Human Milk Oligosaccharides Variation in Gestational Diabetes Mellitus Mothers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Review and Project Registration
2.2. Subjects and Sample Collection
2.2.1. Study Subjects
2.2.2. Determination of Milk Type
2.2.3. Milk Sample Collection and Storage
2.2.4. Data Collection and Definition of Variables
2.3. Quantification of HMOs
2.4. Statistical Analysis Methods
3. Results
3.1. Basic Information on Maternity and Infancy of Both Groups
3.2. Milk Type
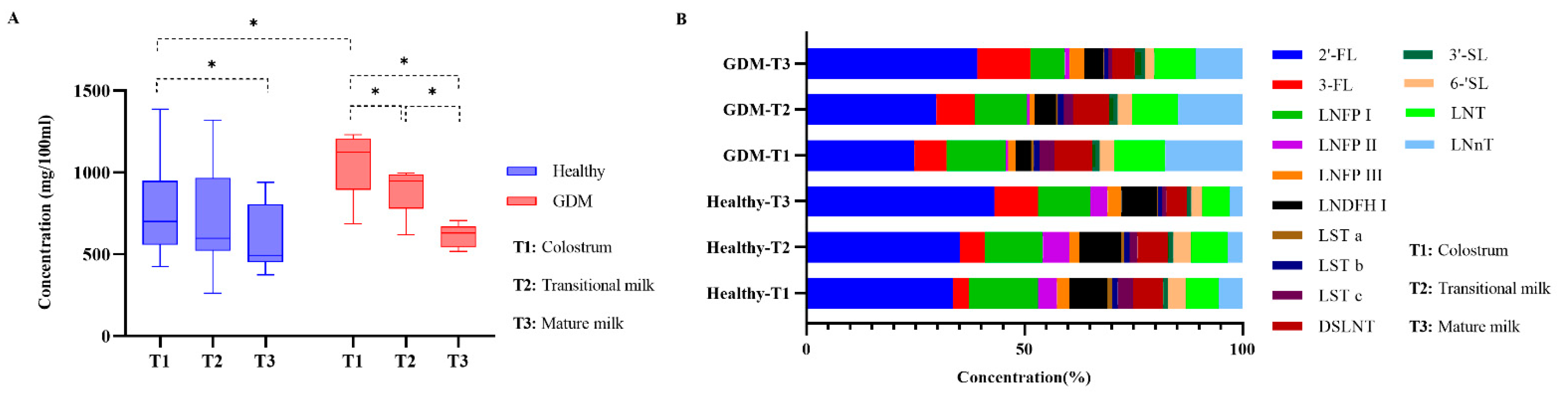
3.3. Changes of Total HMOs in GDM and Healthy Se+Le+ Mothers during Lactation
3.4. Changes of Individual HMOs in GDM and Healthy Se+Le+ Mothers during Lactation
3.5. Relations between Basic Maternal and Infant Information and HMO Concentrations
3.6. Infant Growth and Development in Relation to HMO Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sundekilde, U.K.; Downey, E.; O’Mahony, J.A.; O’Shea, C.A.; Ryan, C.A.; Kelly, A.L.; Bertram, H.C. The Effect of Gestational and Lactational Age on the Human Milk Metabolome. Nutrients 2016, 8, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donovan, S.M. The Role of Lactoferrin in Gastrointestinal and Immune Development and Function: A Preclinical Perspective. J. Pediatr. 2016, 173, S16–S28. [Google Scholar] [CrossRef] [Green Version]
- Eidelman, A.I. Breastfeeding and the Use of Human Milk: An Analysis of the American Academy of Pediatrics 2012 Breastfeeding Policy Statement. Breastfeed Med. 2012, 7, 323–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonnerdal, B. Bioactive Proteins in Human Milk: Health, Nutrition, and Implications for Infant Formulas. J. Pediatr. 2016, 173, S4–S9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.C.; Chen, P.H. Health consequences of nutrition in childhood and early infancy. Pediatr. Neonatol. 2009, 50, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X. Human Milk Oligosaccharides (HMOS): Structure, Function, and Enzyme-Catalyzed Synthesis. Adv. Carbohydr. Chem. Biochem. 2015, 72, 113–190. [Google Scholar] [CrossRef]
- Triantis, V.; Bode, L.; van Neerven, R.J.J. Immunological Effects of Human Milk Oligosaccharides. Front. Pediatr. 2018, 6, 190. [Google Scholar] [CrossRef] [Green Version]
- Kunz, C.; Rudloff, S.; Baier, W.; Klein, N.; Strobel, S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Annu. Rev. Nutr. 2000, 20, 699–722. [Google Scholar] [CrossRef]
- Soyyilmaz, B.; Miks, M.H.; Rohrig, C.H.; Matwiejuk, M.; Meszaros-Matwiejuk, A.; Vigsnaes, L.K. The Mean of Milk: A Review of Human Milk Oligosaccharide Concentrations throughout Lactation. Nutrients 2021, 13, 2737. [Google Scholar] [CrossRef]
- Totten, S.M.; Wu, L.D.; Parker, E.A.; Davis, J.C.; Hua, S.; Stroble, C.; Ruhaak, L.R.; Smilowitz, J.T.; German, J.B.; Lebrilla, C.B. Rapid-throughput glycomics applied to human milk oligosaccharide profiling for large human studies. Anal. Bioanal. Chem. 2014, 406, 7925–7935. [Google Scholar] [CrossRef]
- Doherty, A.M.; Lodge, C.J.; Dharmage, S.C.; Dai, X.; Bode, L.; Lowe, A.J. Human Milk Oligosaccharides and Associations with Immune-Mediated Disease and Infection in Childhood: A Systematic Review. Front. Pediatr. 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezoff, E.A.; Hawkins, J.A.; Ollberding, N.J.; Karns, R.; Morrow, A.L.; Helmrath, M.A. The human milk oligosaccharide 2′-fucosyllactose augments the adaptive response to extensive intestinal. Am. J. Physiol. Liver Physiol. 2016, 310, G427–G438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chichlowski, M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. The influence of milk oligosaccharides on microbiota of infants: Opportunities for formulas. Annu. Rev. Food Sci. Technol. 2011, 2, 331–351. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.T.; Chen, C.; Kling, D.E.; Liu, B.; McCoy, J.M.; Merighi, M.; Heidtman, M.; Newburg, D.S. The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology 2013, 23, 169–177. [Google Scholar] [CrossRef]
- Ferreira, A.L.; Alves, R.; Figueiredo, A.; Alves-Santos, N.; Freitas-Costa, N.; Batalha, M.; Yonemitsu, C.; Manivong, N.; Furst, A.; Bode, L.; et al. Human Milk Oligosaccharide Profile Variation Throughout Postpartum in Healthy Women in a Brazilian Cohort. Nutrients 2020, 12, 790. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.C.; Zhao, Z.W.; Zhao, A.; Zhang, J.; Wu, W.; Ren, Z.X.; Wang, P.Y.; Zhang, Y.M. Neutral Human Milk Oligosaccharides Are Associated with Multiple Fixed and Modifiable Maternal and Infant Characteristics. Nutrients 2020, 12, 826. [Google Scholar] [CrossRef] [Green Version]
- Eussen, S.R.B.M.; Mank, M.; Kottler, R.; Hoffmann, X.K.; Behne, A.; Rapp, E.; Stahl, B.; Mearin, M.L.; Koletzko, B. Presence and Levels of Galactosyllactoses and Other Oligosaccharides in Human Milk and Their Variation during Lactation and According to Maternal Phenotype. Nutrients 2021, 13, 2324. [Google Scholar] [CrossRef]
- Han, S.M.; Derraik, J.G.B.; Binia, A.; Sprenger, N.; Vickers, M.H.; Cutfield, W.S. Maternal and Infant Factors Influencing Human Milk Oligosaccharide Composition: Beyond Maternal Genetics. J. Nutr. 2021, 151, 1383–1393. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Hou, W.; Meng, X.; Zhao, A.; Zhao, W.; Pan, J.; Tang, J.; Huang, Y.; Li, H.; Jia, W.; Liu, F.; et al. Development of Multimarker Diagnostic Models from Metabolomics Analysis for Gestational Diabetes Mellitus (GDM). Mol. Cell. Proteom. 2018, 17, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Ravnsborg, T.; Svaneklink, S.; Andersen, L.L.T.; Larsen, M.R.; Jensen, D.M.; Overgaard, M. First-trimester proteomic profiling identifies novel predictors of gestational diabetes mellitus. PLoS ONE 2019, 14, e0214457. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhao, D.; Chen, Y.; Zhang, K.; Chen, X.; Lin, J.; Li, C.; Iqbal, J.; Zhao, Y.; Liang, Y.; et al. Comparative Proteomics Analysis of Serum Proteins in Gestational Diabetes during Early and Middle Stages of Pregnancy. Proteom. Clin. Appl. 2019, 13, e1800060. [Google Scholar] [CrossRef] [PubMed]
- Burlina, S.; Banfi, C.; Brioschi, M.; Visentin, S.; Dalfra, M.G.; Traldi, P.; Lapolla, A. Is the placental proteome impaired in well-controlled gestational diabetes? J. Mass. Spectrom. 2019, 54, 359–365. [Google Scholar] [CrossRef]
- Lee, C.L.; Chiu, P.C.; Pang, P.C.; Chu, I.K.; Lee, K.F.; Koistinen, R.; Koistinen, H.; Seppala, M.; Morris, H.R.; Tissot, B.; et al. Glycosylation failure extends to glycoproteins in gestational diabetes mellitus: Evidence from reduced alpha2-6 sialylation and impaired immunomodulatory activities of pregnancy-related glycodelin-A. Diabetes 2011, 60, 909–917. [Google Scholar] [CrossRef] [Green Version]
- Jantscher-Krenn, E.; Aigner, J.; Reiter, B.; Kofeler, H.; Csapo, B.; Desoye, G.; Bode, L.; van Poppel, M.N.M. Evidence of human milk oligosaccharides in maternal circulation already during pregnancy: A pilot study. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E347–E357. [Google Scholar] [CrossRef]
- Jantscher-Krenn, E.; Treichler, C.; Brandl, W.; Schonbacher, L.; Kofeler, H.; van Poppel, M.N.M. The association of human milk oligosaccharides with glucose metabolism in overweight and obese pregnant women. Am. J. Clin. Nutr. 2019, 110, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Hirschmugl, B.; Brandl, W.; Csapo, B.; van Poppel, M.; Kofeler, H.; Desoye, G.; Wadsack, C.; Jantscher-Krenn, E. Evidence of Human Milk Oligosaccharides in Cord Blood and Maternal-to-Fetal Transport across the Placenta. Nutrients 2019, 11, 2640. [Google Scholar] [CrossRef] [Green Version]
- Hoch, D.; Brandl, W.; Strutz, J.; Kofeler, H.C.; van Poppel, M.N.M.; Bode, L.; Hiden, U.; Desoye, G.; Jantscher-Krenn, E. Human Milk Oligosaccharides in Cord Blood Are Altered in Gestational Diabetes and Stimulate Feto-Placental Angiogenesis In Vitro. Nutrients 2021, 13, 4257. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; Totten, S.M.; Huang, J.; Grapov, D.; Durham, H.A.; Lammi-Keefe, C.J.; Lebrilla, C.; German, J.B. Human milk secretory immunoglobulin a and lactoferrin N-glycans are altered in women with gestational diabetes mellitus. J. Nutr. 2013, 143, 1906–1912. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, J.; Li, C.; Xu, Y.; Wang, X.; Lu, Y.; Zhang, T.; Cao, H.; Huang, L.; Wang, Z. Pregnancy-Related Diseases and Delivery Mode can Affect the Content of Human Milk Oligosaccharides: A Preliminary Study. J. Agric. Food Chem. 2022, 70, 5207–5217. [Google Scholar] [CrossRef] [PubMed]
- Alderete, T.L.; Autran, C.; Brekke, B.E.; Knight, R.; Bode, L.; Goran, M.I.; Fields, D.A. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am. J. Clin. Nutr. 2015, 102, 1381–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.C.; Lewis, Z.T.; Krishnan, S.; Bernstein, R.M.; Moore, S.E.; Prentice, A.M.; Mills, D.A.; Lebrilla, C.B.; Zivkovic, A.M. Growth and Morbidity of Gambian Infants are Influenced by Maternal Milk Oligosaccharides and Infant Gut Microbiota. Sci. Rep. 2017, 7, 40466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, M.W.; Lind, M.V.; Laursen, R.P.; Yonemitsu, C.; Larnkjaer, A.; Molgaard, C.; Michaelsen, K.F.; Bode, L. Human Milk Oligosaccharide Composition Is Associated with Excessive Weight Gain During Exclusive Breastfeeding-An Explorative Study. Front. Pediatr. 2019, 7, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagstrom, H.; Rautava, S.; Ollila, H.; Kaljonen, A.; Turta, O.; Makela, J.; Yonemitsu, C.; Gupta, J.; Bode, L. Associations between human milk oligosaccharides and growth in infancy and early childhood. Am. J. Clin. Nutr. 2020, 111, 769–778. [Google Scholar] [CrossRef]
- Tran, T.S.; Hirst, J.E.; Do, M.A.; Morris, J.M.; Jeffery, H.E. Early prediction of gestational diabetes mellitus in Vietnam: Clinical impact of currently recommended diagnostic criteria. Diabetes Care 2013, 36, 618–624. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association Professional Practice Committee. 15. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S232–S243. [Google Scholar] [CrossRef]
- ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol. 2018, 131, e49–e64. [CrossRef]
- World Health Organization. Child Growth Standards. Available online: https://www.who.int/tools/child-growth-standards/software (accessed on 13 March 2023).
- World Health Organization. WHO Child Growth Standards: Head Circumference-For-Age, Arm Circumference-For-Age, Triceps Skinfold-For-Age and Subscapular Skinfold-For-Age: Methods and Development. Available online: https://www.who.int/publications/i/item/9789241547185 (accessed on 13 March 2023).
- Plows, J.F.; Berger, P.K.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Najera, J.A.; Khwajazada, S.; Bode, L.; Goran, M.I. Longitudinal Changes in Human Milk Oligosaccharides (HMOs) Over the Course of 24 Months of Lactation. J. Nutr. 2021, 151, 876–882. [Google Scholar] [CrossRef]
- Gu, F.; Wang, S.; Beijers, R.; de Weerth, C.; Schols, H.A. Structure-Specific and Individual-Dependent Metabolization of Human Milk Oligosaccharides in Infants: A Longitudinal Birth Cohort Study. J. Agric. Food Chem. 2021, 69, 6186–6199. [Google Scholar] [CrossRef]
- Bhargava, P.; Li, C.; Stanya, K.J.; Jacobi, D.; Dai, L.; Liu, S.; Gangl, M.R.; Harn, D.A.; Lee, C.H. Immunomodulatory glycan LNFPIII alleviates hepatosteatosis and insulin resistance through direct and indirect control of metabolic pathways. Nat. Med. 2012, 18, 1665–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dotz, V.; Lemmers, R.F.H.; Reiding, K.R.; Hipgrave Ederveen, A.L.; Lieverse, A.G.; Mulder, M.T.; Sijbrands, E.J.G.; Wuhrer, M.; van Hoek, M. Plasma protein N-glycan signatures of type 2 diabetes. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2613–2622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manhardt, C.T.; Punch, P.R.; Dougher, C.W.L.; Lau, J.T.Y. Extrinsic sialylation is dynamically regulated by systemic triggers in vivo. J. Biol. Chem. 2017, 292, 13514–13520. [Google Scholar] [CrossRef] [Green Version]
- Jantscher-Krenn, E.; Zherebtsov, M.; Nissan, C.; Goth, K.; Guner, Y.S.; Naidu, N.; Choudhury, B.; Grishin, A.V.; Ford, H.R.; Bode, L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012, 61, 1417–1425. [Google Scholar] [CrossRef]
- Hundshammer, C.; Minge, O. In Love with Shaping You-Influential Factors on the Breast Milk Content of Human Milk Oligosaccharides and Their Decisive Roles for Neonatal Development. Nutrients 2020, 12, 3568. [Google Scholar] [CrossRef]
- Battarbee, A.N.; Venkatesh, K.K.; Aliaga, S.; Boggess, K.A. The association of pregestational and gestational diabetes with severe neonatal morbidity and mortality. J. Perinatol. 2020, 40, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Autran, C.A.; Kellman, B.P.; Kim, J.H.; Asztalos, E.; Blood, A.B.; Spence, E.C.H.; Patel, A.L.; Hou, J.; Lewis, N.E.; Bode, L. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut 2018, 67, 1064–1070. [Google Scholar] [CrossRef]
- Craft, K.M.; Thomas, H.C.; Townsend, S.D. Sialylated variants of lacto-N-tetraose exhibit antimicrobial activity against Group B Streptococcus. Org. Biomol. Chem. 2019, 17, 1893–1900. [Google Scholar] [CrossRef]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [Green Version]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef] [Green Version]
- Elison, E.; Vigsnaes, L.K.; Rindom Krogsgaard, L.; Rasmussen, J.; Sorensen, N.; McConnell, B.; Hennet, T.; Sommer, M.O.; Bytzer, P. Oral supplementation of healthy adults with 2′-O-fucosyllactose and lacto-N-neotetraose is well tolerated and shifts the intestinal microbiota. Br. J. Nutr. 2016, 116, 1356–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of Infant Formula with Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponzo, V.; Ferrocino, I.; Zarovska, A.; Amenta, M.B.; Leone, F.; Monzeglio, C.; Rosato, R.; Pellegrini, M.; Gambino, R.; Cassader, M.; et al. The microbiota composition of the offspring of patients with gestational diabetes mellitus (GDM). PLoS ONE 2019, 14, e0226545. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Healthy Group (n = 8) | GDM Group (n = 7) | p Value | ||
|---|---|---|---|---|
| Mother’s basic information | ||||
| Reproductive age (years, ±s) | 31.13 ± 1.89 | 31.71 ± 2.43 | 0.606 | |
| Gestation (weeks, ±s) | 39.71 ± 0.87 | 39.71 ± 0.41 | 0.996 | |
| Production (%) | Primiparous | 6 (75.0) | 6 (85.7) | 1 |
| Multiparous | 2 (25.0) | 1 (14.3) | ||
| Occupation (%) | Professionals | 1 (12.5) | 0 | 1 |
| Office clerk | 7 (87.5) | 7 (100) | ||
| Region (%) | Beijing area | 1 (12.5) | 0 | 1 |
| Non-Beijing area | 7 (87.5) | 7 (100) | ||
| Education level (Maternity) (%) | Undergraduate and below | 2 (25.0) | 4 (66.7) | 0.277 |
| Postgraduate and above | 6 (75.0) | 2 (33.3) | ||
| Education level (Spouse) (%) | Undergraduate and below | 1 (12.5) | 3 (50.0) | 0.245 |
| Postgraduate and above | 7 (87.5) | 3 (50.0) | ||
| Monthly income per family member (%) | ≤15,000 | 3 (37.5) | 4 (66.7) | 0.592 |
| >15,000 | 5 (62.5) | 2 (33.3) | ||
| Premature rupture of membranes (%) | Yes | 0 | 1 (14.3) | 0.467 |
| No | 8 (100) | 6 (85.7) | ||
| Delivery (%) | Vaginal delivery | 6 (75.0) | 5 (71.4) | 1 |
| Cesarean delivery | 2 (25.0) | 2 (28.6) | ||
| Body temperature (°C, median(Q1–Q3)) | 36.5 (36.3, 36.6) | 36.5 (36.2, 36.5) | 0.292 | |
| Pulse (bpm, median(Q1–Q3)) | 90 (72, 100) | 80 (80, 90) | 0.314 | |
| Systolic pressure (mmHg, ±s) | 120.00 ± 7.69 | 119.71 ± 7.54 | 0.943 | |
| Diastolic pressure (mmHg, ±s) | 75.25 ± 7.55 | 75.14 ± 5.55 | 0.976 | |
| Height (cm, ±s) | 165.63 ± 4.50 | 161.14 ± 6.12 | 0.127 | |
| Pre-pregnancy weight (kg, ±s) | 55.50 ± 3.96 | 58.57 ± 5.65 | 0.240 | |
| Current weight (kg, ±s) | 68.75 ± 6.48 | 68.21 ± 5.16 | 0.864 | |
| Gestational weight gain (kg, ±s) | 13.25 ± 5.18 | 9.64 ± 4.77 | 0.186 | |
| Allergic diseases (Maternity) | Yes | 4 (50.0) | 3 (42.9) | 1 |
| No | 4 (50.0) | 4 (57.1) | ||
| Allergic diseases (Spouse) | Yes | 3 (37.5) | 3 (42.9) | 1 |
| No | 5 (62.5) | 4 (57.1) | ||
| Infant’s basic information | ||||
| Gender (%) | Male | 2 (25.0) | 4 (57.1) | 0.315 |
| Female | 6 (75.0) | 3 (42.9) | ||
| Birth weight (g, ±s) | 3336.25 ± 267.53 | 3420.00 ± 320.73 | 0.590 | |
| Birth length (cm, ±s) | 49.50 ± 1.07 | 50.29 ± 1.25 | 0.212 | |
| Birth head circumference (cm, median(Q1–Q3)) | 34 (33, 35) | 34 (34, 34.5) | 0.598 | |
| HMO Types | Healthy Group (n = 8) | GDM Group (n = 7) | |||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | ||
| NN | LNT | 50.25 (42.54, 98.76) b | 48.85 (30.33, 82.38) c | 29.69 (26.53, 39.70) bc | 130.69 (87.26, 147.64) b | 106.91 (62.44, 119.97) c | 59.62 (21.26, 67.26) bc |
| LNnT | 34.17 (29.75, 42.69 b | 17.38 (11.65, 37.01) | 15.90 (9.55, 22.49) b | 174.68 (34.05, 240.20) ab* | 126.62 (27.55, 176.77) ac* | 74.15 (17.48, 82.12) bc* | |
| FN | 2′-FL | 230.65 (173.45, 336.75) | 220.44 (175.24, 326.92) | 261.97 (202.52, 328.61) | 264.78 (250.50, 300.70) | 254.42 (233.85, 296.42) | 239.60 (218.65, 298.89) |
| 3-FL | 21.89 (5.59, 85.70) | 31.76 (7.32, 71.57) | 46.48 (11.43, 74.96) | 73.91 (55.36, 136.45) | 70.08 (56.30, 102.10) | 70.23 (61.08, 106.02) | |
| LNFP-I | 117.46 (53.84, 169.65) b | 65.25 (45.60, 144.66) | 87.79 (16.29, 108.42) b | 156.63 (125.22, 176.69) ab | 103.76 (83.93, 123.60) ac | 47.95 (39.87, 56.68) bc | |
| LNFP-II | 31.97 (10.44, 46.02) | 37.33 (17.08, 40.75) | 21.59 (14.35, 28.25) | 4.93 (4.22, 5.46) * | 4.30 (3.30, 20.51) * | 6.44 (4.11, 7.03) | |
| LNFP-III | 21.72 (15.79, 29.27) a | 17.28 (11.60, 24.36) a | 19.66 (11.91, 26.00) | 17.09 (7.31, 22.74) a | 12.23 (7.28, 20.03) ac | 19.19 (14.20, 21.92) c | |
| LNDFH-I | 66.16 (15.20, 87.10) b | 55.85 (49.31, 88.98) c | 36.76 (22.99, 68.73) bc | 34.50 (25.52, 43.83) b | 40.68 (28.39, 53.96) c | 26.01 (18.08, 31.78) bc | |
| NS | 3′-SL | 8.63 (4.42, 11.14) | 8.09 (3.76, 9.28) | 6.50 (3.38, 9.41) | 19.48 (8.02, 23.01) b* | 19.26 (7.19, 22.43) c | 12.99 (7.58, 19.23) bc* |
| 6′-SL | 30.95 (8.23, 38.37) b | 21.90 (7.53, 35.54) c | 11.19 (1.84, 16.77) bc | 33.81 (23.51, 48.74) b | 28.08 (15.66, 41.50) c | 11.64 (11.34, 21.42) bc | |
| LST a | 8.42 (2.68, 10.76) ab | 4.19 (1.34, 5.51) ac | 1.77 (0.65, 2.44) bc | 7.88 (5.84, 9.77) ab | 4.32 (2.90, 6.12) ac | 1.60 (1.56, 3.01) bc | |
| LST b | 8.96 (4.75, 11.65) b | 7.83 (3.45, 9.95) c | 5.63 (2.95, 7.75) bc | 12.71 (9.06, 15.94) b | 10.44 (6.24, 16.23) c | 5.86 (3.83, 7.90) b | |
| LST c | 27.38 (9.05, 3340) ab | 10.24 (8.73, 18.64) ac | 4.97 (2.60, 7.21) bc | 35.01 (29.49, 41.84) ab | 15.78 (8.51, 25.13) ac | 4.66 (3.11, 8.78) bc | |
| DSLNT | 45.46 (35.14, 57.83) b | 48.88 (28.63, 63.46) c | 25.96 (16.25, 37.37) bc | 89.20 (41.76, 101.94) ab* | 85.00 (27.50, 93.61) ac | 34.03 (8.84, 56.26) bc | |
| 42 Days after Birth | 3 Months after Birth | 6 Months after Birth | ||||
|---|---|---|---|---|---|---|
| Healthy Group | GDM Group | Healthy Group | GDM Group | Healthy Group | GDM Group | |
| Length | 56.00 ± 1.47 | 57.62 ± 1.26 | 63.90 ± 1.44 | 64.75 ± 3.10 | 68.73 ± 1.42 | 68.50 ± 0.58 |
| Weight | 4942.86 ± 479.09 | 5460.00 ± 1238.65 | 7587.50 ± 909.50 | 6962.50 ± 982.66 | 8536.25 ± 617.32 | 8530.00 ± 565.33 |
| Head | 37.57 ± 0.73 | 37.75 ± 1.54 | 41.23 ± 1.24 | 40.00 ± 0.50 | 42.89 ± 1.49 | 43.33 ± 1.04 |
| LAZ | 0.99 ± 0.87 | 0.81 ± 0.89 | 1.36 ± 0.33 | 2.03 ± 2.41 | 1.05 ± 0.63 | 1.42 ± 0.42 |
| WAZ | 1.02 ± 0.81 | 0.80 ± 1.62 | 1.65 ± 1.11 | 1.12 ± 1.64 | 1.11 ± 0.63 | 1.32 ± 0.69 |
| BMIZ | 0.70 ± 0.71 | 0.55 ± 2.64 | 1.23 ± 1.54 | 0.06 ± 0.73 | 0.71 ± 0.71 | 0.75 ± 1.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, Y.; Luo, Y.; Xing, Y.; Liu, H.; Chen, B.; Zhu, L.; Ma, D.; Zhu, J. Human Milk Oligosaccharides Variation in Gestational Diabetes Mellitus Mothers. Nutrients 2023, 15, 1441. https://doi.org/10.3390/nu15061441
Dou Y, Luo Y, Xing Y, Liu H, Chen B, Zhu L, Ma D, Zhu J. Human Milk Oligosaccharides Variation in Gestational Diabetes Mellitus Mothers. Nutrients. 2023; 15(6):1441. https://doi.org/10.3390/nu15061441
Chicago/Turabian StyleDou, Yuqi, Yuanli Luo, Yan Xing, Hui Liu, Botian Chen, Liye Zhu, Defu Ma, and Jing Zhu. 2023. "Human Milk Oligosaccharides Variation in Gestational Diabetes Mellitus Mothers" Nutrients 15, no. 6: 1441. https://doi.org/10.3390/nu15061441
APA StyleDou, Y., Luo, Y., Xing, Y., Liu, H., Chen, B., Zhu, L., Ma, D., & Zhu, J. (2023). Human Milk Oligosaccharides Variation in Gestational Diabetes Mellitus Mothers. Nutrients, 15(6), 1441. https://doi.org/10.3390/nu15061441







