Dried Fruits, Nuts, and Cancer Risk and Survival: A Review of the Evidence and Future Research Directions
Abstract
1. Introduction
2. Dietary Strategies to Prevent Cancer
3. Dried Fruits and Cancer
3.1. Preclinical Studies Relating Dried Fruits and Cancer
3.2. Human Intervention Studies of Dried Fruits and Cancer
3.3. Epidemiological Studies of Dried Fruits and Cancer
3.3.1. Cancer Incidence (or Risk)
3.3.2. Cancer Mortality and Survival
3.4. Research Gaps, Needs, and Priorities Related to Dried Fruits and Cancer
4. Tree Nuts, Peanuts, and Cancer
4.1. Preclinical Studies Related to Nuts and Cancer
4.2. Human Intervention Studies of Nuts and Cancer
4.3. Epidemiological Studies of Nuts and Cancer
4.3.1. Cancer Incidence
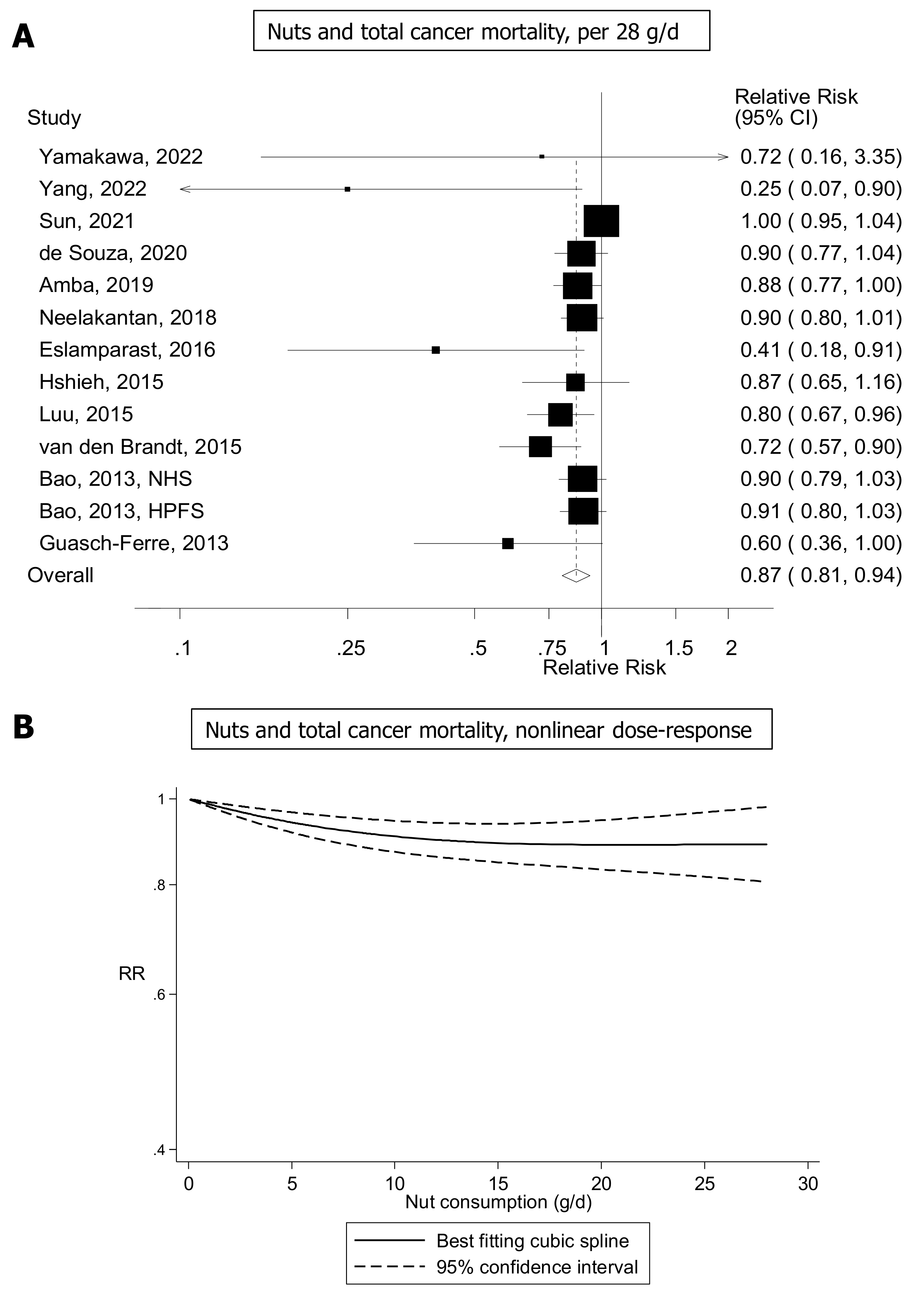
4.3.2. Cancer Mortality and Survival
4.4. Research Gaps, Needs, and Priorities Related to the Study of Nuts and Cancer
5. Summary and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Disclaimer
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund. Continuous Update Project Expert Report 2018. Recommendations and Public Health and Policy. 2018. Available online: https://www.wcrf.org/diet-activity-and-cancer/global-cancer-update-programme/about-the-third-expert-report/ (accessed on 26 January 2023).
- Colditz, G.A.; Wolin, K.Y.; Gehlert, S. Applying What We Know to Accelerate Cancer Prevention. Sci. Transl. Med. 2012, 4, 127rv124. [Google Scholar] [CrossRef]
- Brennan, P.; Davey-Smith, G. Identifying Novel Causes of Cancers to Enhance Cancer Prevention: New Strategies Are Needed. J. Nat. Cancer Inst. 2022, 114, 353–360. [Google Scholar] [CrossRef]
- Zitvogel, L.; Pietrocola, F.; Kroemer, G. Nutrition, Inflammation and Cancer. Nat. Immunol. 2017, 18, 843–850. [Google Scholar] [CrossRef]
- Wild, W.E.; Stewart, B.W. (Eds.) World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-Cancer-Research-For-Cancer-Prevention-2020 (accessed on 26 January 2023).
- Wang, D.D.; Li, Y.; Afshin, A.; Springmann, M.; Mozaffarian, D.; Stampfer, M.J.; Hu, F.B.; Murray, C.J.L.; Willett, W.C. Global Improvement in Dietary Quality Could Lead to Substantial Reduction in Premature Death. J. Nutr. 2019, 149, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2020. Available online: https://www.dietaryguidelines.gov/ (accessed on 26 January 2023).
- United States Code of Federal Regulations. Health Claims: Fiber-Containing Grain Products, Fruits, and Vegetables and Cancer. 21 CFR 101.76. 1993. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-101/subpart-E/section-101.76 (accessed on 26 January 2023).
- International Agency for Research on Cancer; World Health Organization. European Code against Cancer. Available online: https://cancer-code-europe.iarc.fr/index.php/en/ (accessed on 26 January 2023).
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- The American Cancer Society Medical and Editorial Content Team. American Cancer Society Guideline for Diet and Physical Activity. Available online: https://www.cancer.org/healthy/eat-healthy-get-active/acs-guidelines-nutrition-physical-activity-cancer-prevention/guidelines.html# (accessed on 28 January 2023).
- World Cancer Research Fund; American Institute for Cancer Research. Continuous Update Project Expert Report, Wholegrains, Vegetables and Fruit and the Risk of Cancer. 2018. Available online: https://www.wcrf.org/wp-content/uploads/2020/12/Wholegrains-veg-and-fruit.pdf (accessed on 26 January 2023).
- International Nut & Dried Fruit Council. Nuts & Dried Fruits Statistical Yearbook; INC International Nut & Dried Fruit: Reus, Spain, 2022; Available online: https://inc.nutfruit.org/technical-projects/ (accessed on 26 January 2023).
- Sullivan, V.K.; Na, M.; Proctor, D.N.; Kris-Etherton, P.M.; Petersen, K.S. Consumption of Dried Fruits Is Associated with Greater Intakes of Underconsumed Nutrients, Higher Total Energy Intakes, and Better Diet Quality in US Adults: A Cross-Sectional Analysis of the National Health and Nutrition Examination Survey, 2007–2016. J. Acad. Nutr. Diet. 2021, 121, 1258–1272. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.; Liu, X.; Liu, J. Dried Fruit Consumption and Cancer. In Health Benefits of Nuts and Dried Fruits; Alasalvar, C., Salas-Salvado, J., Ros, E., Sabate, J., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–20, Chapter 19. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Sangiovanni, E.; Fumagalli, M.; Colombo, E.; Frigerio, G.; Colombo, F.; Peres de Sousa, L.; Altindisli, A.; Restani, P.; Dell’Agli, M. Evaluation of the Anti-Inflammatory Activity of Raisins (Vitis vinifera L.) in Human Gastric Epithelial Cells: A Comparative Study. Int. J. Mol. Sci. 2016, 17, 1156. [Google Scholar] [CrossRef]
- Kountouri, A.M.; Gioxari, A.; Karvela, E.; Kaliora, A.C.; Karvelas, M.; Karathanos, V.T. Chemopreventive Properties of Raisins Originating from Greece in Colon Cancer Cells. Food Funct. 2013, 4, 366–372. [Google Scholar] [CrossRef]
- Mori, S.; Sawada, T.; Okada, T.; Ohsawa, T.; Adachi, M.; Keiichi, K. New Anti-Proliferative Agent, MK615, from Japanese Apricot “Prunus mume” Induces Striking Autophagy in Colon Cancer Cells In Vitro. World J. Gastroenterol. 2007, 13, 6512–6517. [Google Scholar] [CrossRef]
- Nakagawa, A.; Sawada, T.; Okada, T.; Ohsawa, T.; Adachi, M.; Kubota, K. New Antineoplastic Agent, MK615, from UME (a Variety of) Japanese Apricot Inhibits growth of Breast Cancer Cells In Vitro. Breast J. 2007, 13, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Jasmine, R.; Manikandan, K.; Karthikeyan, K. Evaluating the Antioxidant and Cnticancer Property of Ficus carica Fruits. Afr. J. Biotechnol. 2015, 14, 634–641. [Google Scholar] [CrossRef]
- Uz, R.I.; Bakar, N.H.A.; Swethadri, G.K.; Baig, A.; Idris, M.A.; Maryam, I.U. Non-Toxic Antiproliferative Effect of Ficus carica Fruit Extracts on Estrogen Receptor Positive Breast Cancer Cell (MCF-7). J. Chem. Pharm. Res. 2015, 7, 815–821. [Google Scholar]
- Bu, S.Y.; Lerner, M.; Stoecker, B.J.; Boldrin, E.; Brackett, D.J.; Lucas, E.A.; Smith, B.J. Dried Plum Polyphenols Inhibit Osteoclastogenesis By Downregulating NFATc1 and Inflammatory Mediators. Calcif. Tissue Int. 2008, 82, 475–488. [Google Scholar] [CrossRef]
- Fujii, T.; Ikami, T.; Xu, J.W.; Ikeda, K. Prune Extract (Prunus domestica L.) Suppresses the Proliferation and Induces the Apoptosis of Human Colon Carcinoma Caco-2. J. Nutr. Sci. Vitaminol. 2006, 52, 389–391. [Google Scholar] [CrossRef]
- Khan, F.; Ahmed, F.; Pushparaj, P.N.; Abuzenadah, A.; Kumosani, T.; Barbour, E.; AlQahtani, M.; Gauthaman, K. Ajwa Date (Phoenix dactylifera L.) Extractinhibits Human Breast Adenocarcinoma (MCF7) cells In Vitro By Inducing Apoptosis and Cell Cycle Arrest. PLoS ONE 2016, 11, e0158963. [Google Scholar] [CrossRef]
- Mirza, M.B.; Elkady, A.I.; Al-Attar, A.M.; Syed, F.Q.; Mohammed, F.A.; Hakeem, K.R. Induction of Apoptosis and Cell Cycle Arrest by Ethyl Acetate Fraction of Phoenix dactylifera L. (Ajwa dates) in Prostate Cancer Cells. J. Ethnopharmacol. 2018, 218, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Turan, A.; Celik, I. Antioxidant and Hepatoprotective Properties of Dried Fig Against Oxidative Stress and Hepatotoxicity in Rats. Int. J. Biol. Macromol. 2016, 91, 554–559. [Google Scholar] [CrossRef]
- Khan, F.; Khan, T.J.; Kalamegam, G.; Pushparaj, P.N.; Chaudhary, A.; Abuzenadah, A.; Kumosani, T.; Barbour, E.; Al-Qahtani, M. Anti-cancer Effects of Ajwa Dates (Phoenix dactylifera L.) in Diethylnitrosamine Induced Hepatocellular Carcinoma in Wistar Rats. BMC Complement. Altern. Med. 2017, 17, 418. [Google Scholar] [CrossRef]
- Yang, Y.; Gallaher, D.D. Effect of Dried Plums on Colon Cancer Risk Factors in Rats. Nutr. Cancer 2005, 53, 117–125. [Google Scholar] [CrossRef]
- Ugras, M.Y.; Kurus, M.; Ates, B.; Soylemez, H.; Otlu, A.; Yilmaz, I. Prunus armeniaca L (Apricot) Protects Rat Testes from Detrimental Effects of Low-dose X-rays. Nutr. Res. 2010, 30, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.A.; Smyth, J.A.; Liu, Z.; Bolling, B.W. Aronia Berry (Aronia mitschurinii ‘Viking’) Inhibits Colitis in Mice and Inhibits T Cell Tumour Necrosis Factor-α Secretion. J. Funct. Foods 2018, 44, 48–57. [Google Scholar] [CrossRef]
- Martin, D.A.; Taheri, R.; Brand, M.H.; Draghi, A.; Sylvester, F.A.; Bolling, B.W. Anti-Inflammatory Activity of Aronia Berry Extracts in Murine Splenocytes. J. Funct. Foods 2014, 8, 68–75. [Google Scholar] [CrossRef]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and Gut Health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Chang, S.K.; Kris-Etherton, P.M.; Sullivan, V.K.; Petersen, K.S.; Guasch-Ferré, M.; Jenkins, D. Dried Fruits and Health. Nutrients 2023, in press. [Google Scholar]
- Raveendran, D.; Bhagwat, M.; Chidanand, D.V.; Anandakumar, S.; Sunil, C.K. Highlight on Drying Fruit Slices with Better Retention of Bioactive Compounds. J. Food Proc. Eng. 2022, 45, e14048. [Google Scholar] [CrossRef]
- Serratosa, M.P.; Lopez-Toledano, A.; Merida, J.; Medina, M. Changes in Color and Phenolic Compounds during the Raisining of Grape Cv. Pedro Ximenez. J. Agric. Food Chem. 2008, 56, 2810–2816. [Google Scholar] [CrossRef]
- Catak, J.; Yaman, M.; Ugur, H.; Servi, E.Y.; Mizrak, O.F. Investigation of the Advanced Glycation End Products Precursors in Dried Fruits and Nuts by HPLC using Pre-column Derivatization. J. Food Nutr. Res. 2022, 61, 81–88. [Google Scholar]
- Córdova, R.; Mayén, A.-L.; Knaze, V.; Aglago, E.K.; Schalkwijk, C.; Wagner, K.-H.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Katzke, V.A.; et al. Dietary Intake of Advanced Glycation Endproducts (AGEs) and Cancer Risk Across More Than 20 Anatomical Sites: A Multinational Cohort Study. Cancer Commun. 2022, 42, 1041–1045. [Google Scholar] [CrossRef]
- McWeeny, D.J.; Biltcliffe, D.O.; Powell, R.C.T.; Spark, A.A. The Maillard Reaction and Its Inhibition by Sulfite. J. Food Sci. 1969, 34, 641–643. [Google Scholar] [CrossRef]
- Scrob, T.; Covaci, E.; Hosu, A.; Tanaselia, C.; Casoni, D.; Torok, A.I.; Frentiu, T.; Cimpoiu, C. Effect of In Vitro Simulated Gastrointestinal Digestion on Some Nutritional Characteristics of Several Dried Fruits. Food Chem. 2022, 385, 132713. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Martin, D.A.; Valdez, J.C.; Sudakaran, S.; Rey, F.; Bolling, B.W. Aronia Berry Polyphenols Have Matrix-dependent Effects on the Gut Microbiota. Food Chem. 2021, 359, 129831. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, V.K.; Petersen, K.S.; Kris-Etherton, P.M. Dried Fruit Consumption and Cardiometabolic Health: A Randomised Crossover Trial. Br. J. Nutr. 2020, 124, 912–921. [Google Scholar] [CrossRef]
- Viguiliouk, E.; Jenkins, A.L.; Blanco Mejia, S.; Sievenpiper, J.L.; Kendall, C.W.C. Effect of Dried Fruit on Postprandial Glycemia: A Randomized Acute-feeding Trial. Nutr. Diabetes 2018, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- George, K.S.; Munoz, J.; Ormsbee, L.T.; Akhavan, N.S.; Foley, E.M.; Siebert, S.C.; Kim, J.S.; Hickner, R.C.; Arjmandi, B.H. The Short-Term Effect of Prunes in Improving Bone in Men. Nutrients 2022, 14, 276. [Google Scholar] [CrossRef]
- Mentor-Marcel, R.A.; Bobe, G.; Sardo, C.; Wang, L.S.; Kuo, C.T.; Stoner, G.; Colburn, N.H. Plasma cytokines as Potential Response Indicators to Dietary Freeze-dried Black Raspberries in Colorectal Cancer Patients. Nutr. Cancer 2012, 64, 820–825. [Google Scholar] [CrossRef]
- Mallery, S.R.; Stoner, G.D.; Larsen, P.E.; Fields, H.W.; Rodrigo, K.A.; Schwartz, S.J.; Tian, Q.; Dai, J.; Mumper, R.J. Formulation and in-vitro and in-vivo Evaluation of a Mucoadhesive Gel Containing Freeze Dried Black Raspberries: Implications for Oral Cancer Chemoprevention. Pharm. Res. 2007, 24, 728–737. [Google Scholar] [CrossRef]
- Wang, L.S.; Arnold, M.; Huang, Y.W.; Sardo, C.; Seguin, C.; Martin, E.; Huang, T.H.; Riedl, K.; Schwartz, S.; Frankel, W.; et al. Modulation of Genetic and Epigenetic Biomarkers of Colorectal Cancer in Humans by Black Raspberries: A Phase I Pilot Study. Clin. Cancer Res. 2011, 17, 598–610. [Google Scholar] [CrossRef]
- Wang, L.S.; Burke, C.A.; Hasson, H.; Kuo, C.T.; Molmenti, C.L.; Seguin, C.; Liu, P.; Huang, T.H.; Frankel, W.L.; Stoner, G.D. A Phase Ib Study of the Effects of Black Raspberries on Rectal Polyps in Patients with Familial Adenomatous Polyposis. Cancer Prev. Res. 2014, 7, 666–674. [Google Scholar] [CrossRef]
- McAnulty, L.S.; Collier, S.R.; Landram, M.J.; Whittaker, D.S.; Isaacs, S.E.; Klemka, J.M.; Cheek, S.L.; Arms, J.C.; McAnulty, S.R. Six Weeks Daily Ingestion of Whole Blueberry Powder Increases Natural Killer Cell Counts and Reduces Arterial Stiffness in Sedentary Males and Females. Nutr. Res. 2014, 34, 577–584. [Google Scholar] [CrossRef]
- Student, V.; Vidlar, A.; Bouchal, J.; Vrbkova, J.; Kolar, Z.; Kral, M.; Kosina, P.; Vostalova, J. Cranberry Intervention in Patients with Prostate Cancer Prior to Radical Prostatectomy. Clinical, Pathological and Laboratory Findings. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2016, 160, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Mossine, V.V.; Mawhinney, T.P.; Giovannucci, E.L. Dried Fruit Intake and Cancer: A Systematic Review of Observational Studies. Adv. Nutr. 2020, 11, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Zeegers, M.P.; Goldbohm, R.A.; van den Brandt, P.A. Consumption of Vegetables and Fruits and Urothelial Cancer Incidence: A Prospective Study. Cancer Epidemiol. Biomark. Prev. 2001, 10, 1121–1128. [Google Scholar]
- Botterweck, A.A.; van den Brandt, P.A.; Goldbohm, R.A. A Prospective Cohort Study on Vegetable and Fruit Consumption and Stomach Cancer Risk in The Netherlands. Am. J. Epidemiol. 1998, 148, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, A.G.; Goldbohm, R.A.; Dorant, E.; van den Brandt, P.A. Vegetable and Fruit Consumption and Prostate Cancer Risk: A Cohort Study in The Netherlands. Cancer Epidemiol. Biomark. Prev. 1998, 7, 673–680. [Google Scholar]
- Michels, K.B.; Edward, G.; Joshipura, K.J.; Rosner, B.A.; Stampfer, M.J.; Fuchs, C.S.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. Prospective Study of Fruit and Vegetable Consumption and Incidence of Colon and Rectal Cancers. J. Natl. Cancer Inst. 2000, 92, 1740–1752. [Google Scholar] [CrossRef]
- Mills, P.K.; Beeson, W.L.; Abbey, D.E.; Fraser, G.E.; Phillips, R.L. Dietary Habits and Past Medical History as Related to Fatal Pancreas Cancer Risk among Adventists. Cancer 1988, 61, 2578–2585. [Google Scholar] [CrossRef]
- Tantamango, Y.M.; Knutsen, S.F.; Beeson, W.L.; Fraser, G.; Sabate, J. Foods and Food Groups Associated with the Incidence of Colorectal Polyps: The Adventist Health Study. Nutr. Cancer 2011, 63, 565–572. [Google Scholar] [CrossRef]
- Mills, P.K.; Beeson, W.L.; Phillips, R.L.; Fraser, G.E. Cohort Study of Diet, Lifestyle, and Prostate Cancer in Adventist Men. Cancer 1989, 64, 598–604. [Google Scholar] [CrossRef]
- Fraser, G.E.; Beeson, W.L.; Phillips, R.L. Diet and Lung Cancer in California Seventh-day Adventists. Am. J. Epidemiol. 1991, 133, 683–693. [Google Scholar] [CrossRef]
- Dunneram, Y.; Greenwood, D.C.; Cade, J.E. Diet and Risk of Breast, Endometrial and Ovarian Cancer: UK Women’s Cohort Study. Br. J. Nutr. 2019, 122, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jin, L.; Petrick, J.L.; Zeng, H.; Wang, F.; Tang, L.; Smith-Warner, S.A.; Eliassen, A.H.; Zhang, F.F.; Campbell, P.T.; et al. Specific Botanical Groups of Fruit and Vegetable Consumption and Liver Cancer and Chronic Liver Disease Mortality: A Prospective Cohort Study. Am. J. Clin. Nutr. 2022, 17, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Li, R.; Deng, T.; Lin, Z.; Li, H.; Yang, Y.; Su, Q.; Wang, J.; Yang, Y.; Wang, J.; et al. Association between Dried Fruit Intake and Pan-cancers Incidence Risk: A Two-sample Mendelian Randomization Study. Front. Nutr. 2022, 9, 899137. [Google Scholar] [CrossRef] [PubMed]
- Baghurst, P.A.; McMichael, A.J.; Slavotinek, A.H.; Baghurst, K.I.; Boyle, P.; Walker, A.M. A Case-Control Study of Diet and Cancer of the Pancreas. Am. J. Epidemiol. 1991, 134, 167–179. [Google Scholar] [CrossRef]
- Tayyem, R.F.; Shehadah, I.; Abu-Mweis, S.S.; Bawadi, H.A.; Bani-Hani, K.E.; Al-Jaberi, T.; Al-Nusairr, M.; Heath, D.D. Fruit and Vegetable Intake among Jordanians: Results from a Case-control Study of Colorectal Cancer. Cancer Control 2014, 21, 350–360. [Google Scholar] [CrossRef]
- González, C.A.; Sanz, J.M.; Marcos, G.; Pita, S.; Brullet, E.; Saigi, E.; Badia, A.; Riboli, E. Dietary Factors and Stomach Cancer in Spain: A Multi-centre Case-control Study. Int. J. Cancer 1991, 49, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Yassibaş, E.; Arslan, P.; Yalçin, S. Evaluation of Dietary and Life-style Habits of Patients with Gastric Cancer: A Case-control Study in Turkey. Asian Pac. J. Cancer Prev. 2012, 13, 2291–2297. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.; Bhupathiraju, S.N.; Rosner, B.A.; Sun, Q.; Giovannucci, E.L.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; et al. Fruit and Vegetable Intake and Mortality: Results from 2 Prospective Cohort Studies of US Men and Women and a Meta-Analysis of 26 Cohort Studies. Circulation 2021, 143, 1642–1654. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and Vegetable Intake and the Risk of Cardiovascular Disease, Total Cancer and All-cause Mortality-a Systematic Review and Dose-response Meta-analysis of Prospective Studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Hollman, P.C. Unravelling of the Health Effects of Polyphenols is a Complex Puzzle Complicated by Metabolism. Arch. Biochem. Biophys. 2014, 559, 100–105. [Google Scholar] [CrossRef]
- Bakker, M.F.; Peeters, P.H.; Klaasen, V.M.; Bueno-de-Mesquita, H.B.; Jansen, E.H.; Ros, M.M.; Travier, N.; Olsen, A.; Tjønneland, A.; Overvad, K.; et al. Plasma Carotenoids, Vitamin C, Tocopherols, and Retinol and the Risk of Breast Cancer in the European Prospective Investigation into Cancer and Nutrition Cohort. Am. J. Clin. Nutr. 2016, 103, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Cordova, R.; Kliemann, N.; Huybrechts, I.; Rauber, F.; Vamos, E.P.; Levy, R.B.; Wagner, K.H.; Viallon, V.; Casagrande, C.; Nicolas, G.; et al. Consumption of Ultra-Processed Foods Associated with Weight Gain and Obesity in Adults: A Multi-national Cohort Study. Clin. Nutr. 2021, 40, 5079–5088. [Google Scholar] [CrossRef] [PubMed]
- Nishi, S.K.; Viguiliouk, E.; Blanco Mejia, S.; Kendall, C.W.C.; Bazinet, R.P.; Hanley, A.J.; Comelli, E.M.; Salas Salvadó, J.; Jenkins, D.J.A.; Sievenpiper, J.L. Are Fatty Nuts a Weighty Concern? A Systematic Review and Meta-Analysis and Dose–Response Meta-Regression of Prospective Cohorts and Randomized Controlled Trials. Obes. Rev. 2021, 22, e13330. [Google Scholar] [CrossRef]
- Fitzgerald, E.; Lambert, K.; Stanford, J.; Neale, E.P. The Effect of Nut Consumption (Tree Nuts and Peanuts) on the Gut Microbiota of Humans: A Systematic Review. Br. J. Nutr. 2021, 125, 508–520. [Google Scholar] [CrossRef]
- Schulz, M.D.; Atay, C.; Heringer, J.; Romrig, F.K.; Schwitalla, S.; Aydin, B.; Ziegler, P.K. High-Fat-Diet-Mediated Dysbiosis Promotes Intestinal Carcinogenesis Independently of Obesity. Nature 2014, 514, 508–512. [Google Scholar] [CrossRef]
- Hardman, W.E.; Ion, G.; Akinsete, J.A.; Witte, T.R. Dietary Walnut Suppressed Mammary Gland Tumorigenesis in the C(3)1 TAg Mouse. Nutr. Cancer Int. J. 2011, 63, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.P.; Lamarque, A.L.; Comba, A.; Berra, M.A.; Silva, R.A.; Labuckas, D.O.; Das, U.N. Synergistic Anti-tumor Effects of Melatonin and PUFAs from Walnuts in a Murine Mammary Adenocarcinoma Model. Nutrition 2015, 31, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Bai, M.H.; Zhang, T.; Li, G.D.; Liu, M. Ellagic acid Induces Cell Cycle Arrest and Apoptosis through TGF-beta/Smad3 Signaling Pathway in Human Breast Cancer MCF-7 Cells. Int. J. Oncol. 2015, 46, 1730–1738. [Google Scholar] [CrossRef]
- Hong, M.Y.; Moore, J.; Nakagawa, A.; Nungaray, V. Effects of Mixed Nuts on Colonic Cell Proliferation and Ptgs2 and Rela Gene Expression. Anticancer Res. 2022, 42, 4285–4292. [Google Scholar] [CrossRef]
- Chen, Y.; Nakanishi, M.; Bautista, E.J.; Qendro, V.; Sodergren, E.; Rosenberg, D.W.; Weinstock, G.M. Colon Cancer Prevention with Walnuts: A Longitudinal Study in Mice from the Perspective of a Gut Enterotype-like Cluster. Cancer Prev. Res. 2020, 13, 15–24. [Google Scholar] [CrossRef]
- Nagel, J.M.; Brinkoetter, M.; Magkos, F.; Liu, X.; Chamberland, J.P.; Shah, S.; Zhou, J. Dietary Walnuts Inhibit Colorectal Cancer Growth in Mice by Suppressing Angiogenesis. Nutrition 2012, 28, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Chen, Y.F.; Qendro, V.; Miyamoto, S.; Weinstock, E.; Weinstock, G.M.; Rosenberg, D.W. Effects of Walnut Consumption on Colon Carcinogenesis and Microbial Community Structure. Cancer Prev. Res. 2016, 9, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.A.; Iwahashi, C.K. Whole Almonds and Almond Fractions Reduce Aberrant Crypt Foci in a Rat Model of Colon Carcinogenesis. Cancer Lett. 2001, 165, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Tsoukas, M.A.; Ko, B.J.; Witte, T.R.; Dincer, F.; Hardman, W.E.; Mantzoros, C.S. Dietary Walnut Suppression of Colorectal Cancer in Mice: Mediation by miRNA Patterns and Fatty Acid Incorporation. J. Nutr. Biochem. 2015, 26, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; Jang, H.-H.; Kim, G.; Zouiouich, S.; Cho, S.-Y.; Kim, H.-J.; Kim, J.; Choe, J.-S.; Gunter, M.J.; Ferrari, P.; et al. Taxonomic Composition and Diversity of the Gut Microbiota in Relation to Habitual Dietary Intake in Korean Adults. Nutrients 2021, 13, 366. [Google Scholar] [CrossRef]
- Davis, P.A.; Vasu, V.T.; Gohil, K.; Kim, H.; Khan, I.H.; Cross, C.E.; Yokoyama, W. A High-fat Diet Containing Whole Walnuts (Juglans regia) Reduces Tumour Size and Growth Along with Plasma Insulin-like Growth Factor 1 in the Transgenic Adenocarcinoma of the Mouse Prostate Model. Br. J. Nutr. 2012, 108, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yokoyama, W.; Davis, P.A. TRAMP Prostate Tumor Growth Is Slowed by Walnut Diets through Altered IGF-1 Levels, Energy Pathways, and Cholesterol Metabolism. J. Med. Food 2014, 17, 1281–1286. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Korkmaz, A.; Fuentes-Broto, L.; Hardman, W.E.; Rosales-Corral, S.A. A Walnut-Enriched Diet Reduces the Growth of LNCaP Human Prostate Cancer Xenografts in Nude Mice. Cancer Investig. 2013, 31, 365–373. [Google Scholar] [CrossRef]
- Hu, Y.; McIntosh, G.H.; Le Leu, R.K.; Somashekar, R.; Meng, X.Q.; Gopalsamy, G.; Bambaca, L. Supplementation with Brazil Nuts and Green Tea Extract Regulates Targeted Biomarkers Related to Colorectal Cancer Risk in Humans. Br. J. Nutr. 2016, 116, 1901–1911. [Google Scholar] [CrossRef]
- Pereira, M.A.N.; da Silva Junior, E.C.; Dayse da Silva, I.L.; de Carvalho, B.A.; Ferreira, E.; Andrade, E.F.; Guimarães Guilherme, L.R.; Pereira, L.J. Antitumor Effect of Selenium-Rich Brazil Nuts and Selenomethionine Dietary Supplementation on Pre-existing 4T1 Mammary Tumor Growth in Mice. PLoS ONE 2023, 18, e0278088. [Google Scholar] [CrossRef]
- Hardman, W.E.; Ion, G. Suppression of Implanted MDA-MB 231 Human Breast Cancer Growth in Nude Mice by Dietary Walnut. Nutr. Cancer Int. J. 2008, 60, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.; Salas-Salvado, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D. Mediterranean Diet and Invasive Breast Cancer Risk among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.J.; Fedirko, V.; Jenab, M.; Schomburg, L.; Méplan, C.; Freisling, H.; Bueno-de-Mesquita, H.B.; Hybsier, S.; Becker, N.-P.; Czuban, M.; et al. Selenium Status is Associated with Colorectal Cancer Risk in the European Prospective Investigation of Cancer and Nutrition Cohort. Int. J. Cancer 2015, 136, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of Selenium and Vitamin E on Risk of Prostate Cancer and Other Cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Tanzman, J.S.; Sabate, J. Lack of Effect of Walnuts on Serum Levels of Prostate Specific Antigen: A Brief Report. J. Am. Coll. Nutr. 2007, 26, 317–320. [Google Scholar] [CrossRef]
- Spaccarotella, K.J.; Kris-Etherton, P.M.; Stone, W.L.; Bagshaw, D.M.; Fishell, V.K.; West, S.G.; Lawrence, F.R. The Effect of Walnut Intake on Factors Related to Prostate and Vascular Health in Older Men. Nutr. J. 2008, 7, 13. [Google Scholar] [CrossRef]
- Jia, X.D.; Li, N.; Zhang, W.Z.; Zhang, X.P.; Lapsley, K.; Huang, G.W.; Blumberg, J. A Pilot Study on the Effects of Almond Consumption on DNA Damage and Oxidative Stress in Smokers. Nutr. Cancer 2006, 54, 179–183. [Google Scholar] [CrossRef]
- Bamberger, C.; Rossmeier, A.; Lechner, K.; Wu, L.; Waldmann, E.; Fischer, S.; Stark, R.G.; Altenhofer, J.; Henze, K.; Parhofer, K.G. A Walnut-Enriched Diet Affects Gut Microbiome in Healthy Caucasian Subjects: A Randomized, Controlled Trial. Nutrients 2018, 10, 244. [Google Scholar] [CrossRef]
- Naghshi, S.; Sadeghian, M.; Nasiri, M.; Mobarak, S.; Asadi, M.; Sadeghi, O. Association of Total Nut, Tree Nut, Peanut, and Peanut Butter Consumption with Cancer Incidence and Mortality: A Comprehensive Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Adv. Nutr. 2021, 12, 793–808. [Google Scholar] [CrossRef]
- Long, J.; Ji, Z.; Yuan, P.; Long, T.; Liu, K.; Li, J.; Cheng, L. Nut Consumption and Risk of Cancer: A Meta-analysis of Prospective Studies. Cancer Epidemiol. Biomark. Prev. 2020, 29, 565–573. [Google Scholar] [CrossRef]
- Cao, C.; Gan, X.; He, Y.; Nong, S.; Su, Y.; Liu, Z.; Zhang, Y.; Hu, X.; Peng, X. Association Between Nut Consumption and Cancer Risk: A Meta-Analysis. Nutr. Cancer 2023, 75, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Wu, Y.; Li, Y.; Zhang, X.; Willett, W.C.; Eliassen, A.H.; Rosner, B.; Song, M.; Mucci, L.A.; Giovannucci, E.L. Association of Nut Consumption with Risk of Total Cancer and 5 Specific Cancers: Evidence from 3 Large Prospective Cohort Studies. Am. J. Clin. Nutr. 2021, 114, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, L.; van den Brandt, P.A. Nut and Peanut Butter Consumption and the Risk of Total Cancer: A Prospective Cohort Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2100–2104. [Google Scholar] [CrossRef] [PubMed]
- Von Ruesten, A.; Feller, S.; Bergmann, M.M.; Boeing, H. Diet and Risk of Chronic Diseases: Results from the First 8 Years of Follow-up in the EPIC-Potsdam Study. Eur. J. Clin. Nutr. 2013, 67, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna, R.; Bjørnerud, T.; Bemanian, M.; Aune, D.; Fadnes, L.T. Consumption of Nuts and Seeds and Health Outcomes Including Cardiovascular Disease, Diabetes and Metabolic Disease, Cancer, and Mortality: An Umbrella Review. Adv. Nutr. 2022, 13, nmac077. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.C.; Zhang, R.; Martinez-Gonzalez, M.A.; Zhang, Z.L.; Bonaccio, M.; Dam, R.M.; Qin, L.Q. Nut Consumption in Relation to All-Cause and Cause-Specific Mortality: A Meta-analysis 18 Prospective Studies. Food Funct. 2017, 8, 3893–3905. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Nut Consumption and Risk of Cardiovascular Disease, Total Cancer, All-Cause and Cause-Specific Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. BMC Med. 2016, 14, 207. [Google Scholar] [CrossRef]
- Grosso, G.; Yang, J.; Marventano, S.; Micek, A.; Galvano, F.; Kales, S.N. Nut Consumption on All-Cause, Cardiovascular, and Cancer Mortality Risk: A Systematic Review and Meta-Analysis of Epidemiologic Studies. Am. J. Clin. Nutr. 2015, 101, 783–793. [Google Scholar] [CrossRef]
- Zhang, D.; Dai, C.; Zhou, L.; Li, Y.; Liu, K.; Deng, Y.J.; Li, N.; Zheng, Y.; Hao, Q.; Yang, S.; et al. Meta-Analysis of the Association between Nut Consumption and the Risks of Cancer Incidence and Cancer-Specific Mortality. Aging 2020, 12, 10772–10794. [Google Scholar] [CrossRef]
- Amba, V.; Murphy, G.; Etemadi, A.; Wang, S.; Abnet, C.C.; Hashemian, M. Nut and Peanut Butter Consumption and Mortality in the National Institutes of Health-AARP Diet and Health Study. Nutrients 2019, 11, 1508. [Google Scholar] [CrossRef]
- De Souza, R.J.; Dehghan, M.; Mente, A.; Bangdiwala, S.I.; Ahmed, S.H.; Alhabib, K.F.; Altuntas, Y.; Basiak-Rasała, A.; Dagenais, G.R.; Diaz, R.; et al. Association of Nut Intake with Risk Factors, Cardiovascular Disease, and Mortality in 16 Countries from 5 Continents: Analysis from the Prospective Urban and Rural Epidemiology (PURE) Study. Am. J. Clin. Nutr. 2020, 112, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, B.; Snetselaar, L.G.; Wallace, R.B.; Shadyab, A.H.; Kroenke, C.H.; Haring, B.; Howard, B.V.; Shikany, J.M.; Valdiviezo, C.; et al. Association of Major Dietary Protein Sources with All-Cause and Cause-Specific Mortality: Prospective Cohort Study. J. Am. Heart Assoc. 2021, 10, e015553. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, A.; Yeung, S.; Woo, J.; Lo, K. Joint Associations of Food Groups with All-Cause and Cause-Specific Mortality in the Mr. OS and Ms. OS Study: A Prospective Cohort. Nutrients 2022, 14, 3915. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, M.; Wada, K.; Koda, S.; Uji, T.; Nakashima, Y.; Onuma, S.; Oba, S.; Nagata, C. Associations of Total Nut and Peanut Intakes with All-Cause and Cause-Specific Mortality in a Japanese Community: The Takayama Study. Br. J. Nutr. 2022, 127, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, N.; Koh, W.P.; Yuan, J.M.; van Dam, R.M. Diet-Quality Indexes Are Associated with a Lower Risk of Cardiovascular, Respiratory, and All-Cause Mortality among Chinese Adults. J. Nutr. 2018, 148, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, M.; Kenfield, S.A.; Hu, F.B.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S.; Giovannucci, E.L.; Bao, Y. Nut Consumption and Prostate Cancer Risk and Mortality. Br. J. Cancer 2016, 115, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gu, K.; Wang, F.; Cai, H.; Zheng, W.; Bao, P.; Shu, X.-O. Nut Consumption in Association with Overall Mortality and Recurrence/Disease-Specific Mortality among Long-Term Breast Cancer Survivors. Int. J. Cancer 2022, 150, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Fadelu, T.; Zhang, S.; Niedzwiecki, D.; Ye, X.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.B.; et al. Nut Consumption and Survival in Patients with Stage III Colon Cancer: Results from CALGB 89803 (Alliance). J. Clin. Oncol. 2018, 36, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, I.; Enderle, J.; Burmeister, G.; Koch, M.; Nöthlings, U.; Hampe, J.; Lieb, W. Post-Diagnostic Reliance on Plant-Compared with Animal-based Foods and All-Cause Mortality in Omnivorous Long-Term Colorectal Cancer Survivors. Am. J. Clin. Nutr. 2021, 114, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Eslamparast, T.; Sharafkhah, M.; Poustchi, H.; Hashemian, M.; Dawsey, S.M.; Freedman, N.D.; Boffetta, P.; Abnet, C.C.; Etemadi, A.; Pourshams, A.; et al. Nut consumption and total and cause-specific mortality: Results from the Golestan Cohort Study. Int. J. Epidemiol. 2017, 46, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Hshieh, T.T.; Petrone, A.B.; Gaziano, J.M.; Djoussé, L. Nut consumption and risk of mortality in the Physicians’ Health Study. Am. J. Clin. Nutr. 2015, 101, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Luu, H.N.; Blot, W.J.; Xiang, Y.B.; Cai, H.; Hargreaves, M.K.; Li, H.; Yang, G.; Signorello, L.; Gao, Y.T.; Zheng, W.; et al. Prospective evaluation of the association of nut/peanut consumption with total and cause-specific mortality. JAMA Intern. Med. 2015, 175, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Van den Brandt, P.A.; Schouten, L.J. Relationship of tree nut, peanut and peanut butter intake with total and cause-specific mortality: A cohort study and meta-analysis. Int. J. Epidemiol. 2015, 44, 1038–1049. [Google Scholar] [CrossRef]
- Bao, Y.; Han, J.; Hu, F.B.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S. Association of nut consumption with total and cause-specific mortality. N. Engl. J. Med. 2013, 369, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Bulló, M.; Martínez-González, M.Á.; Ros, E.; Corella, D.; Estruch, R.; Fitó, M.; Arós, F.; Wärnberg, J.; Fiol, M.; et al. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med. 2013, 11, 164. [Google Scholar] [CrossRef]
- Freisling, H.; Viallon, V.; Lennon, H.; Bagnardi, V.; Ricci, C.; Butterworth, A.S.; Sweeting, M.; Muller, D.; Romieu, I.; Bazelle, P.; et al. Lifestyle Factors and Risk of Multimorbidity of Cancer and Cardiometabolic Diseases: A Multinational Cohort Study. BMC Med. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Freisling, H.; Noh, H. Nut Consumption and Cancer. In Health Benefits of Nuts and Dried Fruits; Alasalvar, C., Salas-Salvado, J., Ros, E., Sabate, J., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–24, Chapter 9. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
| Study Type | Participants | Cancer Type | Outcome (95% CI) | Reference |
|---|---|---|---|---|
| Systematic review | n = 437,298 from 16 studies | Pancreatic, prostate, colorectal polyps | Dose-response trend from prospective studies | Mossine et al., 2020 [51] |
| Stomach, pancreatic, colorectal, nasopharyngeal, bladder | Total dried fruit, raisins, or dates reduced incidence from case–control studies | |||
| Cohort | UK Women’s Cohort Study (n = 35,372 women aged 35–69 in England, Wales, and Scotland) | Breast | HR 1.04 (0.98,1.13) | Dunneram et al., 2019 [60] |
| Endometrial | HR 0.60 (0.37, 0.97) | |||
| Ovarian | HR 1.06 (0.89, 1.26) | |||
| Prospective cohort | National Institutes of Health-American Association of Retired Persons Diet and Health Study (n = 485,403 men and women aged 50–71 at baseline in the United States) | Liver | HR (Q5 vs. Q1) 0.73 (0.60, 0.89) | Zhao et al., 2022 [61] |
| Mendelian randomization | UK Biobank (n ~500,000 men and women aged 49–69 in the United Kingdom) | Oral cavity/pharyngeal | IVW OR 0.17 (0.04, 0.69) | Jin et al., 2022 [62] |
| Lung | IVW OR 0.33 (0.17, 0.64) | |||
| Squamous cell lung | IVW OR 0.23 (0.09, 0.60) | |||
| Breast | IVW OR 0.47 (0.32, 0.68) | |||
| Pancreatic | IVW OR 0.03 (0.001, 0.68) | |||
| Cervical | IVW OR 0.99 (0.9897, 0.9998) | |||
| Lung adenocarcinoma, endometrial, thyroid, prostate, bladder, brain | IVW OR not significant |
| Author Year | Cancer Model | Putative Mechanism of Nuts Dietary Factor | Dietary Factor |
|---|---|---|---|
| Breast Cancer-Related Studies | |||
| Hardman and Ion 2008 [90] | Human breast cancer tumors in nude mice | Suppression of cell proliferation or suppression of metastasis | 18% of dietary calories from walnuts |
| Hardman et al., 2011 [75] | C(3)1 TAg transgenic mice, breast cancer | Alterations in cell signaling related to proliferation, differentiation, and apoptosis | Walnuts in the diet |
| Garcia et al., 2015 [76] | Implanted mammary gland adenocarcinoma in BALB/c mouse model | Inhibition of cyclooxygenase and lipoxygenase | 6% walnut oil or 6% walnut flour containing phytomelatonin |
| Chen et al., 2015 [77] | Breast cancer cells | Growth inhibition of breast cancer cells through cell cycle arrest and inhibition of proliferation | Ellagic acid that is abundant in walnuts |
| Colorectal Cancer-Related Studies | |||
| Hong et al., 2022 [78] | Colonic cell proliferation, apoptosis, and gene expression in rat model | Reduced DNA damage possibly via downregulation of RelA inflammation gene expression without changes to colonic cell proliferation and apoptosis | Mixed nuts in the diet |
| Chen et al., 2020 [79] | Mouse tumor bioassay after colonotropic carcinogen exposure | Favorably altering the gut microbiota | Walnuts in a Western diet |
| Nagel et al., 2012 [80] | HT-29 human colon cancer cells in nude mice | Inhibition of tumor growth rate through suppression of angiogenesis | Walnut and flaxseed oil |
| Nakanishi et al., 2016 [81] | Mice treated with organotropic colon carcinogen | Tumor suppression associated with alterations in gut bacteria | Dietary walnut of up to 15% of total caloric intake |
| Davis and Iwahashi 2001 [82] | Aberrant crypt foci (ACF) in rats treated with azoxymethane | ACF and cell turn over reduced | Whole almond-, almond meal- or almond oil-containing diet |
| Prostate Cancer-Related Studies | |||
| Davis et al., 2012 [85] | Transgenic adenocarcinoma of the mouse prostate (TRAMP) | Reduced TRAMP mouse prostate cancer growth and size; declines in plasma IGF-1, resistin, and LDL | Whole almonds as part of a high-fat diet |
| Kim et al., 2014 [86] | TRAMP | Reduced TRAMP mouse prostate cancer growth and size; improved insulin sensitivity and effects on cellular energy status, tumor suppression | Whole walnuts, walnut oil |
| Reiter et al., 2013 [87] | Implanted tumor model in nude mice | Reduced number and growth of LNCaP human prostate cancer cells; decreased oxidative stress | Standard mouse diet supplemented with walnuts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolling, B.W.; Aune, D.; Noh, H.; Petersen, K.S.; Freisling, H. Dried Fruits, Nuts, and Cancer Risk and Survival: A Review of the Evidence and Future Research Directions. Nutrients 2023, 15, 1443. https://doi.org/10.3390/nu15061443
Bolling BW, Aune D, Noh H, Petersen KS, Freisling H. Dried Fruits, Nuts, and Cancer Risk and Survival: A Review of the Evidence and Future Research Directions. Nutrients. 2023; 15(6):1443. https://doi.org/10.3390/nu15061443
Chicago/Turabian StyleBolling, Bradley W., Dagfinn Aune, Hwayoung Noh, Kristina S. Petersen, and Heinz Freisling. 2023. "Dried Fruits, Nuts, and Cancer Risk and Survival: A Review of the Evidence and Future Research Directions" Nutrients 15, no. 6: 1443. https://doi.org/10.3390/nu15061443
APA StyleBolling, B. W., Aune, D., Noh, H., Petersen, K. S., & Freisling, H. (2023). Dried Fruits, Nuts, and Cancer Risk and Survival: A Review of the Evidence and Future Research Directions. Nutrients, 15(6), 1443. https://doi.org/10.3390/nu15061443