2023, 112, 148–157. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Chełchowska, M.; Szamotulska, K.; Rowicka, G.; Klemarczyk, W.; Strucińska, M.; Gajewska, J. Bone status and adipokine levels in children on vegetarian and omnivorous diets. Clin. Nutr. 2019, 38, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Kułaga, Z.; Różdżyńska-Świątkowska, A.; Grajda, A.; Gurzkowska, B.; Wojtyło, M.; Góźdź, M.; Światek-Leśniak, A.; Litwin, M. Percentile charts for growth and nutritional status assessment in Polish children and adolescents from birth to 18 years of age. Standardy Medyczne 2015, 12, 119–135. [Google Scholar]
- Ambroszkiewicz, J.; Klemarczyk, W.; Mazur, J.; Gajewska, J.; Rowicka, G.; Strucińska, M.; Chełchowska, M. Serum hepcidin and soluble transferrin receptor in the assessment of iron metabolism in children on a vegetarian diet. Biol. Trace Elem. Res. 2017, 180, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Szponar, L. Album of Photographs of Food Products; National Food and Nutrition Institute: Warsaw, Poland, 2008. [Google Scholar]
- Wajszczyk, B.; Chwojnowska, Z.; Nasiadko, D.; Rybaczuk, M. Dieta 5.0 Software for Individual and Group Nutrition Assessment and Diet Planning; National Food and Nutrition Institute: Warsaw, Poland, 2015. [Google Scholar]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Zywienia Dla Populacji Polskiej i Ich Zastosowania; Jarosz, M., Ed.; NIZP-PZH: Warsaw, Poland, 2020; pp. 26–148.
- Blau, N.; Duran, M.; Gibson, K.M. Laboratory Guide to the Methods in Biochemical Genetic; Springer: Berlin/Heidelberg, Germany, 2008; p. 74. [Google Scholar]
- Kniskern, M.A.; Johnston, C.S. Protein dietary reference intakes may be inadequate for vegetarians if low amounts of animal protein are consumed. Nutrition 2011, 27, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, N.S.; Jaceldo-Siegl, K.; Sabate, J.; Fraser, G.E. Nutrients profiles of vegetarian and nonvegetarian dietary patterns. J. Acad. Nutr. Diet 2013, 113, 1610–1619. [Google Scholar] [CrossRef] [Green Version]
- Camilieri, G.M.; Verger, E.O.; Huneau, J.F.; Carpentier, F.; Dubuisson, C.; Mariotti, F. Plant and animal protein intakes are differently associated with nutrient adequacy of the diet of French adults. J. Nutr. 2013, 143, 1466–1473. [Google Scholar] [CrossRef] [Green Version]
- Parker, H.W.; Vadiveloo, M.K. Diet quality of vegetarian diets compared with nonvegetarian diets: A systemic review. Nutr. Rev. 2019, 77, 144–166. [Google Scholar] [CrossRef]
- Sobiecki, J.G.; Appleby, P.N.; Bradbury, K.E.; Key, T.J. High compliance with dietary recommendations in a cohort of meat esters, fish eaters, vegetarians, and vegans: Results from European Prospective Investigation into Cancer and Nutrition—Oxford study. Nutr. Res. 2016, 36, 464–477. [Google Scholar] [CrossRef] [Green Version]
- Leser, S. The 2013 FAO report on dietary protein quality evaluation in human nutrition: Recommendations and implications. 2013 Br. Nutr. Found. Nutr. Bull. 2013, 38, 421–442. [Google Scholar] [CrossRef]
- FAO/WHO/UNU (Food and Agriculture Organization/World Health Organization/United Nations University). Protein and Amino Acid Requirements in Human Nutrition; Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 2007; Volume 935, pp. 1–265.
- Rouy, E.; Vico, L.; Laroche, N.; Benoit, V.; Rousseau, B.; Blachier, F.; Tomé, D.; Blais, A. Protein quality affects bone status during moderate protein restriction in growing mice. Bone 2014, 59, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhai, L.; Wei, W.; Dong, J. Effect of methionine restriction on bone density and NK cell activity. BioMed Res. Int. 2016, 2016, 3571810. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, S.; Trefflich, I.; Ueland, P.M.; Menzel, J.; Penczynski, K.J.; Abraham, K.; Weikert, C. Amino acid intake and plasma concentrations and their interplay with gut microbiota in vegans and omnivores in Germany. Eur. J. Nutr. 2022, 61, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Nebl, J.; Drabert, K.; Haufe, S.; Wasserfurth, P.; Eindorf, J.; Tegtbur, U.; Hahn, A.; Tsikas, D. Exercise-induced oxidative stress, nitric oxide and plasma amino acid profile in recreational runners with vegetarian and non-vegetarian dietary patterns. Nutrients 2019, 11, 1875. [Google Scholar] [CrossRef] [Green Version]
- Hovinen, T.; Korkalo, L.; Freese, R.; Skaffari, E.; Isohanni, P.; Niemi, M.; Nevalainen, J.; Gylling, H.; Zamboni, N.; Erkkola, M.; et al. Vegan diet in young children remodels metabolism and challenges the statuses of essential nutrients. EMBO Mol. Med. 2021, 13, e13492. [Google Scholar] [CrossRef]
- Mariotti, F.; Gardner, C.D. Dietary protein and amino acids in vegetarian diets: A Review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef] [Green Version]
- Fini, M.; Torricelli, P.; Giavaresi, G.; Carpi, A.; Nicolini, A.; Giardino, R. Effect of L-lysine and L-arginine on primary osteoblast cultures from normal and osteopenic rats. Biomed. Pharmacoter. 2001, 55, 213–220. [Google Scholar] [CrossRef]
- Bihuniak, J.D.; Insogna, K.L. The effect of dietary protein and amino acids on skeletal metabolism. Mol. Cell. Endocrinol. 2015, 410, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Xiu, S.; Chhetri, J.K.; Sun, L.; Mu, Z.; Wang, L. Association of serum prealbumin with risk of osteoporosis in older adults with type 2 diabetes mellitus: A cross-sectional study. Ther. Adv. Chronic Dis. 2019, 10, 204062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caso, G.; Scalfi, L.; Marra, M.; Covino, A.; Muscaritoli, M.; McNurlan, M.A.; Garlick, P.J.; Contaldo, F. Albumin synthesis is diminished in men consuming a predominantly vegetarian diet. J. Nutr. 2000, 130, 528–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menzel, J.; Abraham, K.; Stangl, G.I.; Ueland, P.M.; Obeid, R.; Schulze, M.B.; Herter-Aeberli, I.; Schwerdtle, T.; Weikert, C. Vegan diet and bone health—Results from the cross-sectional RBVD study. Nutrients 2021, 13, 685. [Google Scholar] [CrossRef]
- Aggarwal, R.; Bains, K. Protein, lysine and vitamin D: Critical role in muscle and bone health. Crit. Rev. Food Sci. Nutr. 2022, 62, 2548–2559. [Google Scholar] [CrossRef] [PubMed]
- MacDonell, R.; Hamrick, M.W.; Isales, C.M. Protein/amino-acid modulation of bone cell function. Bonekey Rep. 2016, 5, 827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, A.; MacGregor, A.; Spector, T.; Cassidy, A. Amino acid intakes are associated with bone mineral density and prevalence of low bone mass in women: Evidence from discordant monozygotic twins. J. Bone Miner. Res. 2015, 31, 326–335. [Google Scholar] [CrossRef] [Green Version]
- Afshinnia, F.; Pennathur, S. Association of hypoalbuminemia with osteoporosis: Analysis of the National Health and Nutrition Examination Servey. J. Clin. Endocrinol. Metab. 2016, 101, 2468–2474. [Google Scholar] [CrossRef] [Green Version]
- Craddock, J.C.; Neale, E.P.; Peoples, G.E.; Probst, Y.C. Vegetarian-based dietary patterns and their relation with inflammatory and immune biomarkers: A systemic review and meta-analysis. Adv. Nutr. 2019, 10, 433–451. [Google Scholar] [CrossRef]
- Findlay, D.M.; Atkins, G.J. Relationship between serum RANKL and RANKL in bone. Osteoporos. Int. 2011, 22, 2597–2602. [Google Scholar] [CrossRef]
- Lederer, A.K.; Maul-Pavicic, A.; Hannibal, L.; Hettich, M.; Steinborn, C.; Grundemann, C.; Zimmermann-Klemd, A.M.; Muller, A.; Sehnert, B.; Salzer, U.; et al. Vegan diet reduces neutrophils, monocytes, and platelets related to branched-chain amino acids—A randomized, controlled trial. Clin. Nutr. 2020, 39, 3241–3250. [Google Scholar] [CrossRef]
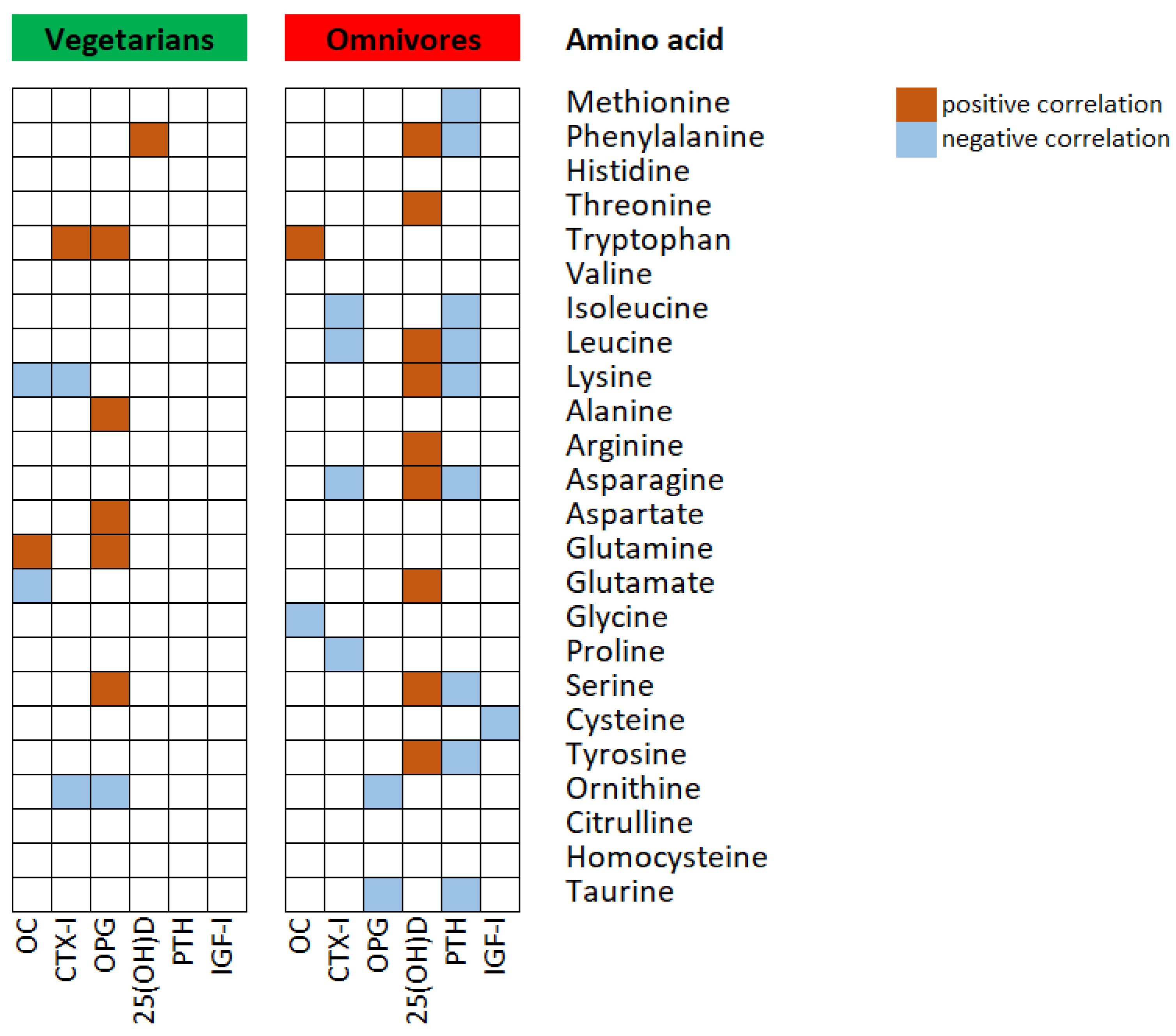
| Vegetarians | Omnivores | p Value | |
|---|---|---|---|
| Characteristics | |||
| n | 51 | 25 | |
| Girls, n (%) | 25 (49%) | 12 (48%) | |
| Age (years) | 6.0 (5.0–8.5) | 5.5 (4.5–7.5) | 0.1027 |
| BMI (kg/m2) | 14.9 ± 0.8 | 15.1 ± 1.2 | 0.5731 |
| Dietary intake | |||
| n | 41 | 20 | |
| Energy (kcal/d) | 1396 (1068–1662) | 1476 (1285–1698) | 0.4082 |
| Protein, % of energy | 12.8 ± 1.8 | 15.8 ± 3.0 | 0.0002 |
| Fat, % of energy | 30.5 ± 5.0 | 32.0 ± 3.3 | 0.3792 |
| Carbohydrates, % of energy | 56.7 ± 5.9 | 52.2 ± 3.8 | 0.0125 |
| Protein (g/d) | 35.5 (28.8–47.7) | 54.9 (44.2–66.1) | 0.0009 |
| Animal protein (g/d) | 13.0 (7.5–17.5) | 36.8 (26.7–46.0) | 0.0001 |
| Plant protein (g/d) | 22.1 (18.1–26.5) | 18.7 (14.2–21.7) | 0.0106 |
| Fiber (g/d | 16.7 (12.4–21.3) | 14.8 (11.1–16.9) | 0.0034 |
| Calcium (mg/d) | 504.7 (311.7–552.5) | 616.5 (429.2–755.0) | 0.0149 |
| Phosphorus (mg/d) | 774.4 (552.1–938.6) | 897.6 (708.0–1038.5) | 0.1403 |
| Magnesium (mg/d) | 229.8 ± 96.5 | 217.4 ± 95.4 | 0.6609 |
| Vitamin D (µg/d) | 1.70 (0.97–5.30) | 2.30 (1.28–5.66) | 0.0116 |
| Amino Acids (mg/d) | Vegetarians (n = 41) | Omnivores (n = 20) | Median Difference (%) | p Value |
|---|---|---|---|---|
| Essential amino acids | ||||
| Methionine | 750 (623–921) | 1322 (1001–1622) | −43.3 | 0.0002 |
| Phenylalanine | 1666 (1301–2112) | 2431 (1956–2981) | −31.5 | 0.0021 |
| Histidine | 889 (712–1146) | 1486 (1123–1929) | −39.5 | 0.0007 |
| Threonine | 1341 (1054–1743) | 2254 (1771–2769) | −40.0 | 0.0004 |
| Tryptophan | 440 (342–574) | 670 (552–808) | −34.3 | 0.0017 |
| Valine | 2081 (1587–2569) | 3150 (2605–3754) | −33.9 | 0.0008 |
| Isoleucine | 1674 (1292–2055) | 2670 (2096–3233) | −37.3 | 0.0005 |
| Leucine | 2743 (2056–3363) | 4274 (3456–5331) | −35.8 | 0.0005 |
| Lysine | 1963 (1477–2368) | 3762 (2867–4728) | −47.8 | 0.0001 |
| Non-essential amino acids | ||||
| Alanine | 1638 (1266–2018) | 2632 (1886–3162) | −37.8 | 0.0006 |
| Arginine | 1948 (1541–2529) | 2840 (1974–3151) | −31.4 | 0.0078 |
| Aspartate | 3255 (2578–4110) | 4866 (3576–5388) | −33.1 | 0.0001 |
| Glutamate | 7346 (5770–9629) | 11,157 (8677–14,037) | −34.2 | 0.0026 |
| Glycine | 1400 (1100–1763) | 2130 (1520–2410) | −34.3 | 0.0033 |
| Proline | 2794 (1909–3257) | 4166 (3413–5063) | −32.9 | 0.0007 |
| Serine | 1807 (1464–2350) | 2703 (2199–3117) | −33.1 | 0.0028 |
| Cysteine | 658 (502–855) | 778 (549–961) | −15.4 | 0.1273 |
| Tyrosine | 1245 (966–1527) | 2041 (1648–2422) | −39.0 | 0.0006 |
| Amino Acids (µmol/L) | Vegetarian Children (n = 51) | Omnivorous Children (n = 25) | Median Difference (%) | p Value |
|---|---|---|---|---|
| Essential amino acids | ||||
| Methionine | 20.5 (17.9–24.7) | 19.2 (17.0–24.8) | +6.3 | 0.7699 |
| Phenylalanine | 55.9 (45.7–64.5) | 54.1 (50.3–65.5) | +3.3 | 0.7574 |
| Histidine | 72.7 (65.2–84.1) | 76.5 (68.5–82.2) | −5.0 | 0.5363 |
| Threonine | 97.4 (93.8–118.7) | 100.8 (84.7–122.8) | −3.4 | 0.7099 |
| Tryptophan | 29.1 (21.4–36.6) | 29.9 (22.1–35.5) | −2.7 | 0.7139 |
| Valine | 183.8 (166.6–208.2) | 204.5 (186.3–244.7) | −10.1 | 0.0253 |
| Isoleucine | 49.7 (43.9–62.0) | 60.4 (52.6–73.0) | −17.7 | 0.0231 |
| Leucine | 105.4 (93.3–129.4) | 122.6 (104.4–145.7) | −14.1 | 0.0315 |
| Lysine | 118.8 (86.5–146.5) | 141.6 (115.6–167.4) | −16.1 | 0.0297 |
| Non-essential amino acids | ||||
| Alanine | 327.3 (272.2–391.3) | 307.0 (247.1–385.5) | +6.2 | 0.6520 |
| Arginine | 92.7 (81.5–105.9) | 94.0 (83.1–107.0) | −1.4 | 0.9446 |
| Asparagine | 58.2 (45.4–69.6) | 51.4 (43.9–60.9) | +11.7 | 0.2397 |
| Aspartate | 18.1 (14.4–22.8) | 19.6 (16.8–23.5) | −7.7 | 0.0694 |
| Glutamine | 573.7 (537.0–665.0) | 550.8 (522.9–612.8) | +4.0 | 0.0907 |
| Glutamate | 53.8 (39.9–71.2) | 59.0 (43.3–91.2) | −8.6 | 0.0985 |
| Glycine | 265.7 (215.1–301.9) | 242.8 (201.6–263.0) | +8.6 | 0.1821 |
| Proline | 158.6 (128.6–231.9) | 144.5 (106.3–198.4) | +8.9 | 0.2497 |
| Serine | 143.6 (130.0–164.2) | 138.4 (129.1–160.5) | +5.6 | 0.8430 |
| Cysteine | 147.7 (125.2–163.2) | 155.2 (144.3–181.2) | −4.8 | 0.0840 |
| Tyrosine | 57.4 (48.7–68.2) | 53.7 (49.5–65.8) | +6.4 | 0.8247 |
| Ornithine | 45.0 (31.4–61.9) | 51.2 (42.1–67.2) | −12.1 | 0.1696 |
| Citrulline | 30.3 (25.9–33.6) | 28.6 (24.4–32.3) | +5.6 | 0.2148 |
| Homocysteine | 5.9 (5.3–6.9) | 6.1 (5.1–7.4) | −3.7 | 0.9041 |
| Taurine | 116.0 (91.7–132.4) | 127.5 (110.2–140.9) | −9.0 | 0.0800 |
| Vegetarian Children (n = 51) | Omnivorous Children (n = 25) | p Value | |
|---|---|---|---|
| Albumin (mg/mL) | 51.5 (45.0–56.9) | 62.9 (57.3–68.1) | 0.0001 |
| Prealbumin (µg/mL) | 244.1 (215.3–289.6) | 255.4 (243.7–277.1) | 0.6920 |
| 25-hydroxyvitamin D (ng/mL) | 29.9 (25.6–37.7) | 30.9 (26.7–37.0) | 0.8691 |
| PTH (pg/mL) | 22.3 (16.4–33.4) | 17.4 (10.8–23.9) | 0.1733 |
| IGF-I (ng/mL) | 159.6 (115.6–225.9) | 134.8 (103.6–19.3) | 0.0654 |
| OC (ng/mL) | 74.8 (55.9–104.1) | 75.3 (64.2–89.7) | 0.5555 |
| CTX-I (ng/mL) | 1.917 (1.541–2.209) | 1.711 (1.436–1.928) | 0.0343 |
| OC/CTX-I | 0.39 (0.31–0.50) | 0.42 (0.37–0.64) | 0.0915 |
| OPG (pmol/L) | 4.0 (3.4–4.9) | 4.3 (3.8–5.1) | 0.5455 |
| Unstandardized Coefficient | Standardization Factor | t | p Value | Covariance Statistics | |||
|---|---|---|---|---|---|---|---|
| B | Standard Error | β | Tolerance | VIF | |||
| Constant | 2.742 | 0.582 | 4.71 | 0.000 | |||
| Alanine | 0.004 | 0.001 | 0.366 | 3.00 | 0.004 | 0.927 | 1.078 |
| Ornithine | −0.017 | 0.005 | −0.368 | −3.06 | 0.004 | 0.953 | 1.049 |
| Aspartate | 0.058 | 0.024 | 0.298 | 2.39 | 0.021 | 0.888 | 1.126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambroszkiewicz, J.; Gajewska, J.; Mazur, J.; Kuśmierska, K.; Klemarczyk, W.; Rowicka, G.; Strucińska, M.; Chełchowska, M. Dietary Intake and Circulating Amino Acid Concentrations in Relation with Bone Metabolism Markers in Children Following Vegetarian and Omnivorous Diets. Nutrients 2023, 15, 1376. https://doi.org/10.3390/nu15061376
Ambroszkiewicz J, Gajewska J, Mazur J, Kuśmierska K, Klemarczyk W, Rowicka G, Strucińska M, Chełchowska M. Dietary Intake and Circulating Amino Acid Concentrations in Relation with Bone Metabolism Markers in Children Following Vegetarian and Omnivorous Diets. Nutrients. 2023; 15(6):1376. https://doi.org/10.3390/nu15061376
Chicago/Turabian StyleAmbroszkiewicz, Jadwiga, Joanna Gajewska, Joanna Mazur, Katarzyna Kuśmierska, Witold Klemarczyk, Grażyna Rowicka, Małgorzata Strucińska, and Magdalena Chełchowska. 2023. "Dietary Intake and Circulating Amino Acid Concentrations in Relation with Bone Metabolism Markers in Children Following Vegetarian and Omnivorous Diets" Nutrients 15, no. 6: 1376. https://doi.org/10.3390/nu15061376
APA StyleAmbroszkiewicz, J., Gajewska, J., Mazur, J., Kuśmierska, K., Klemarczyk, W., Rowicka, G., Strucińska, M., & Chełchowska, M. (2023). Dietary Intake and Circulating Amino Acid Concentrations in Relation with Bone Metabolism Markers in Children Following Vegetarian and Omnivorous Diets. Nutrients, 15(6), 1376. https://doi.org/10.3390/nu15061376











