Dried Fruits: Bioactives, Effects on Gut Microbiota, and Possible Health Benefits—An Update
Abstract
1. Introduction
2. Methodologies
3. Bioactives/Phytochemicals, Dietary Fibre, and Antioxidant Activity in Dried Fruits
| Phenolics | Carotenoids (μg/100 g) | Phytoestrogens (μg/100 g) | Dietary Fibre (g/100 g) | Antioxidant Activity (μmol of TE/100 g) a | |
|---|---|---|---|---|---|
| Apples | Flavan-3-ols Flavonols Phenolic acids Chalcones/dihydrochalcones | Lutein + zeaxanthin (18) | - | 8.7 | 6681 |
| Apricots | Flavan-3-ols Flavonols Flavones Phenolic acids Chalcones/dihydrochalcones | β-Carotene (2163) | Isoflavones (39.8) Lignans (401) Coumestan (4.2) | 7.3 | 3234 |
| Cranberries | Anthocyanins Flavan-3-ols Flavonols Phenolic acids Proanthocyanidins | β-Carotene (27) Lutein + zeaxanthin (138) | - | 5.3 | - |
| Dates | Anthocyanins Flavonols Phenolic acids Proanthocyanidins | β-Carotene (6) Lutein + zeaxanthin (75) | Isoflavones (5.1) Lignans (324) Coumestan (0.8) | 8.0 | 2387–3895 b |
| Figs | Anthocyanins Flavan-3-ols Flavonols Flavones Phenolic acids Proanthocyanidins | β-Carotene (6) Lutein + zeaxanthin (32) | - | 9.8 | 3383 |
| Peaches | Anthocyanins Flavan-3-ols Flavonols Phenolic acids | α-Carotene (3) β-Carotene (1074) β-Cryptoxanthin (444) Lutein + zeaxanthin (559) | - | 8.2 | 4222 |
| Pears | Flavan-3-ols Phenolic acids Chalcones/dihydrochalcones | β-Carotene (2) Lutein + zeaxanthin (50) | - | 7.5 | 9496 |
| Prunes | Flavan-3-ols Flavonols Phenolic acids | α-Carotene (57) β-Carotene (394) β-Cryptoxanthin (93) Lutein + zeaxanthin (148) | Isoflavones (4.2) Lignans (178) Coumestan (1.8) | 7.1 | 8578 |
| Raisins | Anthocyanins Flavan-3-ols Flavonols Flavones Phenolic acids Stilbenes | - | Isoflavones (8.1) Lignans (22) Coumestan (0.2) | 3.7 | 3037–10,450 c |
| References | [1,9] | [10] | [11] | [10] | [14] |
4. Bioaccessibility and Bioavailability of Compounds in Dried Fruits
5. Dried Fruits, Gut Health, and Microbiota
5.1. In Vivo Animal Studies
5.2. Human Clinical Trials
6. Epidemiological Evidence for Health Benefits of Dried Fruits
6.1. CVD
6.2. T2D
6.3. Body Weight
7. Clinical Trial Evidence for Dried Fruits and Health
7.1. Cardiometabolic Diseases
7.2. Bone Health
8. Dried Fruits and Diet Quality
9. Dietary Recommendations for Dried Fruit Consumption
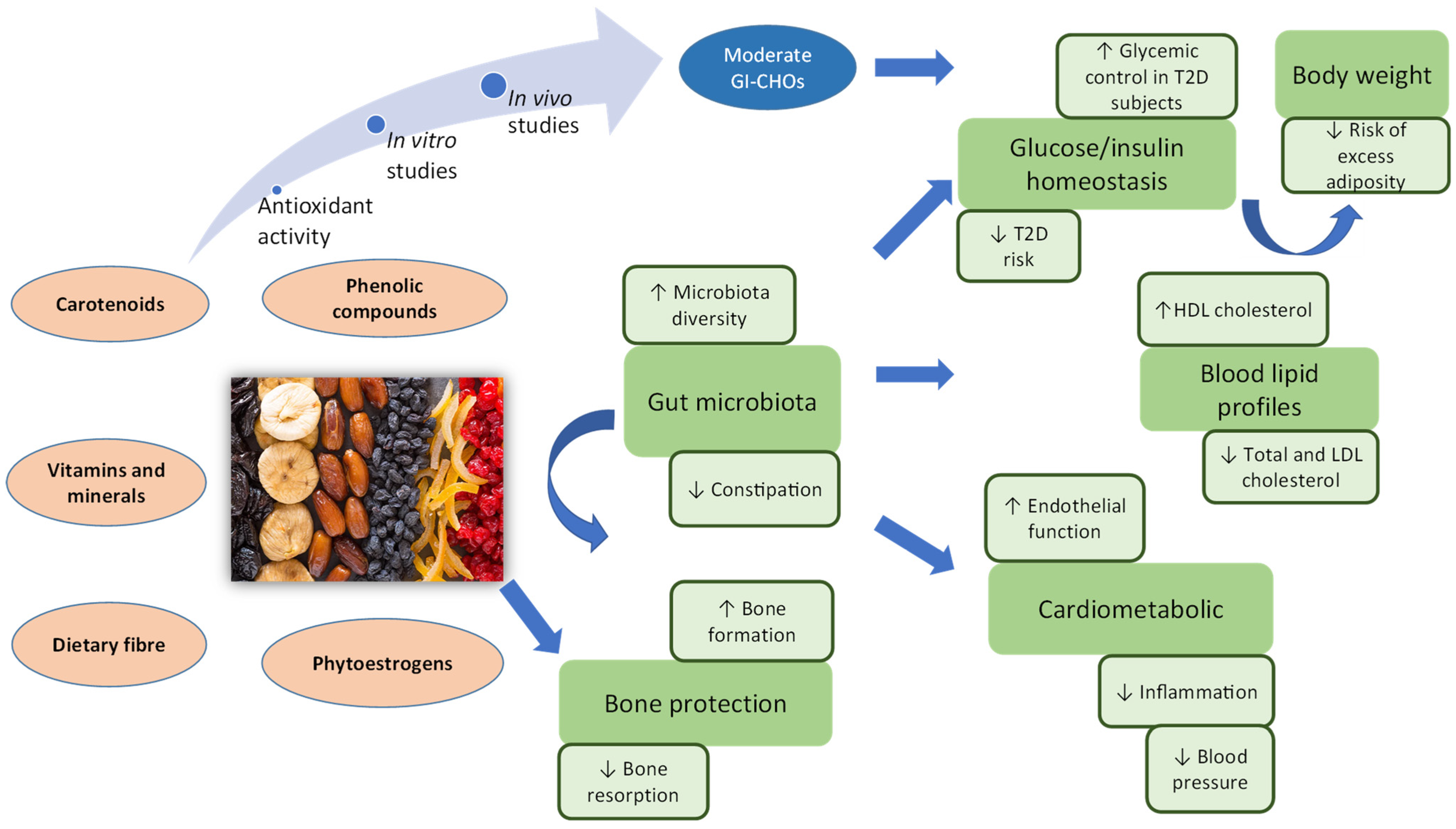
10. Potential Mechanisms Involved for Health Benefits of Dried Fruits
11. Limitation of Studies in Dried Fruits and Future Recommendations
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Review of Dried Fruits: Phytochemicals, Antioxidant Efficacies, and Health Benefits. J. Funct. Foods 2016, 21, 113–132. [Google Scholar] [CrossRef]
- Carughi, A.; Gallaher, D.; Mandalari, G. Bioavailability of Nutrients and Phytochemicals from Dried Fruits. In Health Benefits of Nuts and Dried Fruits; Alasalvar, C., Salas-Salvadó, J., Ros, E., Sabaté, J., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 369–396. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific Opinion on the Substantiation of a Health Claim Related to Prunes and Contribution to Normal Bowel Function Pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2014, 12, 3892. [Google Scholar] [CrossRef]
- Alasalvar, C.; Salas-Salvadó, J.; Ros, E.; Sabaté, J. Health Benefits of Nuts and Dried Fruits: An Overview. In Health Benefits of Nuts and Dried Fruits; Alasalvar, C., Salas-Salvadó, J., Ros, E., Sabaté, J., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 1–9. [Google Scholar]
- Arjmandi, B.H.; George, K.S. Bone Health and Osteoprotection. In Health Benefits of Nuts and Dried Fruits; Alasalvar, C., Salas-Salvadó, J., Ros, E., Sabaté, J., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 469–486. [Google Scholar]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition. Available online: https://www.dietaryguidelines.gov (accessed on 23 January 2023).
- Health Promotion Knowledge Gateway. Food-Based Dietary Guidelines in Europe. Available online: https://knowledge4policy.ec.europa.eu/health-promotion-knowledge-gateway/topic/food-based-dietary-guidelines-europe_en (accessed on 18 January 2023).
- Alasalvar, C.; Salas-Salvadó, J.; Ros, E. Bioactives and Health Benefits of Nuts and Dried Fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef]
- Alasalvar, C.; Chang, S.K.; Shahidi, F. Dried Fruits: Nutrients, Natural Antioxidants, and Phytochemicals. In Health Benefits of Nuts and Dried Fruits; Alasalvar, C., Salas-Salvadó, J., Ros, E., Sabaté, J., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 335–368. [Google Scholar]
- U.S. Department of Agriculture (USDA). National Nutrient Database for Standard Reference Legacy Release. 2018. Available online: https://ndb.nal.usda.gov/ndb/search/list (accessed on 1 March 2023).
- Thompson, L.U.; Boucher, B.A.; Liu, Z.; Cotterchio, M.; Kreiger, N. Phytoestrogen Content of Foods Consumption in Canada, Including Isoflavones, Lignans, and Coumestan. Nutr. Cancer 2006, 54, 184–201. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture (USDA). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005.
- Silva Caldas, A.P.; Bressan, J. Dried Fruits as Components of Health Dietary Patters. In Health Benefits of Nuts and Dried Fruits; Alasalvar, C., Salas-Salvadó, J., Ros, E., Sabaté, J., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 513–526. [Google Scholar]
- U.S. Department of Agriculture (USDA). Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2.0; U.S. Department of Agriculture: Beltsville, MD, USA, 2010.
- Ishiwata, K.; Yamaguchi, T.; Takamura, H.; Matoba, T. DPPH Radical-Scavenging Activity and Polyphenol Content in Dried Fruits. Food Sci. Technol. Res. 2004, 10, 152–156. [Google Scholar] [CrossRef]
- Vinson, J.A.; Zubik, L.; Bose, P.; Samman, N.; Proch, J. Dried Fruits: Excellent In Vitro and In Vivo antioxidants. J. Am. Coll. Nutr. 2005, 24, 44–50. [Google Scholar] [CrossRef]
- Rababah, T.M.; Ereifej, K.; Howard, L. Effect of Ascorbic Acid and Dehydration on Concentrations of Total Phenolics, Antioxidant Capacity, Anthocyanins, and Color in Fruits. J. Agric. Food Chem. 2005, 53, 4444–4447. [Google Scholar] [CrossRef]
- Threlfall, R.; Morris, J.; Meullenet, J.F. Product Development and Nutraceutical Analysis to Enhance the Value of Dried Fruit. J. Food Qual. 2007, 30, 552–566. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, Y. Review of In Vitro Digestion Models for Rapid Screening of Emulsion-based Systems. Food Funct. 2010, 1, 32–59. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Lan, T.; Geng, T.; Ju, Y.; Cheng, G.; Que, Z.; Gao, G.; Fang, Y.; Sun, X. Nutritional Properties and Biological Activities of Kiwifruit (Actinidia) and Kiwifruit Products under Simulated Gastrointestinal In Vitro Digestion. Food Nutr. Res. 2019, 63, 1674. [Google Scholar] [CrossRef]
- Scrob, T.; Hosu, A.; Cimpoiu, C. The Influence of In Vitro Gastrointestinal Digestion of Brassica Oleracea Florets on the Antioxidant Activity and Chlorophyll, Carotenoid and Phenolic Content. Antioxidants 2019, 8, 212. [Google Scholar] [CrossRef]
- Panagopoulou, E.A.; Chiou, A.; Kasimatis, T.-D.; Bismpikis, M.; Mouraka, P.; Karathanos, V.T. Dried Dates: Polar Phenols and Their Fate during in Vitro Digestion. J. Food Meas. Charact. 2021, 15, 1899–1906. [Google Scholar] [CrossRef]
- Schmite, B.d.F.P.; Bitobrovec, A.; Hacke, A.C.M.; Pereira, R.P.; Los Weinert, P.; Dos Anjos, V.E. In Vitro Bioaccessibility of Al, Cu, Cd, and Pb Following Simulated Gastro-Intestinal Digestion and Total Content of These Metals in Different Brazilian Brands of Yerba Mate Tea. Food Chem. 2019, 281, 285–293. [Google Scholar] [CrossRef]
- Scrob, T.; Covaci, E.; Hosu, A.; Tanaselia, C.; Casoni, D.; Torok, A.I.; Frentiu, T.; Cimpoiu, C. Effect of In Vitro Simulated Gastrointestinal Digestion on Some Nutritional Characteristics of Several Dried Fruits. Food Chem. 2022, 385, 132713. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Pasli, A.A.; Ozcelik, B.; Capanoglu, E. Evaluating the In Vitro Bioaccessibility of Phenolics and Antioxidant Activity during Consumption of Dried Fruits with Nuts. LWT-Food Sci. Technol. 2014, 56, 284–289. [Google Scholar] [CrossRef]
- Moles, L.; Otaegui, D. The Impact of Diet on Microbiota Evolution and Human Health. Is Diet an Adequate Tool for Microbiota Modulation? Nutrients 2020, 12, 1654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ju, Z.; Zuo, T. Time for Food: The Impact of Diet on Gut Microbiota and Human Health. Nutrition 2018, 51, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.M.; Lamuela-Raventós, R.M. Gut Health and Microbiota. In Health Benefits of Nuts and Dried Fruits; Alasalvar, C., Salas-Salvadó, J., Ros, E., Sabaté, J., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 487–495. [Google Scholar]
- Cremonesi, P.; Curone, G.; Biscarini, F.; Cotozzolo, E.; Menchetti, L.; Riva, F.; Marongiu, M.L.; Castiglioni, B.; Barbato, O.; Munga, A. Dietary Supplementation with Goji Berries (Lycium barbarum) Modulates the Microbiota of Digestive Tract and Caecal Metabolites in Rabbits. Animals 2022, 12, 121. [Google Scholar] [CrossRef]
- Tian, B.; Zhang, Z.; Zhao, J.; Ma, Q.; Liu, H.; Nie, C.; Ma, Z.; An, W.; Li, J. Dietary Whole Goji Berry (Lycium barbarum) Intake Improves Colonic Barrier Function by Altering Gut Microbiota Composition in Mice. Int. J. Food Sci. Technol. 2021, 56, 103–114. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, G.; Zhang, S.; Ross, C.F.; Zhu, M. Goji Berry Modulates Gut Microbiota and Alleviates Colitis in IL-10-deficient Mice. Mol. Nutr. Food Res. 2018, 62, 1800535. [Google Scholar] [CrossRef]
- Cai, X.; Han, Y.; Gu, M.; Song, M.; Wu, X.; Li, Z.; Li, F.; Goulette, T.; Xiao, H. Dietary Cranberry Suppressed Colonic Inflammation and Alleviated Gut Microbiota Dysbiosis in Dextran Sodium Sulfate-Treated Mice. Food Funct. 2019, 10, 6331–6341. [Google Scholar] [CrossRef]
- Wijayabahu, A.T.; Waugh, S.G.; Ukhanova, M.; Mai, V. Dietary Raisin Intake Has Limited Effect on Gut Microbiota Composition in Adult Volunteers. Nutr. J. 2019, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The Role of the Gut Microbiome in Systemic Inflammatory Disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morató, J.; Matthan, N.R.; Liu, J.; de la Torre, R.; Chen, C.-Y.O. Cranberries Attenuate Animal-Based Diet-Induced Changes in Microbiota Composition and Functionality: A Randomized Crossover Controlled Feeding Trial. J. Nutr. Biochem. 2018, 62, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Bekiares, N.; Krueger, C.G.; Meudt, J.J.; Shanmuganayagam, D.; Reed, J.D. Effect of Sweetened Dried Cranberry Consumption on Urinary Proteome and Fecal Microbiome in Healthy Human Subjects. Omi. J. Integr. Biol. 2017, 22, 145–153. [Google Scholar] [CrossRef]
- Lever, E.; Scott, S.M.; Louis, P.; Emery, P.W.; Whelan, K. The Effect of Prunes on Stool Output, Gut Transit Time and Gastrointestinal Microbiota: A Randomised Controlled Trial. Clin. Nutr. 2019, 38, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, M.A.; Dancz, C.E.; Dahl, M.; Volpe, K.A.; Horton, C.J.; Ozel, B.Z. Effect of Prunes on Gastrointestinal Function after Benign Gynecological Surgery: A Randomized Control Trial. Langenbeck’s Arch. Surg. 2022, 407, 3803–3810. [Google Scholar] [CrossRef] [PubMed]
- Eid, N.; Osmanova, H.; Natchez, C.; Walton, G.; Costabile, A.; Gibson, G.; Rowland, I.; Spencer, J.P.E. Impact of Palm Date Consumption on Microbiota Growth and Large Intestinal Health: A Randomised, Controlled, Cross-over, Human Intervention Study. Br. J. Nutr. 2015, 114, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of Dietary Polyphenols: The Role of Metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z. Health Effects of Dietary Risks in 195 Countries, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Centritto, F.; Iacoviello, L.; di Giuseppe, R.; De Curtis, A.; Costanzo, S.; Zito, F.; Grioni, S.; Sieri, S.; Donati, M.B.; de Gaetano, G. Dietary Patterns, Cardiovascular Risk Factors and C-Reactive Protein in a Healthy Italian Population. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Czekajlo, A.; Rozanska, D.; Zatonska, K.; Szuba, A.; Regulska-Ilow, B. Association between Dietary Patterns and Cardiovascular Risk Factors in a Selected Population of Lower Silesia (PURE Study Poland). Ann. Agric. Environ. Med. 2018, 25, 635–641. [Google Scholar] [CrossRef]
- Sullivan, V.K.; Petersen, K.S.; Kris-Etherton, P.M. Dried Fruit Consumption and Cardiometabolic Health: A Randomised Crossover Trial. Br. J. Nutr. 2020, 124, 912–921. [Google Scholar] [CrossRef]
- Borgi, L.; Muraki, I.; Satija, A.; Willett, W.C.; Rimm, E.B.; Forman, J.P. Fruit and Vegetable Consumption and the Incidence of Hypertension in Three Prospective Cohort Studies. Hypertension 2016, 67, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, J.M.; Manson, J.E.; Branch, L.G.; Colditz, G.A.; Willett, W.C.; Buring, J.E. A Prospective Study of Consumption of Carotenoids in Fruits and Vegetables and Decreased Cardiovascular Mortality in the Elderly. Ann. Epidemiol. 1995, 5, 255–260. [Google Scholar] [CrossRef]
- Lai, H.T.M.; Threapleton, D.E.; Day, A.J.; Williamson, G.; Cade, J.E.; Burley, V.J. Fruit Intake and Cardiovascular Disease Mortality in the UK Women’s Cohort Study. Eur. J. Epidemiol. 2015, 30, 1035–1048. [Google Scholar] [CrossRef]
- Muraki, I.; Imamura, F.; Manson, J.E.; Hu, F.B.; Willett, W.C.; van Dam, R.M.; Sun, Q. Fruit Consumption and Risk of Type 2 Diabetes: Results from Three Prospective Longitudinal Cohort Studies. BMJ 2013, 347, f5001. [Google Scholar] [CrossRef]
- Sullivan, V.K.; Na, M.; Proctor, D.N.; Kris-Etherton, P.M.; Petersen, K.S. Consumption of Dried Fruits Is Associated with Greater Intakes of Underconsumed Nutrients, Higher Total Energy Intakes, and Better Diet Quality in US Adults: A Cross-Sectional Analysis of the National Health and Nutrition Examination Survey, 2007–2016. J. Acad. Nutr. Diet. 2021, 121, 1258–1272. [Google Scholar] [CrossRef]
- Keast, D.R.; O’Neil, C.E.; Jones, J.M. Dried Fruit Consumption Is Associated with Improved Diet Quality and Reduced Obesity in US Adults: National Health and Nutrition Examination Survey, 1999–2004. Nutr. Res. 2011, 31, 460–467. [Google Scholar] [CrossRef]
- Fulgoni, V.L., III; Painter, J.; Carughi, A. Association of Raisin Consumption with Nutrient Intake, Diet Quality, and Health Risk Factors in US Adults: National Health and Nutrition Examination Survey 2001–2012. Food Nutr. Res. 2017, 61, 1378567. [Google Scholar] [CrossRef]
- Tinker, L.F.; Schneeman, B.O.; Davis, P.A.; Gallaher, D.D.; Waggoner, C.R. Consumption of Prunes as a Source of Dietary Fiber in Men with Mild Hypercholesterolemia. Am. J. Clin. Nutr. 1991, 53, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Clayton, Z.S.; Fusco, E.; Schreiber, L.; Carpenter, J.N.; Hooshmand, S.; Hong, M.Y.; Kern, M. Snack Selection Influences Glucose Metabolism, Antioxidant Capacity and Cholesterol in Healthy Overweight Adults: A Randomized Parallel Arm Trial. Nutr. Res. 2019, 65, 89–98. [Google Scholar] [CrossRef]
- Alalwan, T.A.; Perna, S.; Mandeel, Q.A.; Abdulhadi, A.; Alsayyad, A.S.; D’Antona, G.; Negro, M.; Riva, A.; Petrangolini, G.; Allegrini, P. Effects of Daily Low-Dose Date Consumption on Glycemic Control, Lipid Profile, and Quality of Life in Adults with Pre-and Type 2 Diabetes: A Randomized Controlled Trial. Nutrients 2020, 12, 217. [Google Scholar] [CrossRef]
- Shishehbor, F.; Joola, P.; Malehi, A.S.; Jalalifar, M.A. The Effect of Black Seed Raisin on Some Cardiovascular Risk Factors, Serum Malondialdehyde, and Total Antioxidant Capacity in Hyperlipidemic Patients: A Randomized Controlled Trials. Ir. J. Med. Sci. 2022, 191, 195–204. [Google Scholar] [CrossRef]
- Kanellos, P.T.; Kaliora, A.C.; Tentolouris, N.K.; Argiana, V.; Perrea, D.; Kalogeropoulos, N.; Kountouri, A.M.; Karathanos, V.T. A Pilot, Randomized Controlled Trial to Examine the Health Outcomes of Raisin Consumption in Patients with Diabetes. Nutrition 2014, 30, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Weiter, K.M.; Christian, A.L.; Ritchey, M.B.; Bays, H.E. Raisins Compared with Other Snack Effects on Glycemia and Blood Pressure: A Randomized, Controlled Trial. Postgr. Med. 2014, 126, 37–43. [Google Scholar] [CrossRef]
- Bays, H.; Weiter, K.; Anderson, J. A Randomized Study of Raisins versus Alternative Snacks on Glycemic Control and Other Cardiovascular Risk Factors in Patients with Type 2 Diabetes Mellitus. Phys. Sport. 2015, 43, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.M.; Montgomery, S.; Haddad, E.; Kearney, L.; Tonstad, S. Effect of Consumption of Dried California Mission Figs on Lipid Concentrations. Ann. Nutr. Metab. 2011, 58, 232–238. [Google Scholar] [CrossRef]
- Esfahani, A.; Lam, J.; Kendall, C.W.C. Acute Effects of Raisin Consumption on Glucose and Insulin Reponses in Healthy Individuals. J. Nutr. Sci. 2014, 3, E1. [Google Scholar] [CrossRef] [PubMed]
- Viguiliouk, E.; Jenkins, A.L.; Blanco Mejia, S.; Sievenpiper, J.L.; Kendall, C.W.C. Effect of Dried Fruit on Postprandial Glycemia: A Randomized Acute-Feeding Trial. Nutr. Diabetes 2018, 8, 59. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Dehghan, M.; Mente, A.; Bangdiwala, S.I.; Rangarajan, S.; Srichaikul, K.; Mohan, V.; Avezum, A.; Díaz, R.; Rosengren, A. Glycemic Index, Glycemic Load, and Cardiovascular Disease and Mortality. N. Engl. J. Med. 2021, 384, 1312–1322. [Google Scholar] [CrossRef]
- Damani, J.J.; De Souza, M.J.; VanEvery, H.L.; Strock, N.C.A.; Rogers, C.J. The Role of Prunes in Modulating Inflammatory Pathways to Improve Bone Health in Postmenopausal Women. Adv. Nutr. 2022, 13, 1476–1492. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C. Dried Plums, Prunes and Bone Health: A Comprehensive Review. Nutrients 2017, 9, 401. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Johnson, S.A.; Pourafshar, S.; Navaei, N.; George, K.S.; Hooshmand, S.; Chai, S.C.; Akhavan, N.S. Bone-Protective Effects of Dried Plum in Postmenopausal Women: Efficacy and Possible Mechanisms. Nutrients 2017, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Chai, S.C.; Saadat, R.L.; Payton, M.E.; Brummel-Smith, K.; Arjmandi, B.H. Comparative Effects of Dried Plum and Dried Apple on Bone in Postmenopausal Women. Br. J. Nutr. 2011, 106, 923–930. [Google Scholar] [CrossRef]
- Hooshmand, S.; Kern, M.; Metti, D.; Shamloufard, P.; Chai, S.C.; Johnson, S.A.; Payton, M.E.; Arjmandi, B.H. The Effect of Two Doses of Dried Plum on Bone Density and Bone Biomarkers in Osteopenic Postmenopausal Women: A Randomized, Controlled Trial. Osteoporos. Int. 2016, 27, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.Y.; Kern, M.; Nakamichi-Lee, M.; Abbaspour, N.; Ahouraei Far, A.; Hooshmand, S. Dried Plum Consumption Improves Total Cholesterol and Antioxidant Capacity and Reduces Inflammation in Healthy Postmenopausal Women. J. Med. Food 2021, 24, 1161–1168. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Khalil, D.A.; Lucas, E.A.; Georgis, A.; Stoecker, B.J.; Hardin, C.; Payton, M.E.; Wild, R.A. Dried Plums Improve Indices of Bone Formation in Postmenopausal Women. J. Women’s Health Gend. Based Med. 2002, 11, 61–68. [Google Scholar] [CrossRef]
- De Souza, M.J.; Strock, N.C.A.; Williams, N.I.; Lee, H.; Koltun, K.J.; Rogers, C.; Ferruzzi, M.G.; Nakatsu, C.H.; Weaver, C. Prunes Preserve Hip Bone Mineral Density in a 12-Month Randomized Controlled Trial in Postmenopausal Women: The Prune Study. Am. J. Clin. Nutr. 2022, 116, 897–910. [Google Scholar] [CrossRef]
- George, K.S.; Munoz, J.; Ormsbee, L.T.; Akhavan, N.S.; Foley, E.M.; Siebert, S.C.; Kim, J.-S.; Hickner, R.C.; Arjmandi, B.H. The Short-Term Effect of Prunes in Improving Bone in Men. Nutrients 2022, 14, 276. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Gaffen, D.; Eisner, A.; Fajardo, J.; Payton, M.; Kern, M. Effects of 12 Months Consumption of 100 g Dried Plum (Prunes) on Bone Biomarkers, Density, and Strength in Men. J. Med. Food 2022, 25, 40–47. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 19 January 2023).
- International Nut and Dried Fruit Council. INC Nuts & Dried Fruits Statistical Yearbook 2021/2022. Available online: https://inc.nutfruit.org/inc-releases-2021-2022-statistical-yearbook/ (accessed on 16 January 2023).
- Yang, Q.; Liu, T.; Kuklina, E.V.; Flanders, W.D.; Hong, Y.; Gillespie, C.; Chang, M.-H.; Gwinn, M.; Dowling, N.; Khoury, M.J.; et al. Sodium and Potassium Intake and Mortality among US Adults: Prospective Data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2011, 171, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Srichaikul, K.; Ong, M.; Prasla, Z.; Kohen, Y.; Mandalozano, I.; Paquette, M.; Sahye-Pudaruth, S.; Patel, D.; Kendal, C.W.; Sievenpiper, J.L.; et al. Dried Fruits in the Prevention and Control of Diabetes (Insulin Resistance and Prediabetics); Alasalvar, C., Salas-Salvadó, J., Ros, E., Sabaté, J., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 436–447. [Google Scholar]
- Sullivan, V.; Petersen, K.; Kris-Etherton, P. Dried Fruits and Cardio-Metabolic Syndrome (Endothelial Function, Inflammation, and Blood Pressure). In Health Benefits of Nuts and Dried Fruits; Alasalvar, C., Salas-Salvadó, J., Ros, E., Sabaté, J., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 413–436. [Google Scholar]

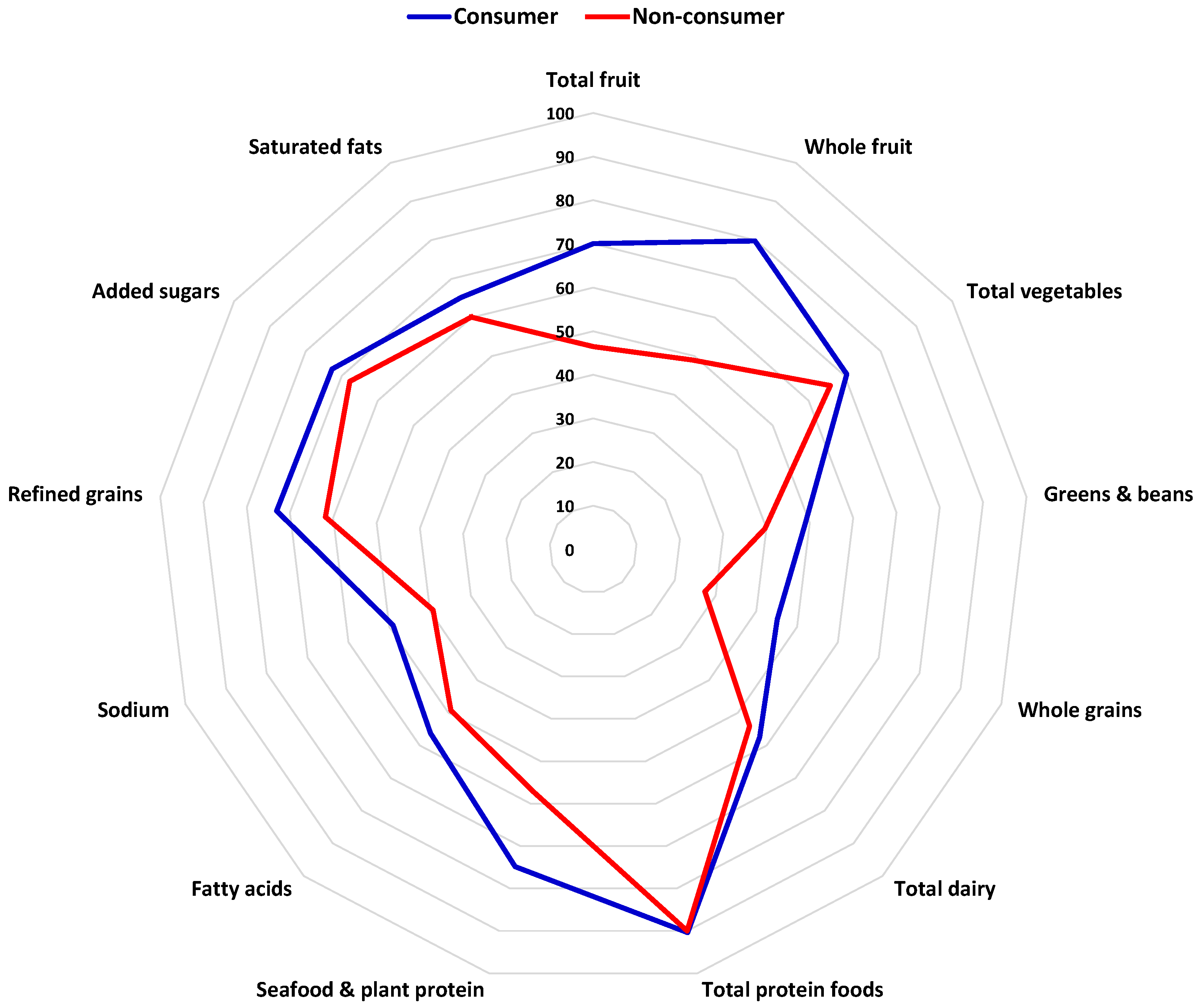
| References | Study Design | Duration (Week) | Participants (n) | Fruit (Dose) | Comparator | Findings |
|---|---|---|---|---|---|---|
| Sullivan et al. [45] | Crossover | 4 | Men and women with BMI 25–36 kg/m2 and ≥1 additional cardiometabolic risk factor, n = 55 | Equal parts (~28 g each) dried plums, Mission figs, Deglet Noor dates, and raisins totalling ¾ cups/day | Energy-matched processed snacks (animal crackers and fruit snack gummies) | Dried fruits increased LDL-C (0.10 mmol/L) and non-HDL-C (0.12 mmol/L) and reduced HDL-C (−0.05 mmol/L) compared to baseline. Dried fruits increased fasting glucose compared to control (0.08 mmol/L). No between-group or within-group differences in total cholesterol, TAG, blood pressure, or insulin. |
| Tinker et al. [53] | Crossover | 4 | Men with elevated total cholesterol (5.2–7.5 mmol/L), n = 41 | Dried plums, ~100 g/day (12 plums) | 360 mL grape juice | Dried plums reduced LDL-C compared to grape juice (−0.17 mmol/L). No difference in total cholesterol, HDL-C, or TAG. |
| Clayton et al. [54] | Parallel | 8 | Men and women with BMI ≥ 25 kg/m2, n = 45 | Dried plums, ~84 g/day | Energy-matched portion (200 kcal) of low-fat muffins | Dried plums reduced LDL-C compared to low-fat muffins (−24.5 mg/dL). Dried plums increased C-peptide compared to baseline (+1.56 ng/mL). No between-group or within-group differences in total cholesterol, HDL-C, blood pressure, TAG, insulin, or glucose. |
| Alalwan et al. [55] | Parallel | 16 | Men and women with T2D, n = 96 | Dates (Khudary cultivar, tamar stage), 3 dates/day | Usual diet | Dates reduced total cholesterol compared to baseline (−0.209 mmol/L). No between-group or within-group differences in HbA1c, TAG, HDL-C, or LDL-C. |
| Shishehbor et al. [56] | Parallel | 5 | Men and women with elevated total cholesterol (>200 mg/dL) or TAG (>200 mg/dL), n = 38 | Raisins, 90 g/day | Usual diet | Raisins reduced DBP compared to control group (−1.56 mm Hg). Raisins reduced LDL-C (−0.68 mmol/L) and total cholesterol (−0.72 mmol/L) compared to baseline. No between-group or within-group differences in SBP, HDL-C, or TAG. |
| Kanellos et al. [57] | Parallel | 24 | Men and postmenopausal women with T2D, n = 48 | Corinthian raisins, 36 g/day | Usual diet | Raisins reduced DBP compared to the control group (−6 mm Hg). No between-group or within-group differences in SBP, total cholesterol, LDL-C, HDL-C, TAG, fasting glucose, or HbA1c. |
| Anderson et al. [58] | Parallel | 12 | Men and women with BMI 25–34.9 kg/m2, blood pressure > 120/80 mm Hg, and fasting glucose 90–150 mg/dL, n = 46 | Raisins, 3 ounces/day | Energy-matched pre-packaged processed snacks (three 100 kcal packages) | Raisins reduced SBP (−5.4 mmHg vs. baseline; −6.3 mmHg vs. snacks), DBP (−5.5 mmHg vs. baseline; −3.6 mmHg vs. snacks), HDL-C (−3.6 mg/dL vs. baseline), and HbA1c (−0.12% vs. baseline; −0.08% vs. snacks). No between-group differences in total cholesterol, LDL-C, TAG, or fasting glucose. |
| Bays et al. [59] | Parallel | 12 | Men and women with T2D and BMI 25–50 kg/m2, n = 46 | Raisins, 3 ounces/day | Energy-matched pre-packaged processed snacks (three 100 kcal packages) | Raisins reduced SBP compared to snacks (−8.7 mm Hg). No between-group differences in fasting glucose, HbA1c, DBP, total cholesterol, LDL-C, HDL-C, or TAG. |
| Peterson et al. [60] | Crossover | 5 (per arm) | Men and women with LDL-C 100–189 mg/dL and BMI 18.5–35 kg/m2, n = 102 | Dried California Mission figs (~120 g/day, 12–15 figs) | Usual diet | Figs increased total cholesterol compared to control (6 mg/dL). No difference in LDL-C, HDL-C, or TAG. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alasalvar, C.; Chang, S.K.; Kris-Etherton, P.M.; Sullivan, V.K.; Petersen, K.S.; Guasch-Ferré, M.; Jenkins, D.J.A. Dried Fruits: Bioactives, Effects on Gut Microbiota, and Possible Health Benefits—An Update. Nutrients 2023, 15, 1611. https://doi.org/10.3390/nu15071611
Alasalvar C, Chang SK, Kris-Etherton PM, Sullivan VK, Petersen KS, Guasch-Ferré M, Jenkins DJA. Dried Fruits: Bioactives, Effects on Gut Microbiota, and Possible Health Benefits—An Update. Nutrients. 2023; 15(7):1611. https://doi.org/10.3390/nu15071611
Chicago/Turabian StyleAlasalvar, Cesarettin, Sui Kiat Chang, Penny M. Kris-Etherton, Valerie K. Sullivan, Kristina S. Petersen, Marta Guasch-Ferré, and David J. A. Jenkins. 2023. "Dried Fruits: Bioactives, Effects on Gut Microbiota, and Possible Health Benefits—An Update" Nutrients 15, no. 7: 1611. https://doi.org/10.3390/nu15071611
APA StyleAlasalvar, C., Chang, S. K., Kris-Etherton, P. M., Sullivan, V. K., Petersen, K. S., Guasch-Ferré, M., & Jenkins, D. J. A. (2023). Dried Fruits: Bioactives, Effects on Gut Microbiota, and Possible Health Benefits—An Update. Nutrients, 15(7), 1611. https://doi.org/10.3390/nu15071611






