Systematic Review and Dose-Response Meta-Analysis on the Relationship between Different Gluten Doses and Risk of Coeliac Disease Relapse
Abstract
1. Introduction
2. Methods
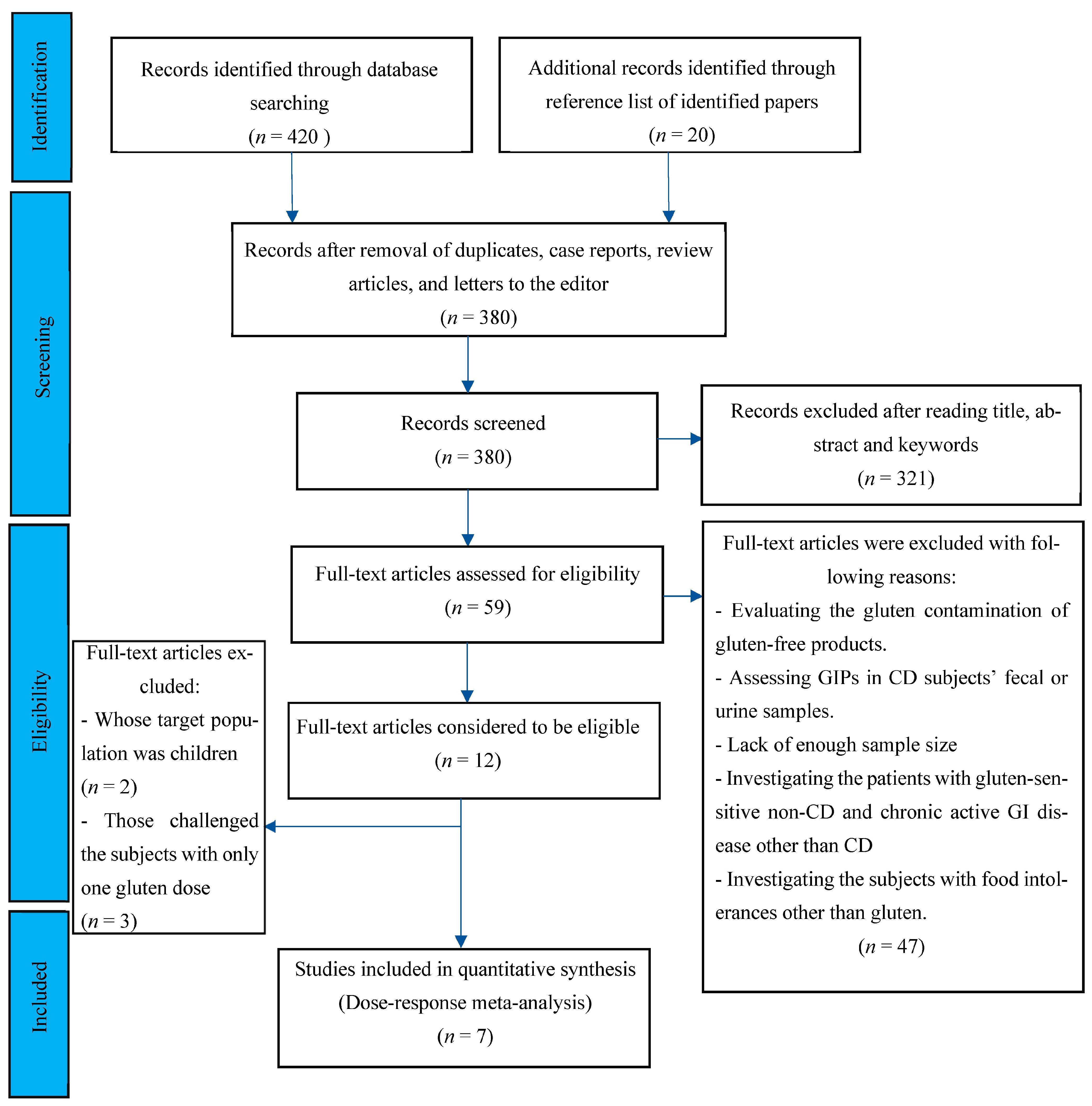
2.1. Search Strategy, Data Retrieval, and Eligibility Criteria
2.2. Study Selection, Quality Assessment, and Data Extraction
2.3. Data Synthesis and Statistical Analysis
3. Results
3.1. Characteristics of Included Studies
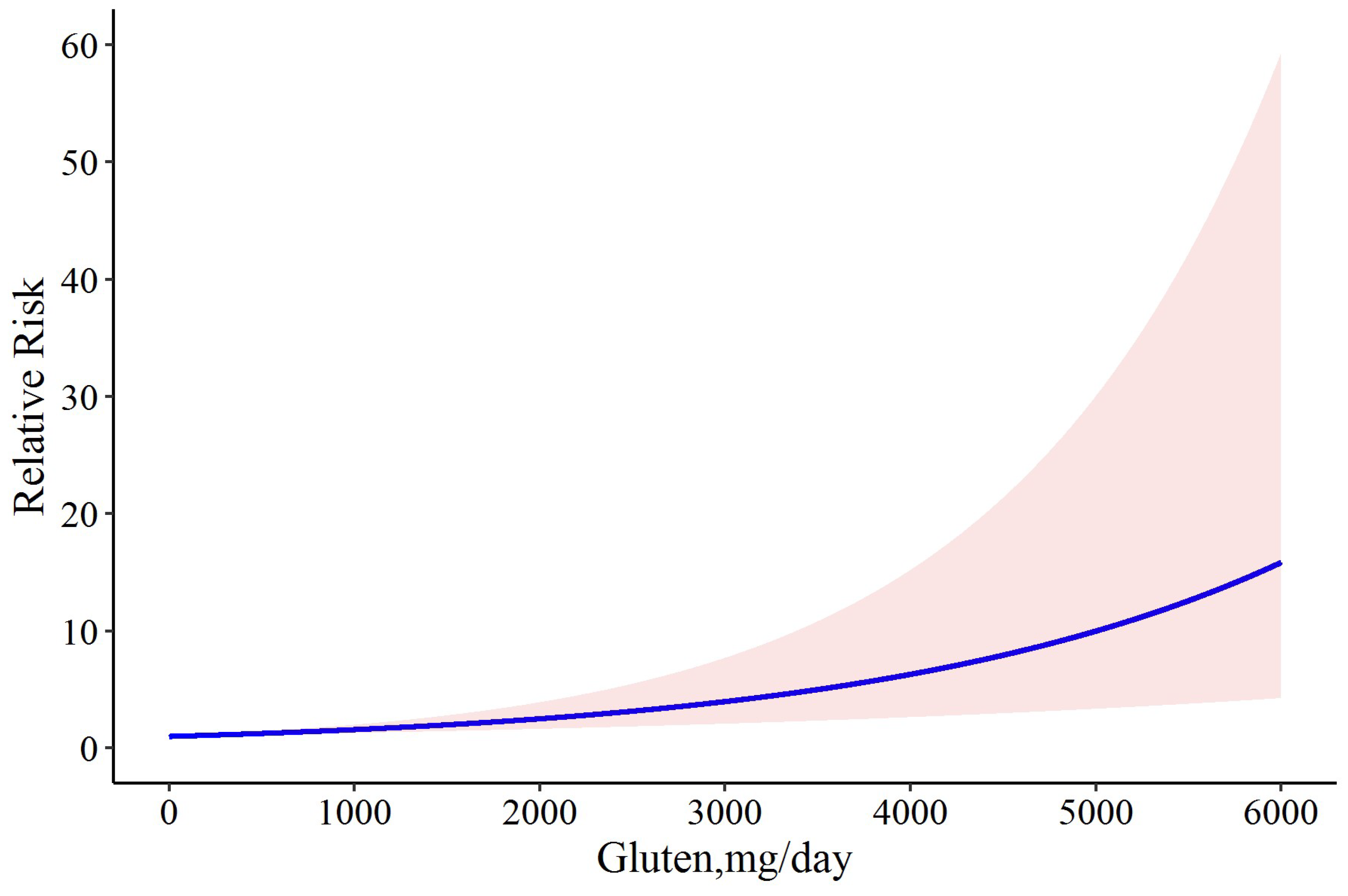
3.2. Dose-Response Meta-Analysis
3.3. Sensitivity Analysis
3.4. Correlation between CD Relapse and Studies’ Features
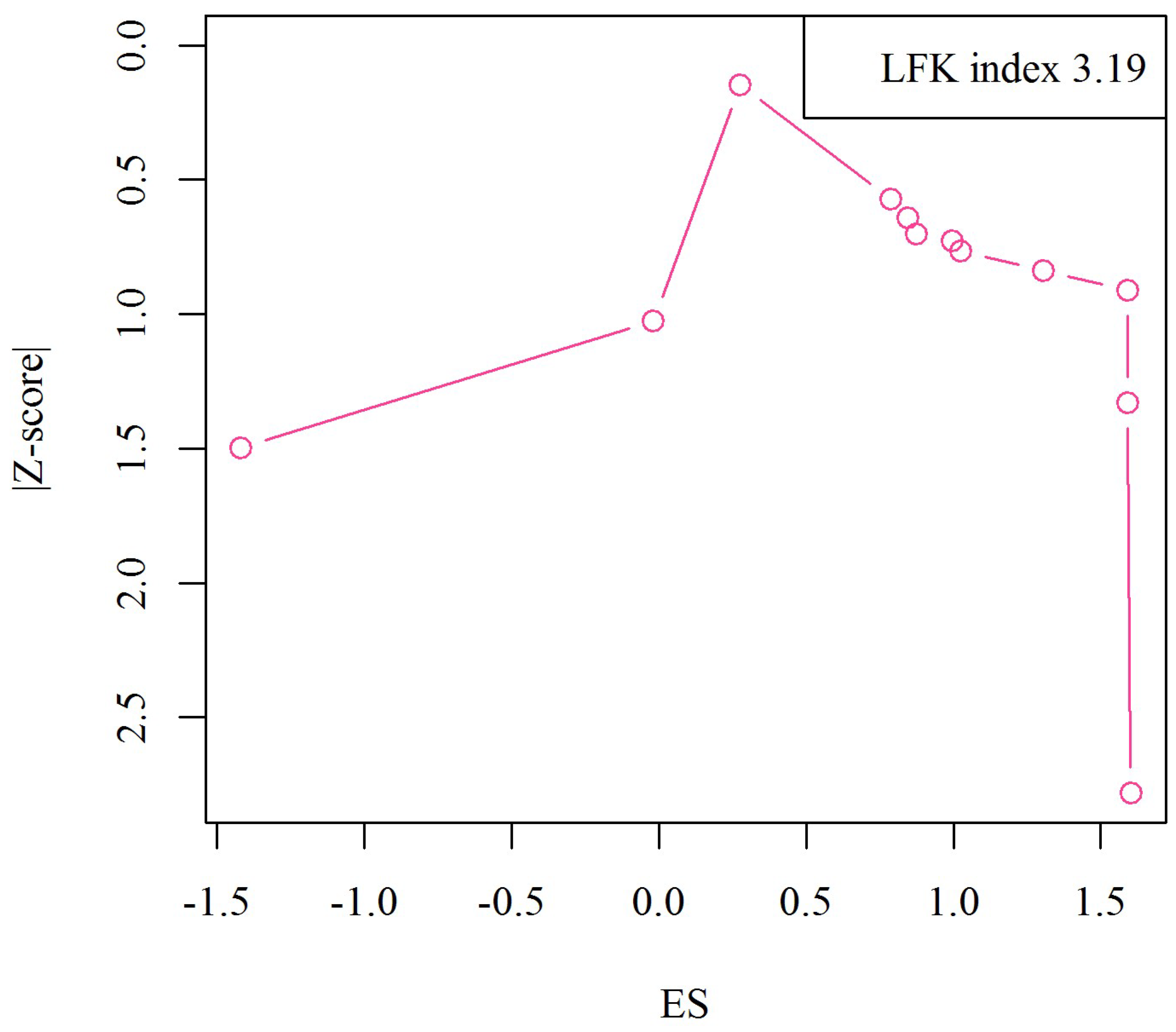
3.5. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vriezinga, S.L.; Auricchio, R.; Bravi, E.; Castillejo, G.; Chmielewska, A.; Crespo Escobar, P.; Kolaček, S.; Koletzko, S.; Korponay-Szabo, I.R.; Mummert, E.; et al. Randomized feeding intervention in infants at high risk for celiac disease. N. Engl. J. Med. 2014, 371, 1304–1315. [Google Scholar] [CrossRef]
- Taraghikhah, N.; Ashtari, S.; Asri, N.; Shahbazkhani, B.; Al-Dulaimi, D.; Rostami-Nejad, M.; Rezaei-Tavirani, M.; Razzaghi, M.R.; Zali, M.R. An updated overview of spectrum of gluten-related disorders: Clinical and diagnostic aspects. BMC Gastroenterol. 2020, 20, 258. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, A.K.; Oxentenko, A.S. Clinical and Histologic Mimickers of Celiac Disease. Clin. Transl. Gastroenterol. 2017, 8, e114. [Google Scholar] [CrossRef]
- Leonard, M.M.; Silvester, J.A.; Leffler, D.; Fasano, A.; Kelly, C.P.; Lewis, S.K.; Goldsmith, J.D.; Greenblatt, E.; Kwok, W.W.; McAuliffe, W.J.; et al. Evaluating Responses to Gluten Challenge: A Randomized, Double-Blind, 2-Dose Gluten Challenge Trial. Gastroenterology 2021, 160, 720–733.e728. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.T.; Dahal-Koirala, S.; Kim, H.S.K.; Qiao, S.W.; Neumann, R.S.; Lundin, K.E.A.; Petersen, J.; Reid, H.H.; Sollid, L.M.; Rossjohn, J. A molecular basis for the T cell response in HLA-DQ2.2 mediated celiac disease. Proc. Natl. Acad. Sci. USA 2020, 117, 3063–3073. [Google Scholar] [CrossRef] [PubMed]
- Lázár-Molnár, E.; Snyder, M. The Role of Human Leukocyte Antigen in Celiac Disease Diagnostics. Clin. Lab. Med. 2018, 38, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Martina, S.; Fabiola, F.; Federica, G.; Chiara, B.; Gioacchino, L.; Gian, L.D.A. Genetic susceptibilty and celiac disease: What role do HLA haplotypes play? Acta Biomed. 2018, 89, 17–21. [Google Scholar]
- Monsuur, A.J.; de Bakker, P.I.W.; Zhernakova, A.; Pinto, D.; Verduijn, W.; Romanos, J.; Auricchio, R.; Lopez, A.; van Heel, D.A.; Crusius, J.B.A. Effective Detection of Human Leukocyte Antigen Risk Alleles in Celiac Disease Using Tag Single Nucleotide Polymorphisms. PLoS ONE 2008, 3, e2270. [Google Scholar] [CrossRef]
- Bruins, M. The clinical response to gluten challenge: A review of the literature. Nutrients 2013, 5, 4614–4641. [Google Scholar] [CrossRef]
- Guarino, M.; Gambuti, E.; Alfano, F.; Strada, A.; Ciccocioppo, R.; Lungaro, L.; Zoli, G.; Volta, U.; De Giorgio, R.; Caio, G. Life-threatening onset of coeliac disease: A case report and literature review. BMJ Open Gastroenterol. 2020, 7, e000406. [Google Scholar] [CrossRef]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Dunaevsky, Y.E.; Tereshchenkova, V.F.; Belozersky, M.A.; Filippova, I.Y.; Oppert, B.; Elpidina, E.N. Effective Degradation of Gluten and Its Fragments by Gluten-Specific Peptidases: A Review on Application for the Treatment of Patients with Gluten Sensitivity. Pharmaceutics 2021, 13, 1603. [Google Scholar] [CrossRef] [PubMed]
- Morón, B.; Bethune, M.T.; Comino, I.; Manyani, H.; Ferragud, M.; López, M.C.; Cebolla, Á.; Khosla, C.; Sousa, C. Toward the Assessment of Food Toxicity for Celiac Patients: Characterization of Monoclonal Antibodies to a Main Immunogenic Gluten Peptide. PLoS ONE 2008, 3, e2294. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Kumar, M.; Pandey, R.; Chauhan, N.S. Physiopathology and Management of Gluten-Induced Celiac Disease. J. Food Sci. 2017, 82, 270–277. [Google Scholar] [CrossRef]
- Spector Cohen, I.; Day, A.; Shaoul, R. Should the Glu Be Ten or Twenty? An Update on the Ongoing Debate on Gluten Safety Limits for Patients with Celiac Disease. Gastrointest. Disord. 2020, 2, 202–211. [Google Scholar] [CrossRef]
- Aljada, B.; Zohni, A.; El-Matary, W. The Gluten-Free Diet for Celiac Disease and Beyond. Nutrients 2021, 13, 3993. [Google Scholar] [CrossRef]
- White, L.E.; Bannerman, E.; Gillett, P.M. Coeliac disease and the gluten-free diet: A review of the burdens; factors associated with adherence and impact on health-related quality of life, with specific focus on adolescence. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2016, 29, 593–606. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Gluten-Free Diet: Gaps and Needs for a Healthier Diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef]
- Cohen, I.S.; Day, A.S.; Shaoul, R. Gluten in Celiac Disease-More or Less? Rambam Maimonides Med. J. 2019, 10, e0007. [Google Scholar] [CrossRef]
- Hollon, J.R.; Cureton, P.A.; Martin, M.L.; Puppa, E.L.L.; Fasano, A. Trace gluten contamination may play a role in mucosal and clinical recovery in a subgroup of diet-adherent non-responsive celiac disease patients. BMC Gastroenterol. 2013, 13, 40. [Google Scholar] [CrossRef]
- Daveson, A.J.M.; Tye-Din, J.A.; Goel, G.; Goldstein, K.E.; Hand, H.L.; Neff, K.M.; Williams, L.J.; Truitt, K.E.; Anderson, R.P. Masked bolus gluten challenge low in FODMAPs implicates nausea and vomiting as key symptoms associated with immune activation in treated coeliac disease. Aliment. Pharmacol. Ther. 2020, 51, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Bruins Slot, I.D.; Bremer, M.G.E.G.; Hamer, R.J.; van der Fels-Klerx, H.J. Part of celiac population still at risk despite current gluten thresholds. Trends Food Sci. Technol. 2015, 43, 219–226. [Google Scholar] [CrossRef]
- Collin, P.; Thorell, L.; Kaukinen, K.; Mäki, M. The safe threshold for gluten contamination in gluten-free products. Can trace amounts be accepted in the treatment of coeliac disease? Aliment. Pharmacol. Ther. 2004, 19, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H.; Segura, V.; Ruiz-Carnicer, Á.; Sousa, C.; Comino, I. Food Safety and Cross-Contamination of Gluten-Free Products: A Narrative Review. Nutrients 2021, 13, 2244. [Google Scholar] [CrossRef]
- Wieser, H.; Ruiz-Carnicer, Á.; Segura, V.; Comino, I.; Sousa, C. Challenges of Monitoring the Gluten-Free Diet Adherence in the Management and Follow-Up of Patients with Celiac Disease. Nutrients 2021, 13, 2274. [Google Scholar] [CrossRef]
- Collin, P.; Mäki, M.; Kaukinen, K. Safe gluten threshold for patients with celiac disease: Some patients are more tolerant than others. Am. J. Clin. Nutr. 2007, 86, 260. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Bascuñán, K.; di Lernia, L.; Bardella, M.T.; Doneda, L.; Soldati, L.; Orlando, S.; Ferretti, F.; Lombardo, V.; Barigelletti, G.; et al. Safety of occasional ingestion of gluten in patients with celiac disease: A real-life study. BMC Med. 2020, 18, 42. [Google Scholar] [CrossRef]
- Gibert, A.; Espadaler, M.; Angel Canela, M.; Sánchez, A.; Vaqué, C.; Rafecas, M. Consumption of gluten-free products: Should the threshold value for trace amounts of gluten be at 20, 100 or 200 p.p.m.? Eur. J. Gastroenterol. Hepatol. 2006, 18, 1187–1195. [Google Scholar] [CrossRef]
- Hischenhuber, C.; Crevel, R.; Jarry, B.; Mäki, M.; Moneret-Vautrin, D.A.; Romano, A.; Troncone, R.; Ward, R. Review article: Safe amounts of gluten for patients with wheat allergy or coeliac disease. Aliment. Pharmacol. Ther. 2006, 23, 559–575. [Google Scholar] [CrossRef]
- Akobeng, A.K.; Thomas, A.G. Systematic review: Tolerable amount of gluten for people with coeliac disease. Aliment. Pharmacol. Ther. 2008, 27, 1044–1052. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Fabiani, E.; Iacono, G.; D’Agate, C.; Francavilla, R.; Biagi, F.; Volta, U.; Accomando, S.; Picarelli, A.; De Vitis, I.; et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am. J. Clin. Nutr. 2007, 85, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.; Schuppan, D.; Pallav, K.; Najarian, R.; Goldsmith, J.D.; Hansen, J.; Kabbani, T.; Dennis, M.; Kelly, C.P. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut 2013, 62, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Greco, L.; Gobbetti, M.; Auricchio, R.; Di Mase, R.; Landolfo, F.; Paparo, F.; Di Cagno, R.; De Angelis, M.; Rizzello, C.G.; Cassone, A.; et al. Safety for patients with celiac disease of baked goods made of wheat flour hydrolyzed during food processing. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2011, 9, 24–29. [Google Scholar] [CrossRef]
- Troncone, R.; Mayer, M.; Spagnuolo, F.; Maiuri, L.; Greco, L. Endomysial antibodies as unreliable markers for slight dietary transgressions in adolescents with celiac disease. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 69–72. [Google Scholar] [CrossRef]
- Mayer, M.; Greco, L.; Troncone, R.; Auricchio, S.; Marsh, M.N. Compliance of adolescents with coeliac disease with a gluten free diet. Gut 1991, 32, 881–885. [Google Scholar] [CrossRef]
- Lebwohl, B.; Cao, Y.; Zong, G.; Hu, F.B.; Green, P.H.R.; Neugut, A.I.; Rimm, E.B.; Sampson, L.; Dougherty, L.W.; Giovannucci, E.; et al. Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: Prospective cohort study. BMJ Clin. Res. Ed. 2017, 357, j1892. [Google Scholar] [CrossRef] [PubMed]
- Syage, J.A.; Kelly, C.P.; Dickason, M.A.; Ramirez, A.C.; Leon, F.; Dominguez, R.; Sealey-Voyksner, J.A. Determination of gluten consumption in celiac disease patients on a gluten-free diet. Am. J. Clin. Nutr. 2018, 107, 201–207. [Google Scholar] [CrossRef]
- Jansson, U.H.; Gudjónsdóttir, A.H.; Ryd, W.; Kristiansson, B. Two different doses of gluten show a dose-dependent response of enteropathy but not of serological markers during gluten challenge in children with coeliac disease. Acta Paediatr. 2001, 90, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Aspasia, S.; Emmanuela-Kalliopi, K.; Nikolaos, T.; Eirini, S.; Ioannis, S.; Anastasia, M. The gluten-free diet challenge in adults with coeliac disease: The Hellenic survey. PEC Innov. 2022, 1, 100037. [Google Scholar] [CrossRef]
- Zysk, W.; Głąbska, D.; Guzek, D. Role of Front-of-Package Gluten-Free Product Labeling in a Pair-Matched Study in Women with and without Celiac Disease on a Gluten-Free Diet. Nutrients 2019, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Gatti, S.; Galeazzi, T.; Monachesi, C.; Padella, L.; Del Baldo, G.; Annibali, R.; Lionetti, E.; Catassi, C. Gluten Contamination in Naturally or Labeled Gluten-Free Products Marketed in Italy. Nutrients 2017, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Rostami, K.; Ensari, A.; Marsh, M.N.; Srivastava, A.; Villanacci, V.; Carroccio, A.; Asadzadeh Aghdaei, H.; Bai, J.C.; Bassotti, G.; Becheanu, G. Gluten induces subtle histological changes in duodenal mucosa of patients with non-coeliac gluten sensitivity: A multicentre study. Nutrients 2022, 14, 2487. [Google Scholar] [CrossRef] [PubMed]
- Wahab, P.J.; Meijer, J.W.; Mulder, C.J. Histologic follow-up of people with celiac disease on a gluten-free diet: Slow and incomplete recovery. Am. J. Clin. Pathol. 2002, 118, 459–463. [Google Scholar] [CrossRef]
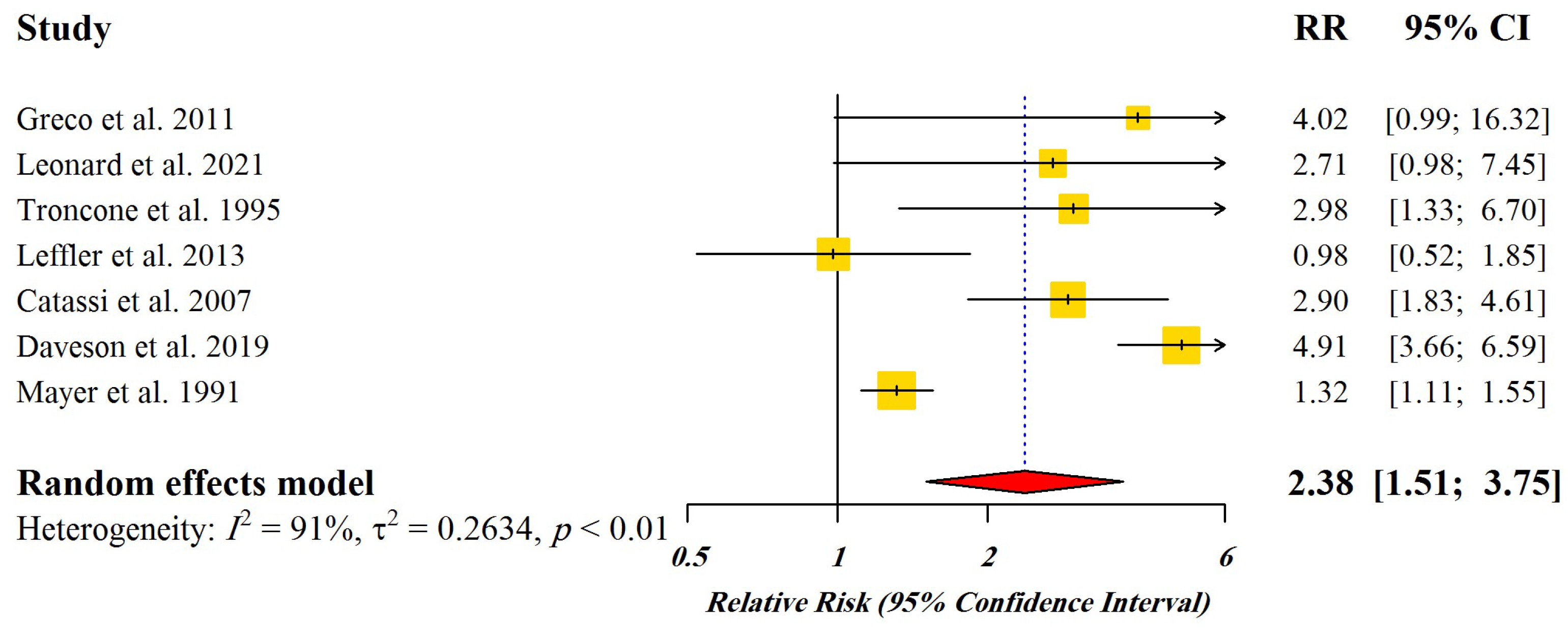


| Study | Study Design | Geographic Location | Interventions | Number of Participants | Challenge Duration | Relapse Assessment Method | Relative Risk (RR) | Confidence Interval 95% |
|---|---|---|---|---|---|---|---|---|
| Daveson et al., 2019 [21] | Randomized, double-blind, sham-controlled gluten challenge trial | United States, New Zealand, Australia | Placebo | 36 | 14 days | Clinical symptoms | 1 (Ref.) | - |
| 6000 mg gluten/day | 36 | 4.9127 | 3.6609–6.5925 | |||||
| Leonard et al., 2021 [4] | Randomized, double-blind, two-dose gluten challenge trial | Boston, USA | 3000 mg gluten/day | 7 | 14 days | Pathological changes | 1 (Ref.) | - |
| 10,000 mg gluten/day | 7 | 2.7081 | 0.9842–7.451 | |||||
| Catassi et al., 2007 [33] | Multi-center, randomized, double-blind, placebo-controlled, trial | Italy | 10 mg gluten/day | 13 | 90 days | Pathological changes | 2.3307 | 1.2271–4.4271 |
| 50 mg gluten/day | 13 | 3.6888 | 1.8887–7.205 | |||||
| Placebo | 13 | 1 (Ref.) | - | |||||
| Leffler et al., 2013 [34] | Randomized, double-blind, two-dose Gluten challenge trial | Israel | 3040 mg gluten/day | 10 | 14 days | Pathological changes | 1 (Ref.) | - |
| 7500 mg gluten/day | 10 | 0.9808 | 0.521–1.8464 | |||||
| Greco et al., 2011 [35] | Randomized, double-blind clinical trial | Italy | 16,025 mg gluten/day | 6 | 60 days | Clinical complications (CD related antibodies and biopsy findings) | 2.3978 | 0.1784–32.2328 |
| 496 mg gluten/day | 2 | 4.9628 | 0.9379–26.2608 | |||||
| 1.6 mg gluten/day | 5 | 1 (Ref.) | - | |||||
| Troncone et al., 1995 [36] | Randomized, double-blind clinical trial | Italy | <500 mg gluten/day | 6 | 7 days | Pathological changes | 2.1972 | 0.5768–8.3694 |
| 500–2000 mg gluten/day | 6 | 2.7850 | 0.7224–10.7375 | |||||
| >2000 mg gluten/day | 7 | 4.9052 | 1.0453–23.0197 | |||||
| Control (strict GFD) | 4 | 1 (Ref.) | - | |||||
| Mayer et al., 1991 [37] | Randomized, double-blind clinical trial | Italy | 60–2000 mg gluten/day | 14 | 30 days | Pathological changes | −0.2418 | 0.1732–0.3376 |
| ˃2000 mg gluten/day | 29 | 1.3163 | 1.1147–1.5543 | |||||
| Control (0 mg gluten/day) | 80 | 1 (Ref.) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostami-Nejad, M.; Asri, N.; Olfatifar, M.; Khorsand, B.; Houri, H.; Rostami, K. Systematic Review and Dose-Response Meta-Analysis on the Relationship between Different Gluten Doses and Risk of Coeliac Disease Relapse. Nutrients 2023, 15, 1390. https://doi.org/10.3390/nu15061390
Rostami-Nejad M, Asri N, Olfatifar M, Khorsand B, Houri H, Rostami K. Systematic Review and Dose-Response Meta-Analysis on the Relationship between Different Gluten Doses and Risk of Coeliac Disease Relapse. Nutrients. 2023; 15(6):1390. https://doi.org/10.3390/nu15061390
Chicago/Turabian StyleRostami-Nejad, Mohammad, Nastaran Asri, Meysam Olfatifar, Babak Khorsand, Hamidreza Houri, and Kamran Rostami. 2023. "Systematic Review and Dose-Response Meta-Analysis on the Relationship between Different Gluten Doses and Risk of Coeliac Disease Relapse" Nutrients 15, no. 6: 1390. https://doi.org/10.3390/nu15061390
APA StyleRostami-Nejad, M., Asri, N., Olfatifar, M., Khorsand, B., Houri, H., & Rostami, K. (2023). Systematic Review and Dose-Response Meta-Analysis on the Relationship between Different Gluten Doses and Risk of Coeliac Disease Relapse. Nutrients, 15(6), 1390. https://doi.org/10.3390/nu15061390







