Natural Product Skatole Ameliorates Lipotoxicity-Induced Multiple Hepatic Damage under Hyperlipidemic Conditions in Hepatocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture, Preparation of Compounds, and Treatment
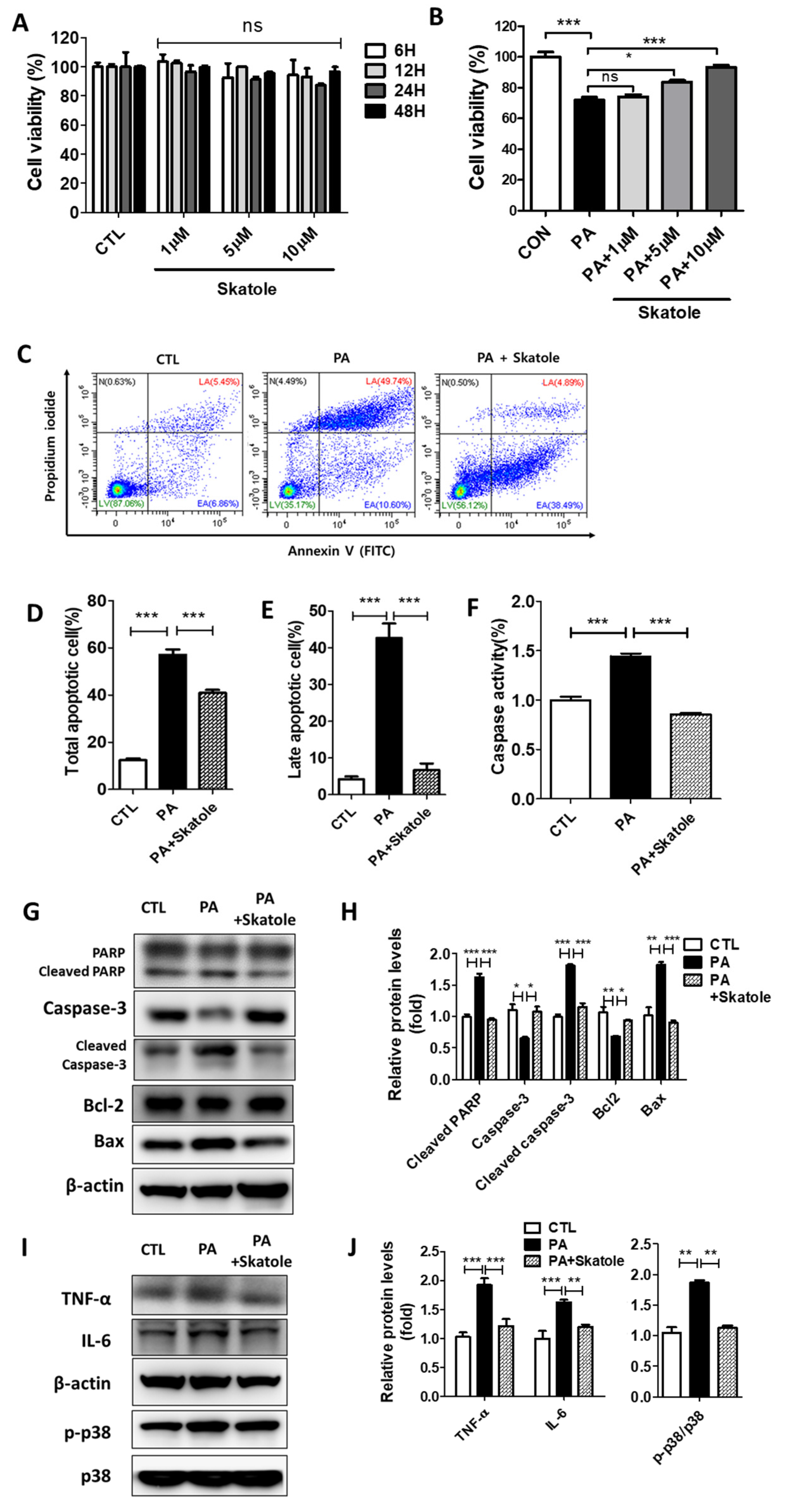
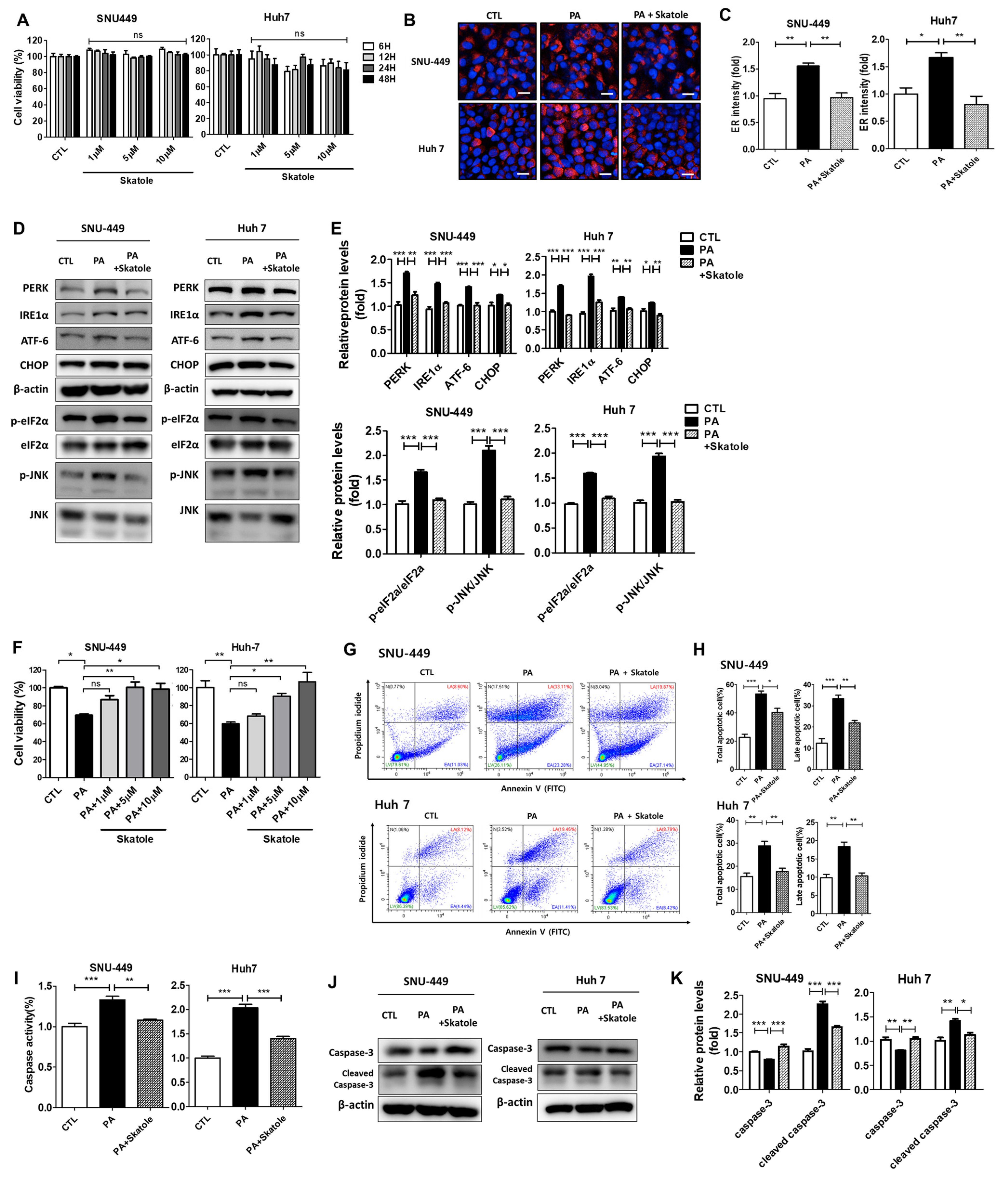
2.2. Cell Viability Assay
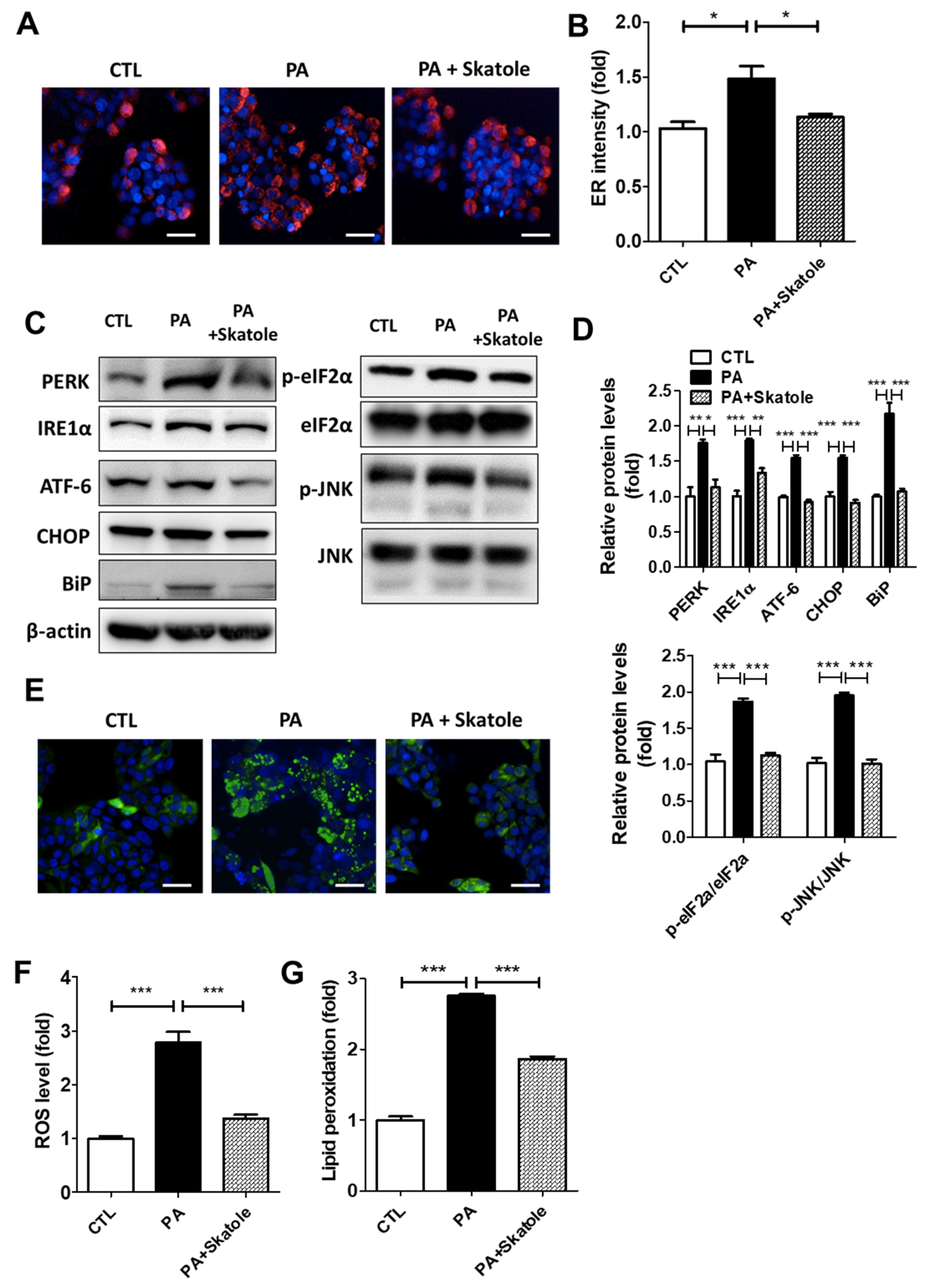
2.3. Endoplasmic Reticulum Staining
2.4. Reactive Oxygen Species (ROS) Production
2.5. Western Blotting
2.6. Apoptosis Assay
2.7. Caspase Activity
2.8. Nile Red Staining
2.9. Lipid Peroxidation Assay
2.10. Glucose Uptake
2.11. Statistical Analysis
3. Results
3.1. Skatole Regulates Lipid and Glucose Metabolism in HepG2 Cells with PA Treatmnet
3.2. Skatole Suppressed PA-Induced ER Stress and ROS Production in HepG2 Cells
3.3. Skatole Attenuates PA-Induced Lipotoxicity by Reducing Apoptosis, Caspase Activity, and Inflammation in HepG2 Cells
3.4. Skatole Is Non-Cytotoxic and Skatole Treatment Reduces ER Stress, Apoptosis, and Caspase Activity in SNU-449 and Huh7 Cells
3.5. Skatole Treatment Reduces Lipid Accumulation and ROS Production and Enhances Glucose Uptake in SNU-449 and Huh7 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, A.E.; Werneburg, N.W.; Canbay, A.; Guicciardi, M.E.; Bronk, S.F.; Rydzewski, R.; Burgart, L.J.; Gores, G.J. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology 2004, 40, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Marusic, M.; Paic, M.; Knobloch, M.; Liberati Prso, A.M. NAFLD, insulin resistance, and diabetes mellitus type 2. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6613827. [Google Scholar] [CrossRef]
- Arroyave-Ospina, J.C.; Wu, Z.M.; Geng, Y.N.; Moshage, H. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: Implications for prevention and therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef]
- Mota, M.; Banini, B.A.; Cazanave, S.C.; Sanyal, A.J. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1049–1061. [Google Scholar] [CrossRef] [Green Version]
- Unger, R.H. Minireview: Weapons of lean body mass destruction: The role of ectopic lipids in the metabolic syndrome. Endocrinology 2003, 144, 5159–5165. [Google Scholar] [CrossRef] [Green Version]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [Green Version]
- Rutkowski, D.T. Liver function and dysfunction—A unique window into the physiological reach of ER stress and the unfolded protein response. Febs J. 2019, 286, 356–378. [Google Scholar] [CrossRef] [Green Version]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell. Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef]
- Akazawa, Y.; Cazanave, S.; Mott, J.L.; Elmi, N.; Bronk, S.F.; Kohno, S.; Charlton, M.R.; Gores, G.J. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J. Hepatol. 2010, 52, 586–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.C.; Kim, S.I.; Hong, Y.; Hwang, J.W.; Cho, G.S.; Cha, H.N.; Han, J.K.; Yun, C.H.; Park, S.Y.; Jang, I.S.; et al. Inhibition of CYP4A reduces hepatic endoplasmic reticulum stress and features of diabetes in mice. Gastroenterology 2014, 147, 860–869. [Google Scholar] [CrossRef]
- Malhi, H.; Gores, G.J. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Sem. Liver Disease 2008, 28, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.H.; Kohli, R.; Gores, G.J. Mechanisms of lipotoxicity in NAFLD and clinical implications. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 131–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rada, P.; Gonzalez-Rodriguez, A.; Garcia-Monzon, C.; Valverde, A.M. Understanding lipotoxicity in NAFLD pathogenesis: Is CD36 a key driver? Cell. Death Disease 2020, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.Q.; Zheng, R.Y.; Li, J.; Hu, L.; Chang, Y.X.; Li, L.; Li, M.H.; Wang, R.Y.; Huang, D.D.; Wu, M.C.; et al. Palmitic acid induces autophagy in hepatocytes via JNK2 activation. Acta Pharmacol. Sin. 2014, 35, 504–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanninger, J.; Neumeier, M.; Hellerbrand, C.; Schacherer, D.; Bauer, S.; Weiss, T.S.; Huber, H.; Schaffler, A.; Aslanidis, C.; Scholmerich, J.; et al. Lipid accumulation impairs adiponectin-mediated induction of activin A by increasing TGFbeta in primary human hepatocytes. Biochim. Biophys. Acta 2011, 1811, 626–633. [Google Scholar] [CrossRef]
- Zhang, H.A.; Yang, X.Y.; Xiao, Y.F. AMPK alpha 1 overexpression alleviates the hepatocyte model of nonalcoholic fatty liver disease via inactivating p38MAPK pathway. Biochem. Biophys. Res. Commun. 2016, 474, 364–370. [Google Scholar] [CrossRef] [Green Version]
- Boden, G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr. Diab Rep. 2006, 6, 177–181. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, S.; Yang, C.; Du, R.; Zhao, J.; Li, J.; Xu, Y.; Qin, Y.; Gao, Y.; Huang, W. Ginsenoside Rg1 ameliorates palmitic acid-induced hepatic steatosis and inflammation in HepG2 cells via the AMPK/NF-kappaB pathway. Int. J. Endocrinol. 2019, 2019, 7514802. [Google Scholar] [CrossRef] [Green Version]
- Trauner, M.; Arrese, M.; Wagner, M. Fatty liver and lipotoxicity. Biochim. Biophys. Acta 2010, 1801, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.; Qiu, S.; Zhao, Y.; Zhao, S.; Sun, D.; Hou, L.; Li, Y.; Zhou, K.; Yu, X.; Yang, C.; et al. Pathogenesis and therapeutic strategies related to non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2022, 23, 7841. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi-Barve, S.; Barve, S.S.; Amancherla, K.; Gobejishvili, L.; Hill, D.; Cave, M.; Hote, P.; McClain, C.J. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology 2007, 46, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Cansancao, K.; Silva Monteiro, L.; Carvalho Leite, N.; Davalos, A.; Tavares do Carmo, M.D.G.; Arantes Ferreira Peres, W. Advanced Liver Fibrosis Is Independently Associated with Palmitic Acid and Insulin Levels in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2018, 10, 1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissar, A.U.; Sharma, L.; Tasduq, S.A. Palmitic acid induced lipotoxicity is associated with altered lipid metabolism, enhanced CYP450 2E1 and intracellular calcium mediated ER stress in human hepatoma cells. Toxicol. Res. 2015, 4, 1344–1358. [Google Scholar] [CrossRef]
- Ogawa, Y.; Imajo, K.; Honda, Y.; Kessoku, T.; Tomeno, W.; Kato, S.; Fujita, K.; Yoneda, M.; Saito, S.; Saigusa, Y.; et al. Palmitate-induced lipotoxicity is crucial for the pathogenesis of nonalcoholic fatty liver disease in cooperation with gut-derived endotoxin. Sci. Rep. 2018, 8, 11365. [Google Scholar] [CrossRef] [Green Version]
- Ricchi, M.; Odoardi, M.R.; Carulli, L.; Anzivino, C.; Ballestri, S.; Pinetti, A.; Fantoni, L.I.; Marra, F.; Bertolotti, M.; Banni, S.; et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J. Gastroenterol. Hepatol. 2009, 24, 830–840. [Google Scholar] [CrossRef]
- Furia, T.E.; Bellanca, N. Fenaroli’s Handbook of Flavor Ingredients; CRC Press, Inc.: Boca Raton, FL, USA, 1975; Volume 2. [Google Scholar]
- Kunapuli, S.P.; Vaidyanathan, C. Indolic compounds in the leaves of Tecoma stans. Phytochemistry 1984, 23, 1826–1827. [Google Scholar] [CrossRef]
- Arctander, S. Perfume & Flavor Chemicals (Aroma Chemicals); Lulu.com: Morrisville, NC, USA, 2019; Volume II. [Google Scholar]
- Mishiro, Y.; Kirimura, K.; Koyama, T. Chromatographic separation of ether-extractable components of aerobically incubated human whole saliva. J. Dent. Res. 1969, 48, 601. [Google Scholar] [CrossRef]
- Yokoyama, M.T.; Carlson, J.R. Microbial metabolites of tryptophan in the intestinal tract with special reference to skatole. Am. J. Clin. Nutr. 1979, 32, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Zgarbová, E.; Vrzal, R. Skatole: A thin red line between its benefits and toxicity. Biochimie 2022, 208, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Karlin, D.A.; Mastromarino, A.J.; Jones, R.D.; Stroehlein, J.R.; Lorentz, O. Fecal skatole and indole and breath methane and hydrogen in patients with large bowel polyps or cancer. J. Cancer Res. Clin. Oncol. 1985, 109, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Prospero, L.; Riezzo, G.; Linsalata, M.; Orlando, A.; D’Attoma, B.; Di Masi, M.; Martulli, M.; Russo, F. Somatization in patients with predominant diarrhoea irritable bowel syndrome: The role of the intestinal barrier function and integrity. BMC Gastroenterol. 2021, 21, 235. [Google Scholar] [CrossRef]
- Nakao, A.; Ball, M. The appearance of a skatole derivative in the urine of schizophrenics. J. Nerv. Ment. Dis. 1960, 130, 417–419. [Google Scholar] [CrossRef]
- Deslandes, B.T.; Gariépy, C.; Houde, A. Review of microbiological and biochemical effects of skatole on animal production. Livest. Prod. Sci. 2001, 71, 193–200. [Google Scholar] [CrossRef]
- Regal, K.A.; Laws, G.M.; Yuan, C.; Yost, G.S.; Skiles, G.L. Detection and characterization of DNA adducts of 3-methylindole. Chem. Res. Toxicol. 2001, 14, 1014–1024. [Google Scholar] [CrossRef]
- Adams, J.D., Jr.; Heins, M.C.; Yost, G.S. 3-Methylindole inhibits lipid peroxidation. Biochem. Biophys. Res. Commun. 1987, 149, 73–78. [Google Scholar] [CrossRef]
- Carlson, J.R.; Dickinson, E.O.; Yokoyama, M.T.; Bradley, B. Pulmonary edema and emphysema in cattle after intraruminal and intravenous administration of 3-methylindole. Am. J. Vet. Res. 1975, 36, 1341–1347. [Google Scholar]
- Bradley, B.J.; Carlson, J.R.; Dickinson, E.O. 3-methylindole-induced pulmonary edema and emphysema in sheep. Am. J. Vet. Res. 1978, 39, 1355–1358. [Google Scholar]
- Hong, Y.; Lee, M.; Kim, C.; Kim, G.-H. Dehydrocostus lactone ameliorates lipid accumulation, insulin resistance, and endoplasmic reticulum stress in palmitate-treated hepatocytes. J. Anal. Sci. Technol. 2022, 13, 38. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, X.; Su, C.; Zhao, L.; Shi, Y. Chitosan nanoparticles induced the antitumor effect in hepatocellular carcinoma cells by regulating ROS-mediated mitochondrial damage and endoplasmic reticulum stress. Artif. Cells Nanomed. Biotechnol. 2019, 47, 747–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Hu, Y.Y.; Tang, M.; Kong, L.; Ying, J.L.; Wu, T.S.; Xue, Y.Y.; Pu, Y.P. Liver toxicity of cadmium telluride quantum dots (CdTe QDs) due to oxidative stress in vitro and in vivo. Int. J. Mol. Sci. 2015, 16, 23279–23299. [Google Scholar] [CrossRef] [Green Version]
- Hur, W.; Kim, S.W.; Lee, Y.K.; Choi, J.E.; Hong, S.W.; Song, M.J.; Bae, S.H.; Park, T.; Um, S.J.; Yoon, S.K. Oleuropein reduces free fatty acid-induced lipogenesis via lowered extracellular signal-regulated kinase activation in hepatocytes. Nutr. Res. 2012, 32, 778–786. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, L.; Wang, P.; Mu, Y.M.; Zhu, X.Y.; Wu, Y.Y.; Yu, H.; Zhang, B.; Chen, S.M.; Sun, X.Z. Saturated free fatty acid, palmitic acid, induces apoptosis in fetal hepatocytes in culture. Exp. Toxicol. Pathol. 2005, 56, 369–376. [Google Scholar] [CrossRef]
- Sullivan, K.E.; Kumar, S.; Liu, X.; Zhang, Y.; de Koning, E.; Li, Y.; Yuan, J.; Fan, F. Uncovering the roles of dihydropyrimidine dehydrogenase in fatty-acid induced steatosis using human cellular models. Sci. Rep. 2022, 12, 14109. [Google Scholar] [CrossRef]
- Takahara, I.; Akazawa, Y.; Tabuchi, M.; Matsuda, K.; Miyaaki, H.; Kido, Y.; Kanda, Y.; Taura, N.; Ohnita, K.; Takeshima, F.; et al. Toyocamycin attenuates free fatty acid-induced hepatic steatosis and apoptosis in cultured hepatocytes and ameliorates nonalcoholic fatty liver disease in mice. PLoS ONE 2017, 12, e0170591. [Google Scholar] [CrossRef] [Green Version]
- Stepankova, M.; Bartonkova, I.; Jiskrova, E.; Vrzal, R.; Mani, S.; Kortagere, S.; Dvorak, Z. Methylindoles and Methoxyindoles are Agonists and Antagonists of Human Aryl Hydrocarbon Receptor. Mol. Pharmacol. 2018, 93, 631–644. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochrome p450 and chemical toxicology. Chem. Res. Toxicol. 2008, 21, 70–83. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Balaguer, P.; Ekstrand, B.; Daujat-Chavanieu, M.; Gerbal-Chaloin, S. Skatole (3-methylindole) is a partial aryl hydrocarbon receptor agonist and induces CYP1A1/2 and CYP1B1 expression in primary human hepatocytes. PLoS ONE 2016, 11, e0154629. [Google Scholar] [CrossRef] [Green Version]
- Vyhlidalova, B.; Poulikova, K.; Bartonkova, I.; Krasulova, K.; Vanco, J.; Travnicek, Z.; Mani, S.; Dvorak, Z. Mono-methylindoles induce CYP1A genes and inhibit CYP1A1 enzyme activity in human hepatocytes and HepaRG cells. Toxicol. Lett. 2019, 313, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Weems, J.M.; Cutler, N.S.; Moore, C.; Nichols, W.K.; Martin, D.; Makin, E.; Lamb, J.G.; Yost, G.S. 3-Methylindole is mutagenic and a possible pulmonary carcinogen. Toxicol. Sci. 2009, 112, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, B.A.; Wan, J.; Hines, R.N.; Yost, G.S. Characterization of the human lung CYP2F1 gene and identification of a novel lung-specific binding motif. J. Biol. Chem. 2003, 278, 15473–15483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, T.M.; Emmerson, K.S. Putative mechanisms of toxicity of 3-methylindole: From free radical to pneumotoxicosis. Annu. Rev. Pharmacol. Toxicol. 1994, 34, 91–115. [Google Scholar] [CrossRef] [PubMed]
- Kurata, K.; Kawahara, H.; Nishimura, K.; Jisaka, M.; Yokota, K.; Shimizu, H. Skatole regulates intestinal epithelial cellular functions through activating aryl hydrocarbon receptors and p38. Biochem. Biophys. Res. Commun. 2019, 510, 649–655. [Google Scholar] [CrossRef]
- Vyhlidalova, B.; Krasulova, K.; Pecinkova, P.; Marcalikova, A.; Vrzal, R.; Zemankova, L.; Vanco, J.; Travnicek, Z.; Vondracek, J.; Karasova, M.; et al. Gut microbial catabolites of tryptophan are ligands and agonists of the aryl hydrocarbon receptor: A detailed characterization. Int. J. Mol. Sci. 2020, 21, 2614. [Google Scholar] [CrossRef] [Green Version]
- Lawson, W.B.; Patchornik, A.; Witkop, B. Substitution, oxidation and group participation in the bromination of indoles. J. Am. Chem. Soc. 1960, 82, 5918–5923. [Google Scholar] [CrossRef]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Wegener, G.; Lund, S. The microbial metabolite indole-3-propionic acid improves glucose metabolism in rats, but does not affect behaviour. Arch. Physiol. Biochem. 2018, 124, 306–312. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, X.; Zhang, H.; Shi, R.; Hui, Y.; Jin, X.; Zhang, W.; Wang, L.; Wang, Q.; Wang, D.; et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 2020, 11, 855. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Jiang, M.; Zhao, J.; Song, Y.; Du, W.; Shi, J. The mechanism underlying the influence of indole-3-propionic acid: A relevance to metabolic disorders. Front. Endocrinol. (Lausanne) 2022, 13, 841703. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, R.; de Mello, V.D.; Mannisto, V.; Lindstrom, J.; Tuomilehto, J.; Pihlajamaki, J.; Uusitupa, M. Indolepropionic acid, a gut bacteria-produced tryptophan metabolite and the risk of type 2 diabetes and non-alcoholic fatty liver disease. Nutrients 2022, 14, 4695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Xin, F.Z.; Xue, Y.; Hu, Z.; Han, Y.; Ma, F.; Zhou, D.; Liu, X.L.; Cui, A.; Liu, Z.; et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Fiore, A.; Murray, P.J. Tryptophan and indole metabolism in immune regulation. Curr. Opin. Immunol. 2021, 70, 7–14. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clement, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Delgado, I.; Anesi, A.; Dexpert, S.; Aubert, A.; Beau, C.; Forestier, D.; Ledaguenel, P.; Magne, E.; Mattivi, F.; et al. Tryptophan metabolic pathways are altered in obesity and are associated with systemic inflammation. Front. Immunol. 2020, 11, 557. [Google Scholar] [CrossRef] [Green Version]
- Wesoly, R.; Weiler, U. Nutritional influences on skatole formation and skatole metabolism in the pig. Animals 2012, 2, 221–242. [Google Scholar] [CrossRef]
- Oh, Y.S.; Bae, G.D.; Baek, D.J.; Park, E.Y.; Jun, H.S. Fatty Acid-Induced Lipotoxicity in Pancreatic Beta-Cells During Development of Type 2 Diabetes. Front. Endocrinol. (Lausanne) 2018, 9, 384. [Google Scholar] [CrossRef]
- Nishi, H.; Higashihara, T.; Inagi, R. Lipotoxicity in Kidney, Heart, and Skeletal Muscle Dysfunction. Nutrients 2019, 11, 1664. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Deng, Z.T.; Huang, S.; Ning, M.; Feng, Y.; Shen, Y.; Zhao, Q.S.; Leng, Y. Alisol B alleviates hepatocyte lipid accumulation and lipotoxicity via regulating RARalpha-PPARgamma-CD36 cascade and attenuates non-alcoholic steatohepatitis in mice. Nutrients 2022, 14, 2411. [Google Scholar] [CrossRef]
- Opazo-Rios, L.; Mas, S.; Marin-Royo, G.; Mezzano, S.; Gomez-Guerrero, C.; Moreno, J.A.; Egido, J. Lipotoxicity and diabetic nephropathy: Novel mechanistic insights and therapeutic opportunities. Int. J. Mol. Sci. 2020, 21, 2632. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.-H.; Hong, Y.; Lee, M.; Keum, B.-R.; Kim, G.-H. Natural Product Skatole Ameliorates Lipotoxicity-Induced Multiple Hepatic Damage under Hyperlipidemic Conditions in Hepatocytes. Nutrients 2023, 15, 1490. https://doi.org/10.3390/nu15061490
Hong S-H, Hong Y, Lee M, Keum B-R, Kim G-H. Natural Product Skatole Ameliorates Lipotoxicity-Induced Multiple Hepatic Damage under Hyperlipidemic Conditions in Hepatocytes. Nutrients. 2023; 15(6):1490. https://doi.org/10.3390/nu15061490
Chicago/Turabian StyleHong, Sin-Hyoung, Yeonhee Hong, Minji Lee, Byeong-Rak Keum, and Gun-Hwa Kim. 2023. "Natural Product Skatole Ameliorates Lipotoxicity-Induced Multiple Hepatic Damage under Hyperlipidemic Conditions in Hepatocytes" Nutrients 15, no. 6: 1490. https://doi.org/10.3390/nu15061490




