HnRNPA2B1 Aggravates Inflammation by Promoting M1 Macrophage Polarization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Mice Experiments
2.3. Disease Activity Index (DAI) Scores Parameters
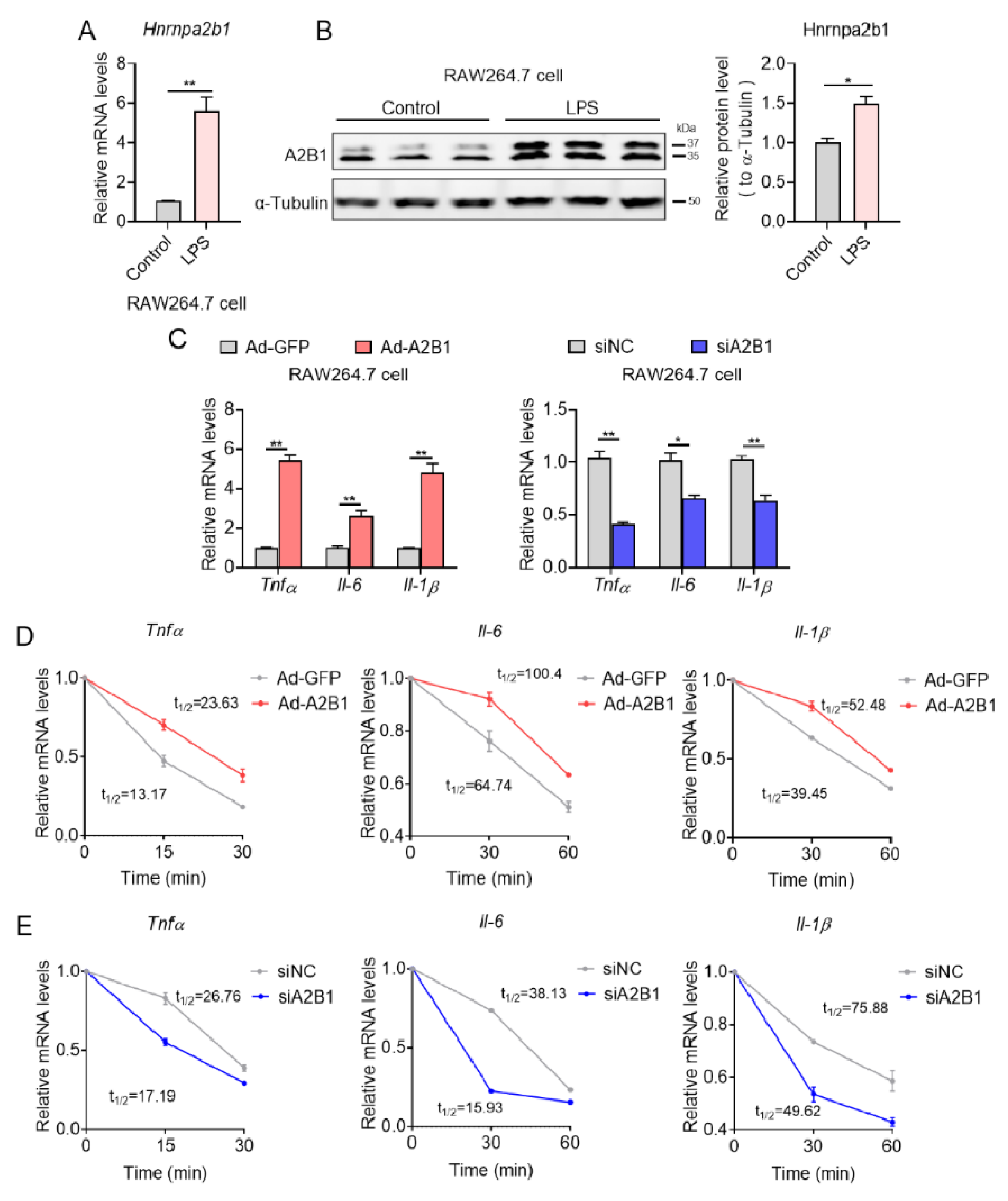
2.4. RNA Extraction, Quantitative PCR
2.5. Western Blotting Analysis
2.6. Cell Isolation and FACS Analysis
2.7. Insulin and Glucose Tolerance Test (ITT and GTT)
2.8. Serum Parameters Analysis
2.9. Histological Analysis and Histopathological Score
2.10. mRNA Stability Assay
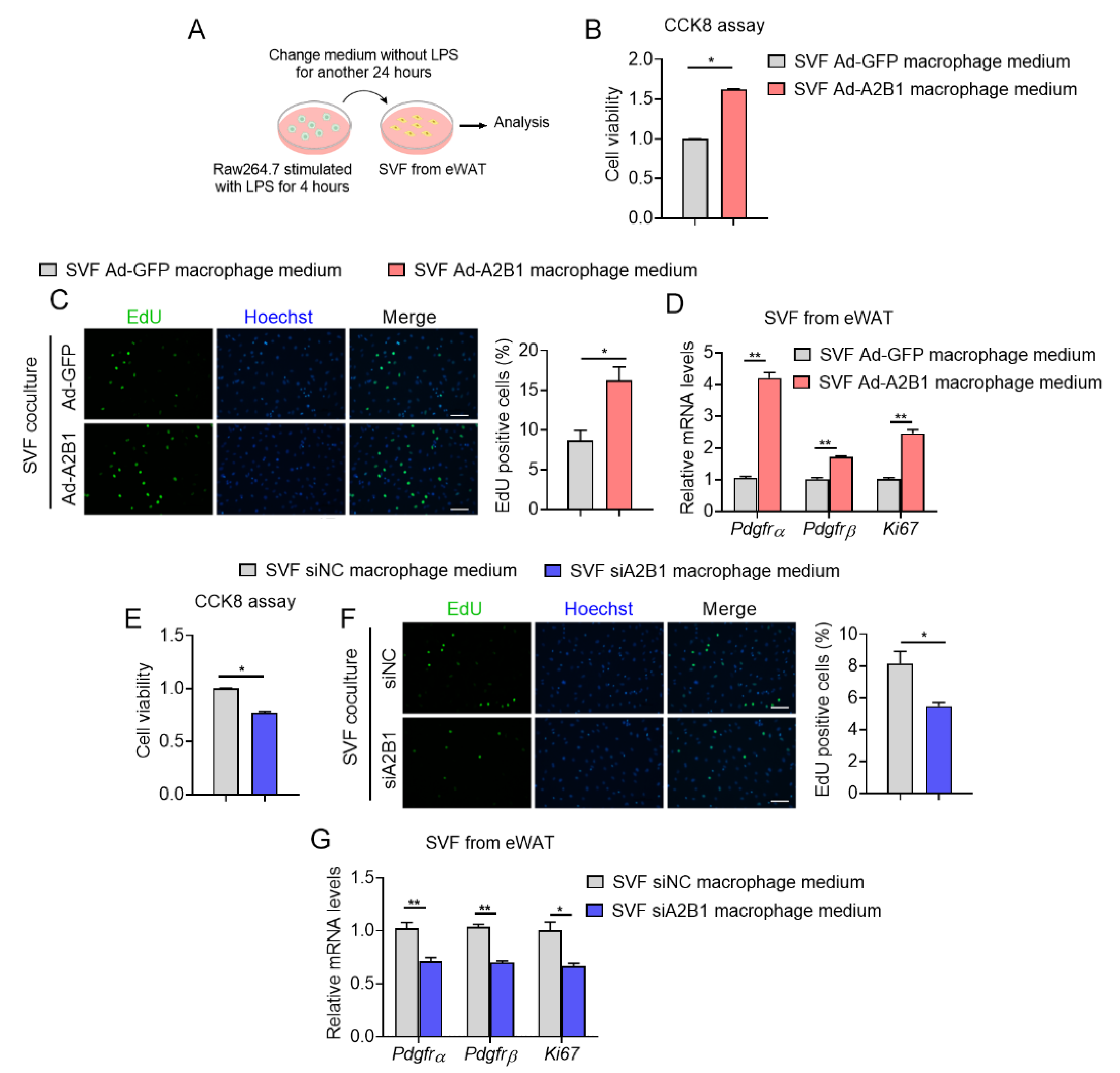
2.11. CCK-8 Assay
2.12. EdU Staining
2.13. Co-Culture Analysis
2.14. Statistical Analysis
3. Results
3.1. Phenotypic Analysis of hnRNPA2B1 Heterozygous Mice
3.2. hnRNPA2B1 Heterozygous Mice Were Protected from DSS-Induced Acute Colitis
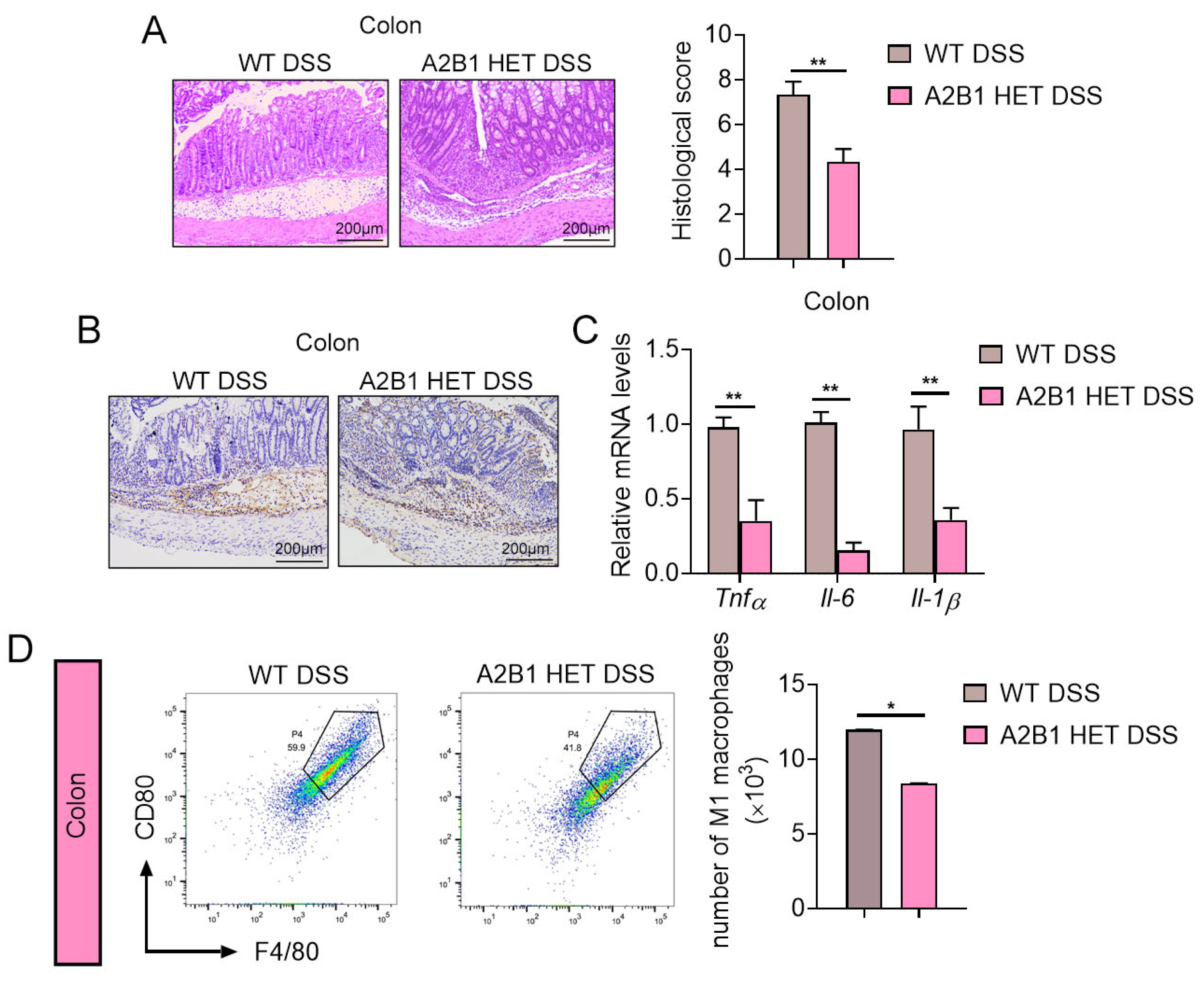
3.3. hnRNPA2B1 Heterozygous Mice Had Attenuated Colon Damage and Inflammation
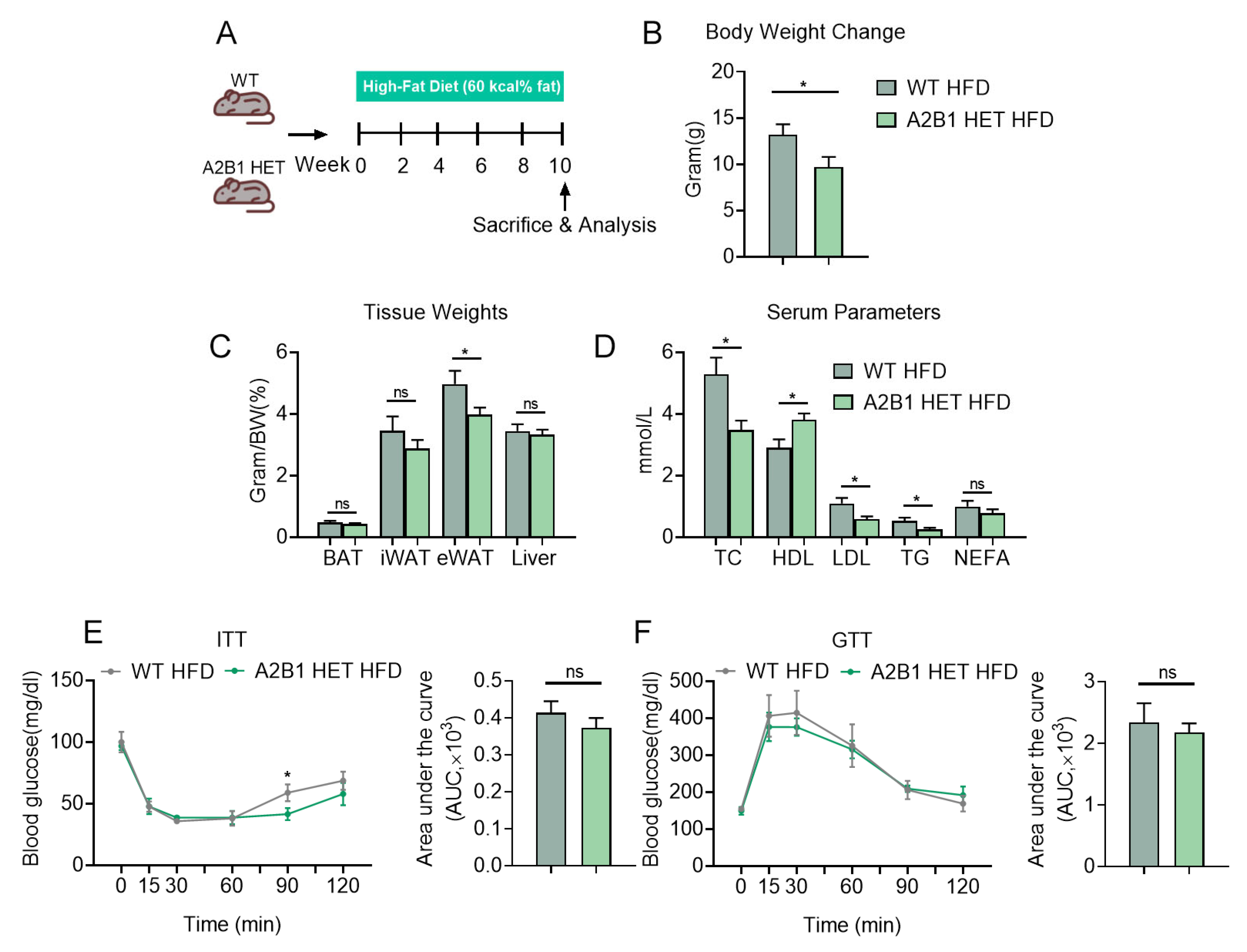
3.4. hnRNPA2B1 Heterozygous Mice Were Protected from HFD-Induced Obesity and Hyperlipidemia
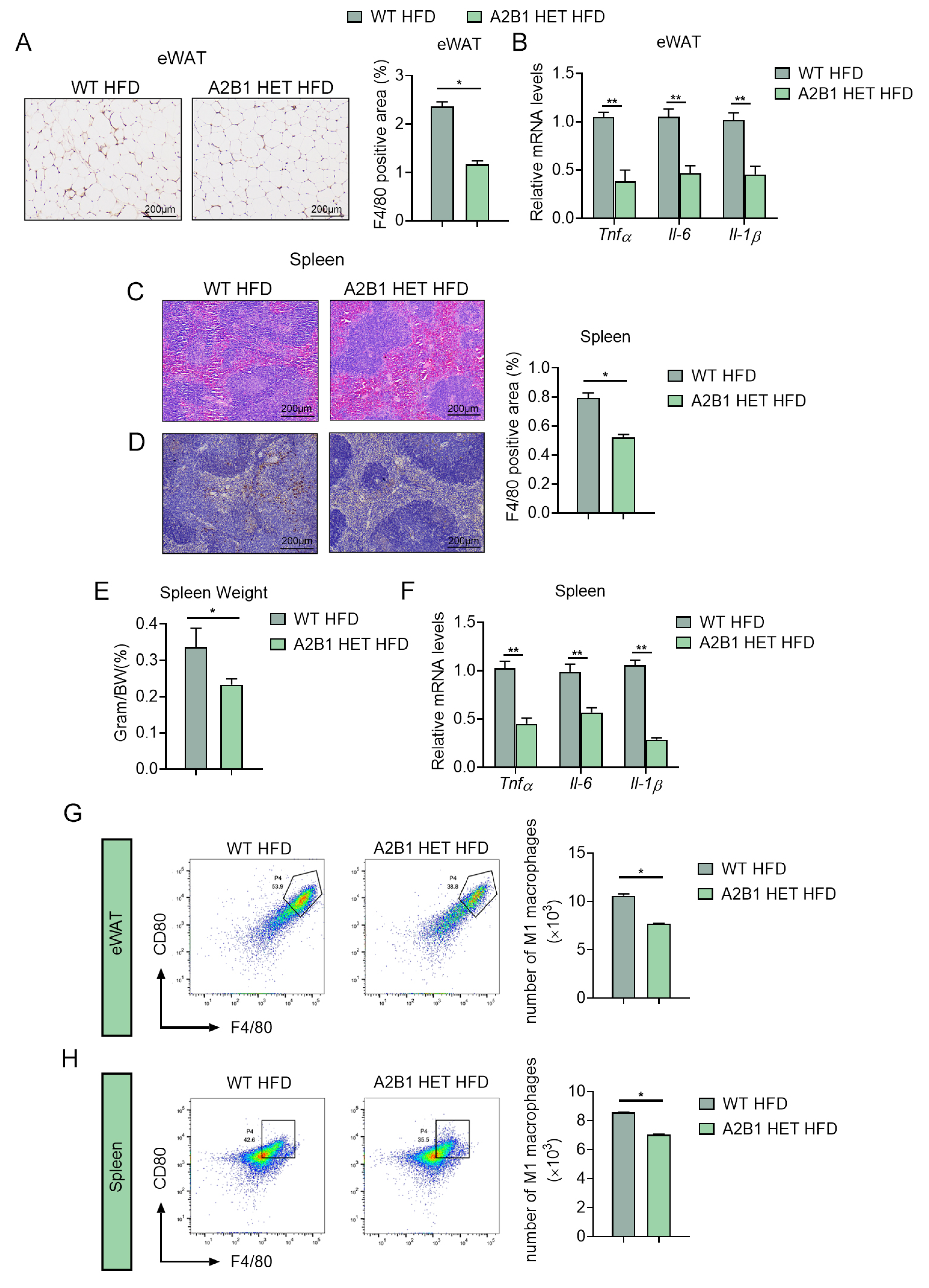
3.5. hnRNPA2B1 Heterozygous Mice Were Resistant to Diet-Induced Inflammation and M1 Macrophage Polarization
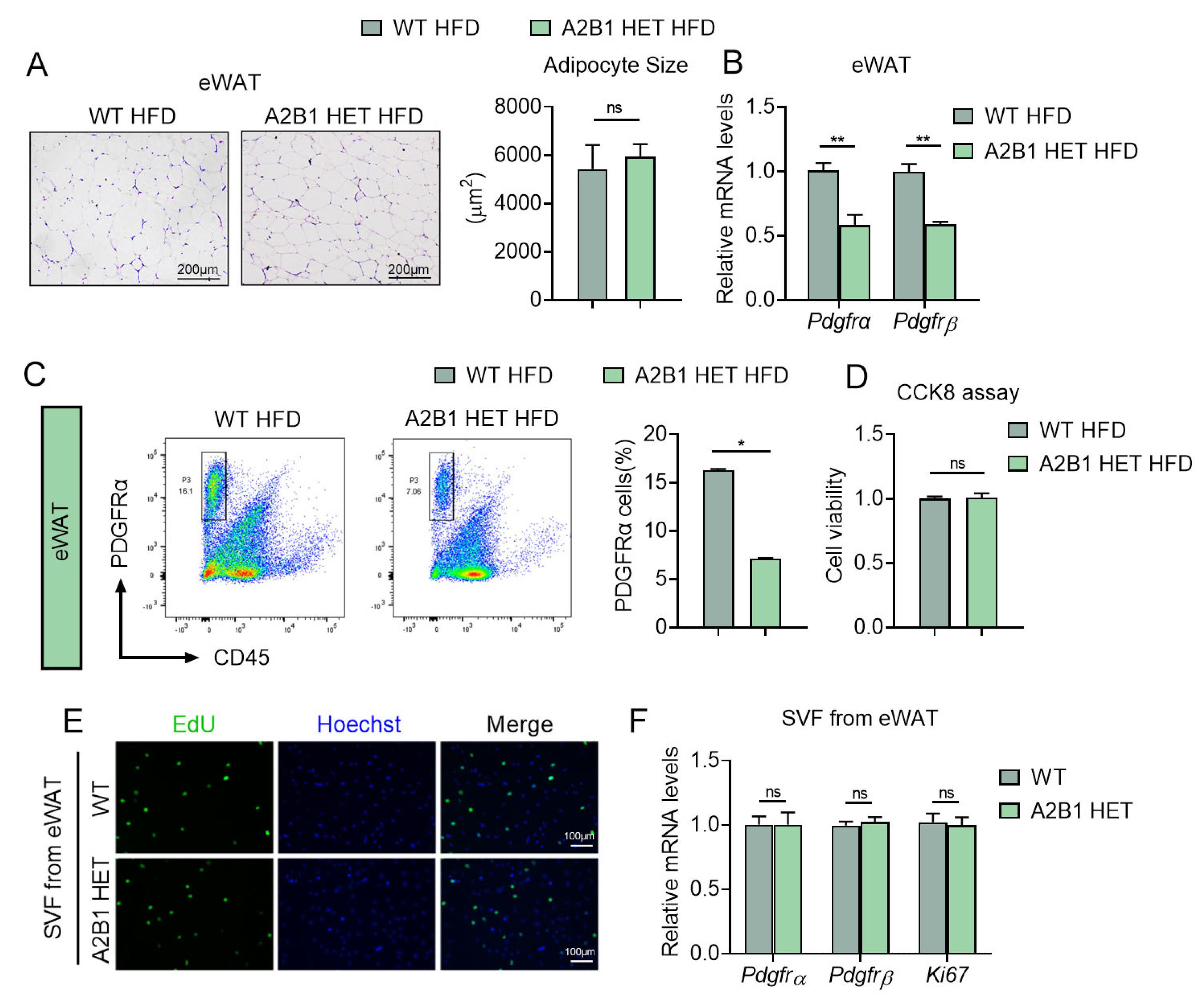
3.6. Modulation of hnRNPA2B1 in Macrophages Affects Preadipocyte Proliferation
3.7. hnRNPA2B1 Controls Proinflammatory Gene mRNA Stability in Macrophages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christ, A.; Lauterbach, M.; Latz, E. Western diet and the immune system: An inflammatory connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Turpin, W.; Goethel, A.; Bedrani, L.; Croitoru Mdcm, K. Determinants of IBD heritability: Genes, bugs, and more. Inflamm. Bowel Dis. 2018, 24, 1133–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.; Yang, Y.; Sun, S.; Dai, Z.; Ren, F.; Wu, Z. Insights into diet-associated oxidative pathomechanisms in inflammatory bowel disease and protective effects of functional amino acids. Nutr. Rev. 2022, 81, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Brestoff, J.R.; Artis, D. Immune regulation of metabolic homeostasis in health and disease. Cell 2015, 161, 146–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and tissue specialization. Annu. Rev. Immunol. 2015, 33, 643–675. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef] [Green Version]
- Geuens, T.; Bouhy, D.; Timmerman, V. The hnRNP family: Insights into their role in health and disease. Hum. Genet. 2016, 135, 851–867. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Cao, X. Nuclear innate sensors for nucleic acids in immunity and inflammation. Immunol. Rev. 2020, 297, 162–173. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, S.L. The roles of hnRNP A2/B1 in RNA biology and disease. Wiley Interdiscip. Rev. RNA 2021, 12, e1612. [Google Scholar] [CrossRef]
- Wang, L.; Wen, M.; Cao, X. Nuclear hnRNPA2B1 initiates and amplifies the innate immune response to DNA viruses. Science 2019, 365, eaav0758. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Ping, X.; Zhang, Y.; Zhang, T.; Wang, L.; Jin, L.; Zhao, W.; Guo, M.; Shen, F.; et al. Local hyperthermia therapy induces browning of white fat and treats obesity. Cell 2022, 185, 949–966.e919. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Zhang, Y.; Zhang, T.; Wang, D.; Ma, X. Heat shock factor 1 in fat biology: Comments on ‘Local hyperthermia therapy induces browning of white fat and treats obesity’. J. Mol. Cell Biol. 2022, 14, mjac026. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Finkel, T.; Ma, X. Local hyperthermia therapy: A novel strategy turning white fat brown. Life Med. 2022, 1, 78–80. [Google Scholar] [CrossRef]
- Campbell, E.L.; MacManus, C.F.; Kominsky, D.J.; Keely, S.; Glover, L.E.; Bowers, B.E.; Scully, M.; Bruyninckx, W.J.; Colgan, S.P. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc. Natl. Acad. Sci. USA 2010, 107, 14298–14303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastorelli, L.; Dozio, E.; Pisani, L.F.; Boscolo-Anzoletti, M.; Vianello, E.; Munizio, N.; Spina, L.; Tontini, G.E.; Peyvandi, F.; Corsi Romanelli, M.M.; et al. Procoagulatory state in inflammatory bowel diseases is promoted by impaired intestinal barrier function. Gastroenterol. Res. Pract. 2015, 2015, 189341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, K.; Yi, J.; Yang, Y.; Deng, M.; Yang, Y.; Wang, T.; Chen, X.; Zhang, Z.; Wang, X. A broad m6A modification landscape in inflammatory bowel disease. Front. Cell Dev. Biol. 2021, 9, 782636. [Google Scholar] [CrossRef]
- Zhou, H.; Xiao, J.; Wu, N.; Liu, C.; Xu, J.; Liu, F.; Wu, L. MicroRNA-223 Regulates the Differentiation and Function of Intestinal Dendritic Cells and Macrophages by Targeting C/EBPβ. Cell Rep. 2015, 13, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Yu, J.; Shi, Q.; Xiao, Y.; Wang, W.; Chen, G.; Zhao, Z.; Wang, R.; Xiao, H.; Hou, C.; et al. Tim-3 promotes intestinal homeostasis in DSS colitis by inhibiting M1 polarization of macrophages. Clin. Immunol. 2015, 160, 328–335. [Google Scholar] [CrossRef]
- Lei, Q.; Gu, H.; Li, L.; Wu, T.; Xie, W.; Li, M.; Zhao, N. TNIP1-mediated TNF-α/NF-κB signalling cascade sustains glioma cell proliferation. J. Cell. Mol. Med. 2020, 24, 530–538. [Google Scholar] [CrossRef] [Green Version]
- Ho, A.T.V.; Palla, A.R.; Blake, M.R.; Yucel, N.D.; Wang, Y.X.; Magnusson, K.E.G.; Holbrook, C.A.; Kraft, P.E.; Delp, S.L.; Blau, H.M. Prostaglandin E2 is essential for efficacious skeletal muscle stem-cell function, augmenting regeneration and strength. Proc. Natl. Acad. Sci. USA 2017, 114, 6675–6684. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Zhang, J.; Chen, L.; Liu, T.; Xu, G.; Li, C.; Yuan, W.; Xu, H.; Su, Z. IL-17 contributed to the neuropathic pain following peripheral nerve injury by promoting astrocyte proliferation and secretion of proinflammatory cytokines. Mol. Med. Rep. 2017, 15, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.Y.; Zhang, J.; Zhu, J.S. The role of m(6)A RNA methylation in human cancer. Mol. Cancer 2019, 18, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comalada, M.; Peppelenbosch, M.P. Impaired innate immunity in Crohn’s disease. Trends Mol. Med. 2006, 12, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mapperley, C.; van de Lagemaat, L.N.; Lawson, H.; Tavosanis, A.; Paris, J.; Campos, J.; Wotherspoon, D.; Durko, J.; Sarapuu, A.; Choe, J.; et al. The mRNA m6A reader YTHDF2 suppresses proinflammatory pathways and sustains hematopoietic stem cell function. J. Exp. Med. 2021, 218, e20200829. [Google Scholar] [CrossRef]
- Bechara, R.; Amatya, N.; Bailey, R.D.; Li, Y.; Aggor, F.E.Y.; Li, D.D.; Jawale, C.V.; Coleman, B.M.; Dai, N.; Gokhale, N.S.; et al. The m(6)A reader IMP2 directs autoimmune inflammation through an IL-17- and TNFα-dependent C/EBP transcription factor axis. Sci. Immunol. 2021, 6, eabd1287. [Google Scholar] [CrossRef]
- Hodson, R. Inflammatory bowel disease. Nature 2016, 540, S97. [Google Scholar] [CrossRef] [Green Version]
- Steinbach, E.C.; Plevy, S.E. The role of macrophages and dendritic cells in the initiation of inflammation in IBD. Inflamm. Bowel Dis. 2014, 20, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Bain, C.C.; Scott, C.L.; Uronen-Hansson, H.; Gudjonsson, S.; Jansson, O.; Grip, O.; Guilliams, M.; Malissen, B.; Agace, W.W.; Mowat, A.M. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013, 6, 498–510. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef] [Green Version]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Wang, C.; Li, Y.; Chen, J.; Yu, L.; Liu, X.; Li, J.; Cao, J.; Deng, Y.; Guo, D.; et al. Incidence of type 2 diabetes and number of events attributable to abdominal obesity in China: A cohort study. J. Diabetes 2016, 8, 190–198. [Google Scholar] [CrossRef]
- Chen, M.; Lu, P.; Ma, Q.; Cao, Y.; Chen, N.; Li, W.; Zhao, S.; Chen, B.; Shi, J.; Sun, Y.; et al. CTNNB1/β-catenin dysfunction contributes to adiposity by regulating the cross-talk of mature adipocytes and preadipocytes. Sci. Adv. 2020, 6, eaax9605. [Google Scholar] [CrossRef] [Green Version]
- Molgat, A.S.; Gagnon, A.; Sorisky, A. Preadipocyte apoptosis is prevented by macrophage-conditioned medium in a PDGF-dependent manner. Am. J. Physiol. Cell Physiol. 2009, 296, C757–C765. [Google Scholar] [CrossRef] [Green Version]
- Molgat, A.S.; Gagnon, A.; Sorisky, A. Macrophage-induced preadipocyte survival depends on signaling through Akt, ERK1/2, and reactive oxygen species. Exp. Cell Res. 2011, 317, 521–530. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, M.; Cao, Y.; Zhang, Y.; Liu, S.; Zhong, Y.; Wang, D.; Li, D.; Xu, L.; Ma, X. HnRNPA2B1 Aggravates Inflammation by Promoting M1 Macrophage Polarization. Nutrients 2023, 15, 1555. https://doi.org/10.3390/nu15071555
Meng M, Cao Y, Zhang Y, Liu S, Zhong Y, Wang D, Li D, Xu L, Ma X. HnRNPA2B1 Aggravates Inflammation by Promoting M1 Macrophage Polarization. Nutrients. 2023; 15(7):1555. https://doi.org/10.3390/nu15071555
Chicago/Turabian StyleMeng, Meiyao, Yuxiang Cao, Yankang Zhang, Shuang Liu, Yinzhao Zhong, Dongmei Wang, Dali Li, Lingyan Xu, and Xinran Ma. 2023. "HnRNPA2B1 Aggravates Inflammation by Promoting M1 Macrophage Polarization" Nutrients 15, no. 7: 1555. https://doi.org/10.3390/nu15071555
APA StyleMeng, M., Cao, Y., Zhang, Y., Liu, S., Zhong, Y., Wang, D., Li, D., Xu, L., & Ma, X. (2023). HnRNPA2B1 Aggravates Inflammation by Promoting M1 Macrophage Polarization. Nutrients, 15(7), 1555. https://doi.org/10.3390/nu15071555




