Reduction of Plasma BCAAs following Roux-en-Y Gastric Bypass Surgery Is Primarily Mediated by FGF21
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Genotyping
2.3. Treatments
2.4. Body Weight and Food Intake
2.5. Glucose Tolerance Test (GTT)
2.6. Tissue and Blood Collection
2.7. Fasting Blood Glucose, Plasma Insulin, and HOMA-IR
2.8. BCAA Assay
2.9. Metabolomics
2.10. Western Blots
2.11. RT-qPCR
2.12. Statistical Analysis
3. Results
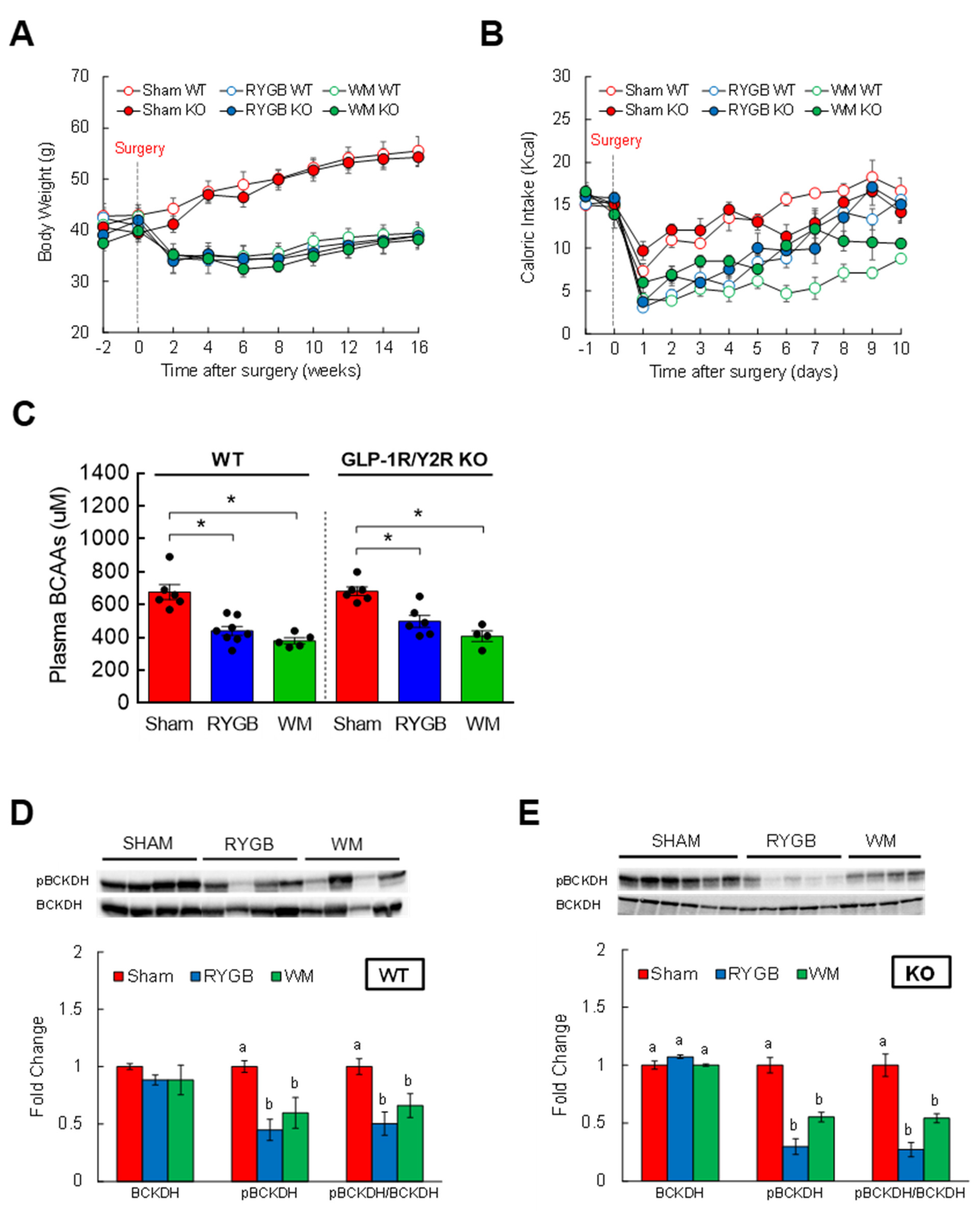
3.1. Body Weight and Caloric Intake after RYGB Surgery
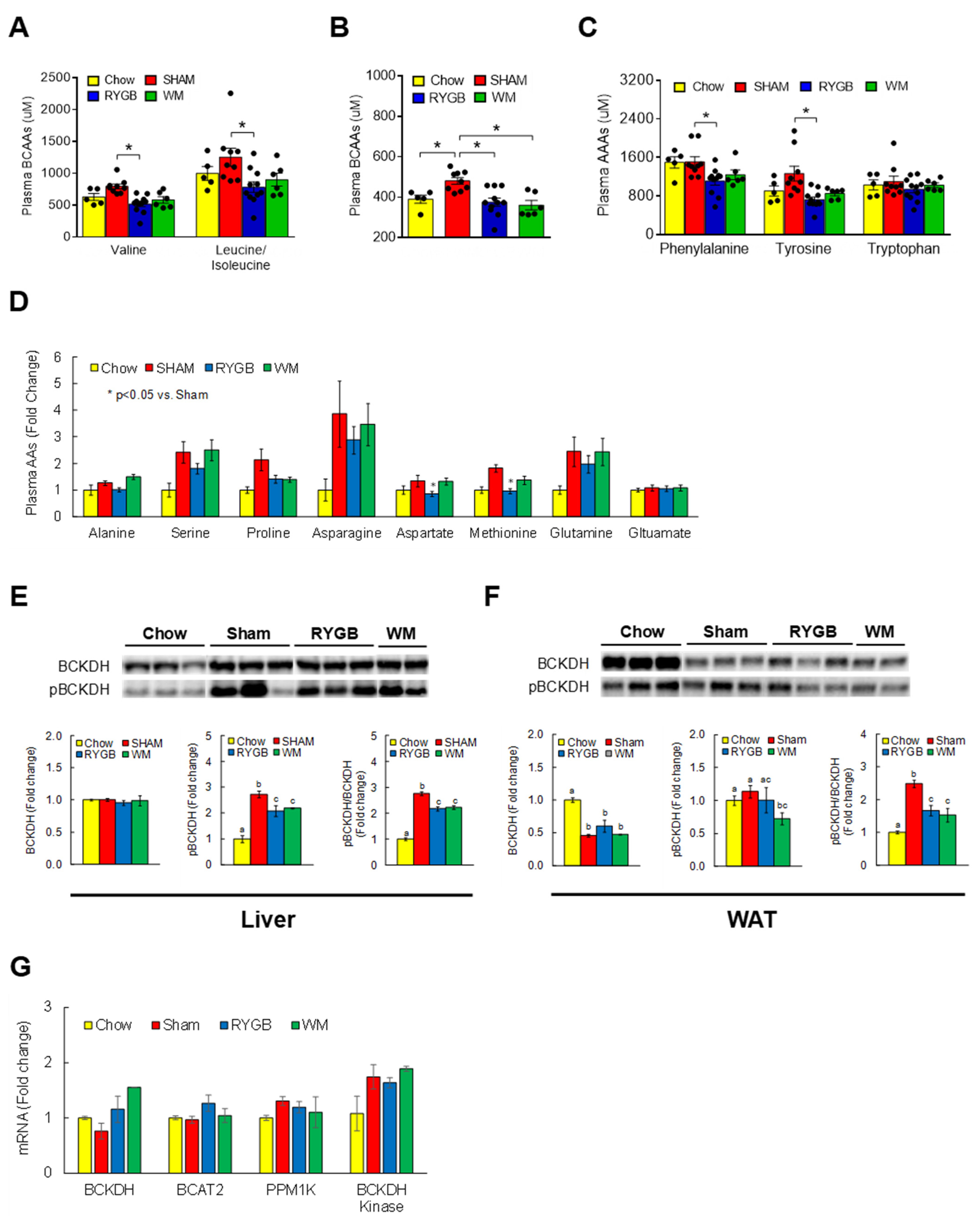
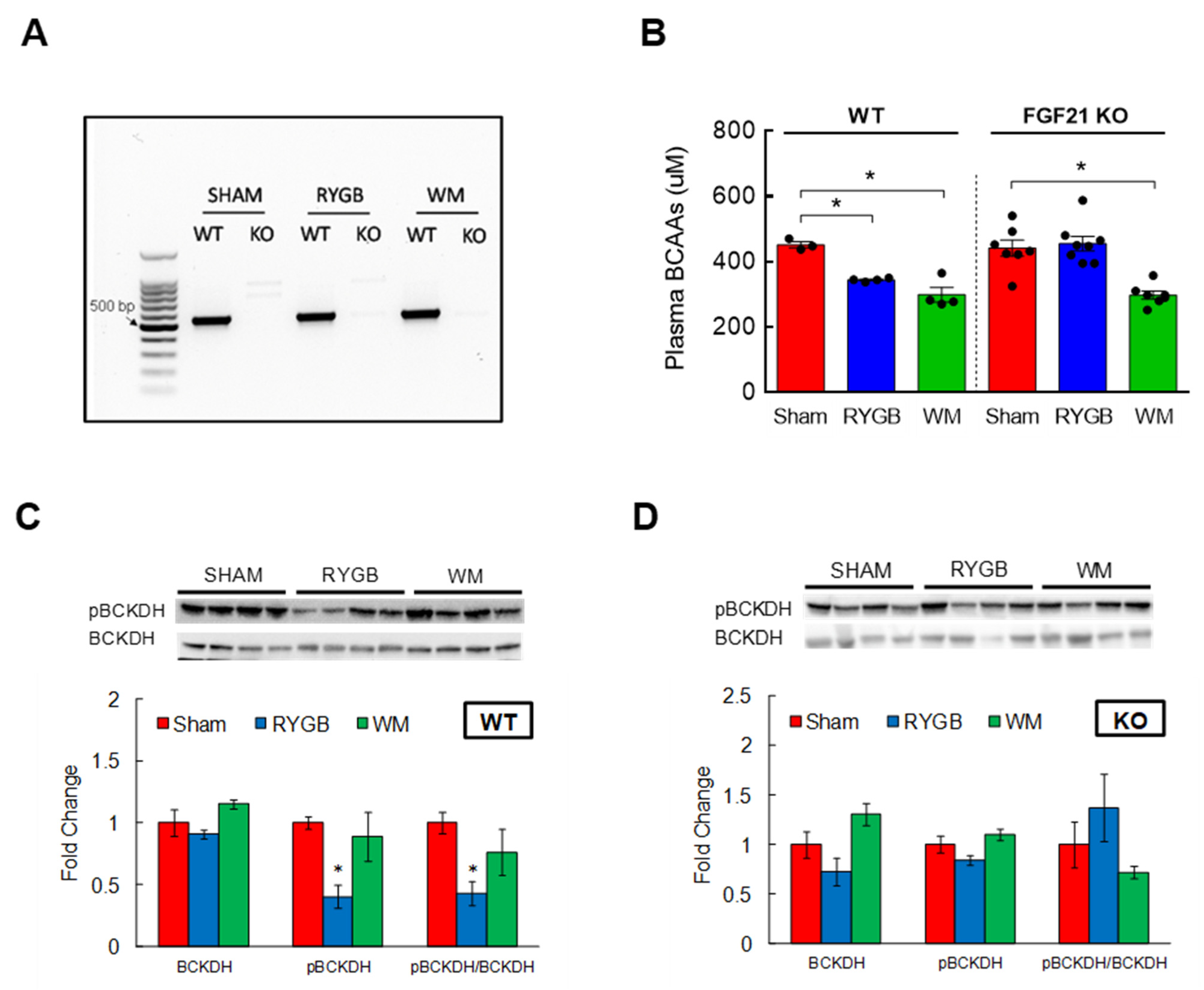
3.2. RYGB Surgery Corrects BCAA Dysregulation
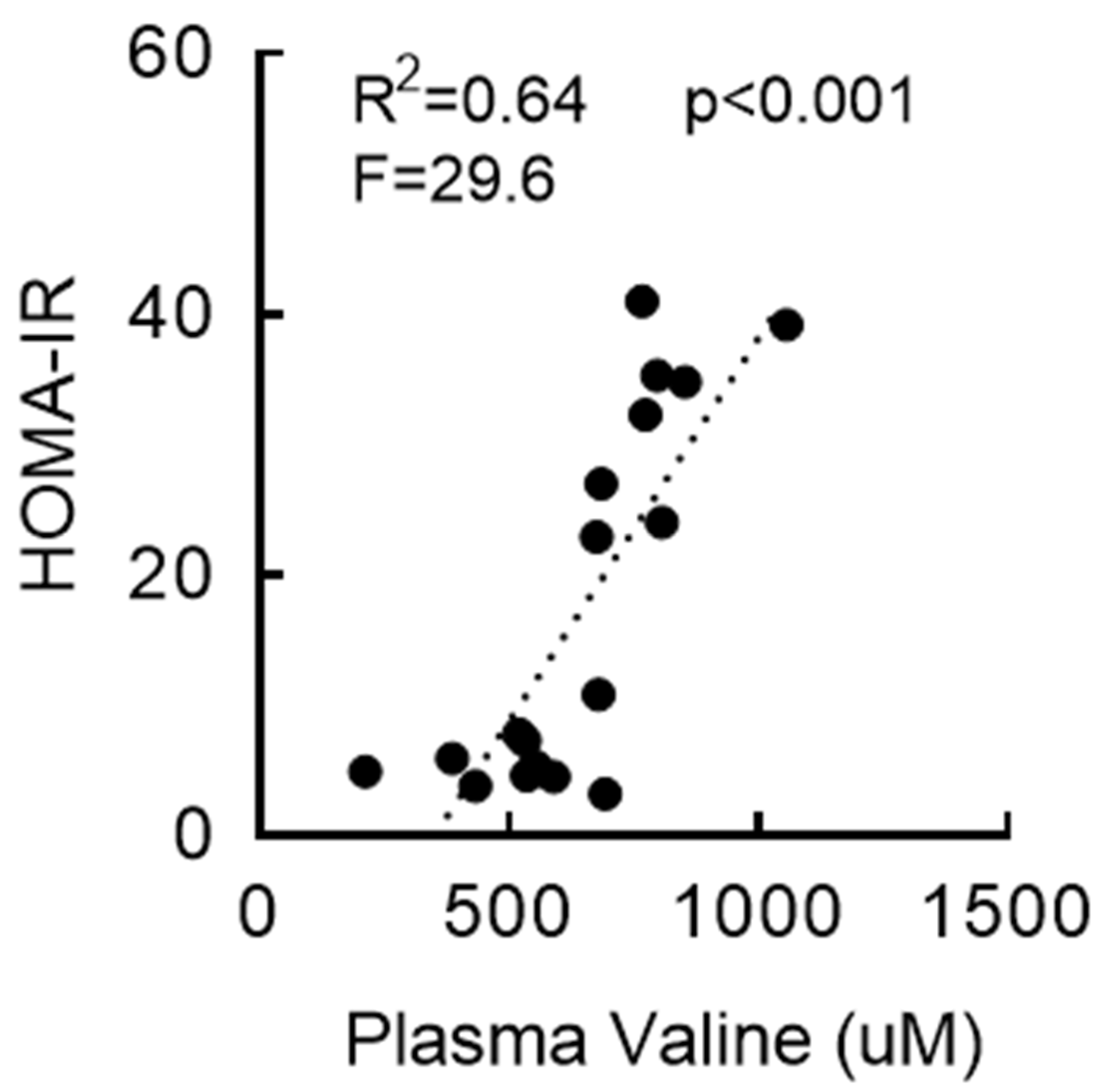
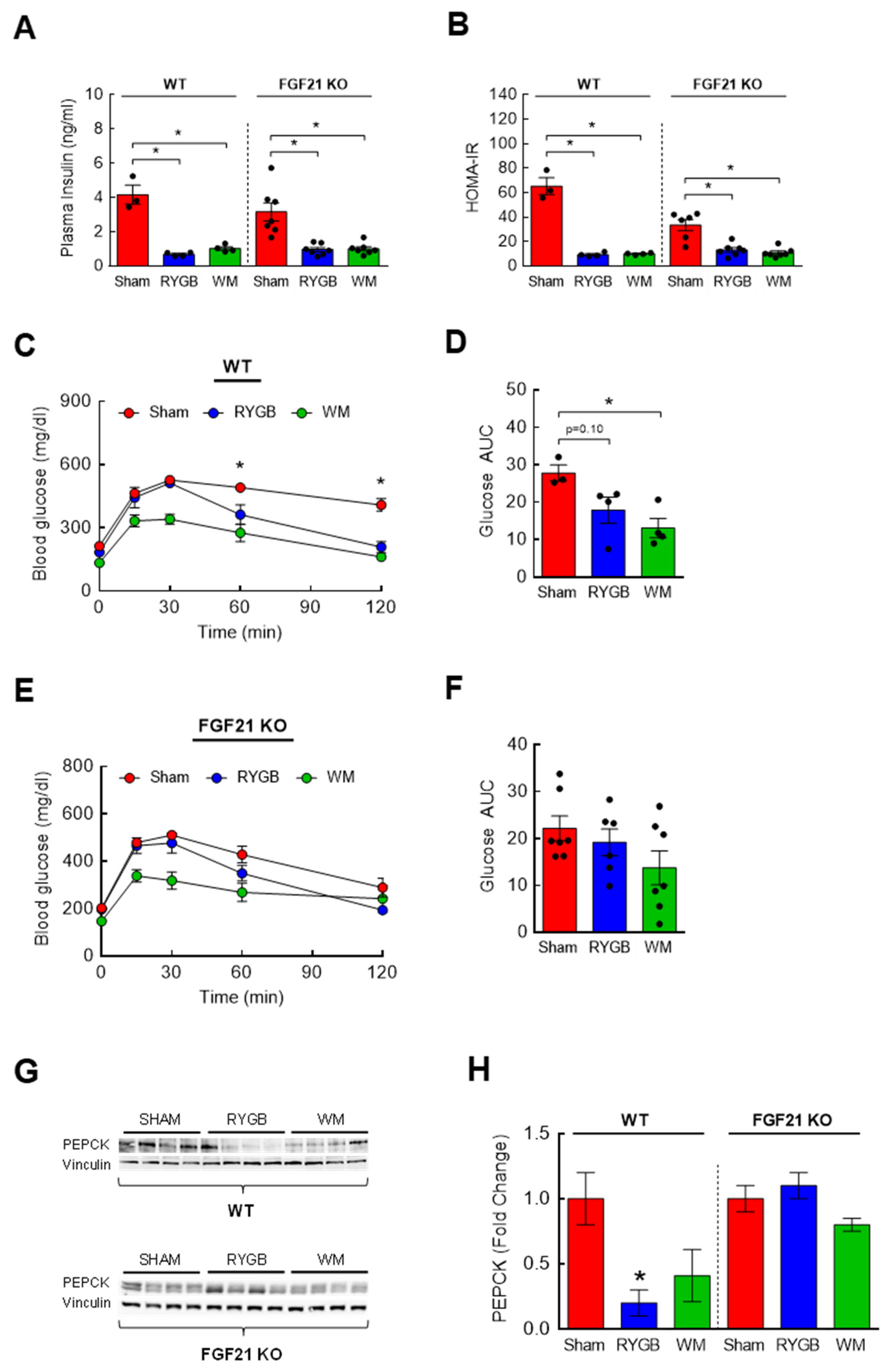
3.3. RYGB-Induced Restoration of BCAA Metabolism Is Associated with Enhanced Insulin Sensitivity
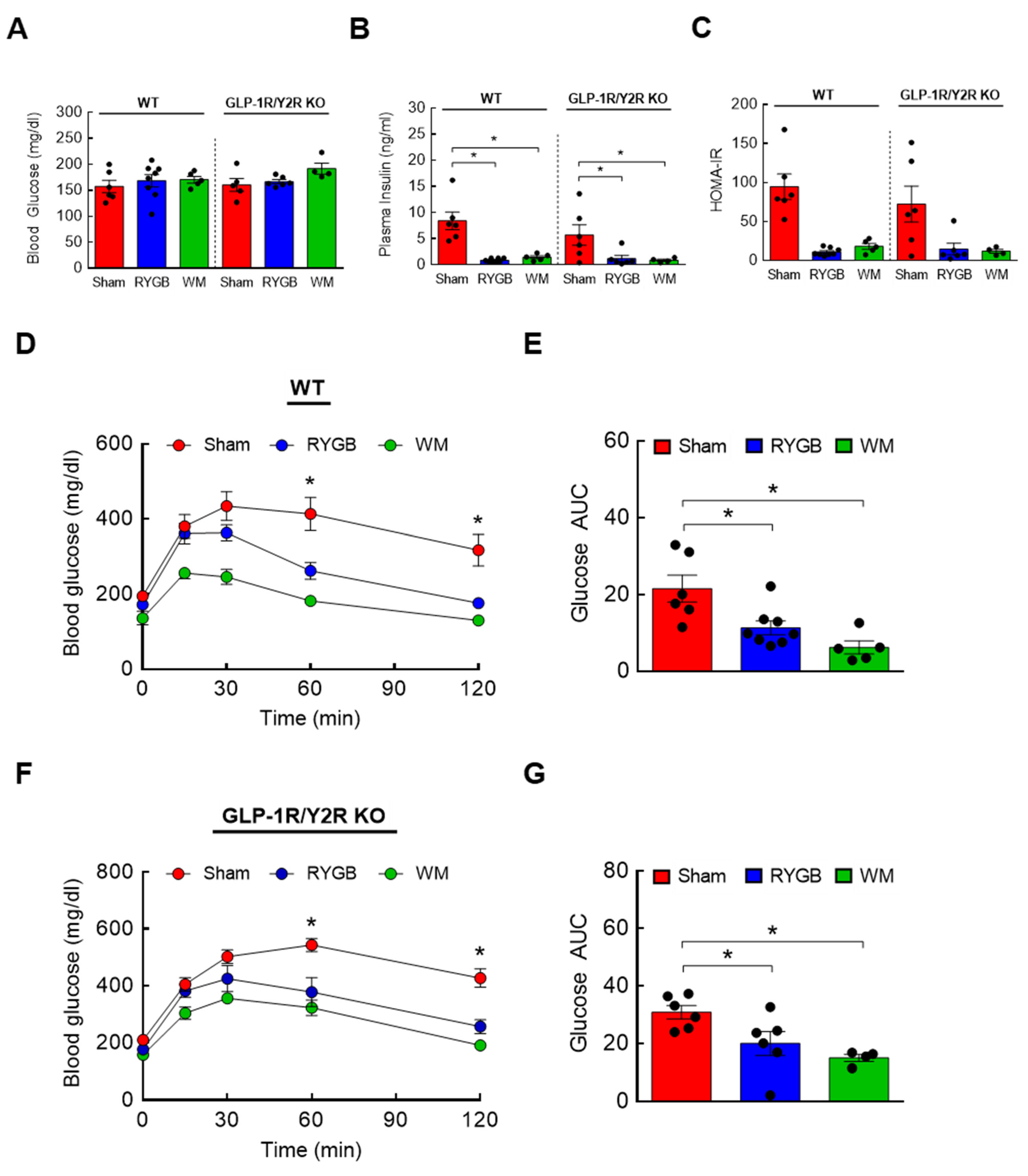
3.4. GLP-1R/Y2R Signaling Is Not Critical for Lowered Plasma BCAAs or Improved Glucose Metabolism following RYGB Surgery
3.5. FGF21 Is Required for RYGB-Induced Reinstatement of BCAA Metabolism
3.6. The Effects of FGF21 Deletion on Glycemic Control following RYGB Surgery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. National Diabetes Statistics Report. Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 29 March 2023).
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuniga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whaley-Connell, A.; Sowers, J.R. Insulin Resistance in Kidney Disease: Is There a Distinct Role Separate from That of Diabetes or Obesity? Cardiorenal. Med. 2017, 8, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Santoleri, D.; Titchenell, P.M. Resolving the Paradox of Hepatic Insulin Resistance. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Stark Casagrande, S.; Fradkin, J.E.; Saydah, S.H.; Rust, K.F.; Cowie, C.C. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 2013, 36, 2271–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camara, S.; Bouenizabila, E.; Hermans, M.P.; Ahn, S.A.; Rousseau, M.F. Novel determinants preventing achievement of major cardiovascular targets in type 2 diabetes. Diabetes Metab. Syndr. 2014, 8, 145–151. [Google Scholar] [CrossRef]
- Scheen, A.J. Dual GIP/GLP-1 receptor agonists: New advances for treating type-2 diabetes. Ann. Endocrinol. 2023, in press. [Google Scholar] [CrossRef]
- Anderson, J.E. Combining Glucagon-Like Peptide 1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors to Target Multiple Organ Defects in Type 2 Diabetes. Diabetes Spectr. 2020, 33, 165–174. [Google Scholar] [CrossRef]
- Ikramuddin, S.; Korner, J.; Lee, W.J.; Connett, J.E.; Inabnet, W.B.; Billington, C.J.; Thomas, A.J.; Leslie, D.B.; Chong, K.; Jeffery, R.W.; et al. Roux-en-Y gastric bypass vs inte nsive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: The Diabetes Surgery Study randomized clinical trial. JAMA 2013, 309, 2240–2249. [Google Scholar] [CrossRef]
- Laferrere, B.; Teixeira, J.; McGinty, J.; Tran, H.; Egger, J.R.; Colarusso, A.; Kovack, B.; Bawa, B.; Koshy, N.; Lee, H.; et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 2479–2485. [Google Scholar] [CrossRef]
- Bose, M.; Olivan, B.; Teixeira, J.; Pi-Sunyer, F.X.; Laferrere, B. Do Incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: What are the evidence? Obes. Surg. 2009, 19, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Troy, S.; Soty, M.; Ribeiro, L.; Laval, L.; Migrenne, S.; Fioramonti, X.; Pillot, B.; Fauveau, V.; Aubert, R.; Viollet, B.; et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 2008, 8, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albaugh, V.L.; Banan, B.; Ajouz, H.; Abumrad, N.N.; Flynn, C.R. Bile acids and bariatric surgery. Mol. Aspects Med. 2017, 56, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; Tsai, P.; Jullig, M.; Liu, A.; Plank, L.; Booth, M. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes. Surg. 2017, 27, 917–925. [Google Scholar] [CrossRef]
- Laferrere, B.; Reilly, D.; Arias, S.; Swerdlow, N.; Gorroochurn, P.; Bawa, B.; Bose, M.; Teixeira, J.; Stevens, R.D.; Wenner, B.R.; et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci. Transl. Med. 2011, 3, 80re2. [Google Scholar] [CrossRef] [Green Version]
- She, P.; Van Horn, C.; Reid, T.; Hutson, S.M.; Cooney, R.N.; Lynch, C.J. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1552–E1563. [Google Scholar] [CrossRef] [Green Version]
- Lips, M.A.; Van Klinken, J.B.; van Harmelen, V.; Dharuri, H.K.; ’t Hoen, P.A.; Laros, J.F.; van Ommen, G.J.; Janssen, I.M.; Van Ramshorst, B.; Van Wagensveld, B.A.; et al. Roux-en-Y gastric bypass surgery, but not calorie restriction, reduces plasma branched-chain amino acids in obese women independent of weight loss or the presence of type 2 diabetes. Diabetes Care 2014, 37, 3150–3156. [Google Scholar] [CrossRef] [Green Version]
- Mutch, D.M.; Fuhrmann, J.C.; Rein, D.; Wiemer, J.C.; Bouillot, J.L.; Poitou, C.; Clement, K. Metabolite profiling identifies candidate markers reflecting the clinical adaptations associated with Roux-en-Y gastric bypass surgery. PLoS ONE 2009, 4, e7905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, S.H.; Hoppel, C.L.; Lok, K.H.; Zhao, L.; Wong, S.W.; Minkler, P.E.; Hwang, D.H.; Newman, J.W.; Garvey, W.T. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J. Nutr. 2009, 139, 1073–1081. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Park, J.Y.; Kim, O.Y.; Ham, B.M.; Kim, H.J.; Kwon, D.Y.; Jang, Y.; Lee, J.H. Metabolic profiling of plasma in overweight/obese and lean men using ultra performance liquid chromatography and Q-TOF mass spectrometry (UPLC-Q-TOF MS). J. Proteome. Res. 2010, 9, 4368–4375. [Google Scholar] [CrossRef]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.; DeLany, J.P. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity 2010, 18, 1695–1700. [Google Scholar] [CrossRef] [Green Version]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Zhao, S.; Yan, W.; Xia, Y.; Chen, X.; Wang, W.; Zhang, J.; Gao, C.; Peng, C.; Yan, F.; et al. Branched Chain Amino Acids Cause Liver Injury in Obese/Diabetic Mice by Promoting Adipocyte Lipolysis and Inhibiting Hepatic Autophagy. EBioMedicine 2016, 13, 157–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, F.; Krebs, M.; Dombrowski, L.; Brehm, A.; Bernroider, E.; Roth, E.; Nowotny, P.; Waldhausl, W.; Marette, A.; Roden, M. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 2005, 54, 2674–2684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, F.; Marette, A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J. Biol. Chem. 2001, 276, 38052–38060. [Google Scholar] [CrossRef] [PubMed]
- Cummings, N.E.; Williams, E.M.; Kasza, I.; Konon, E.N.; Schaid, M.D.; Schmidt, B.A.; Poudel, C.; Sherman, D.S.; Yu, D.; Arriola Apelo, S.I.; et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol. 2018, 596, 623–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, L.; Cummings, N.E.; Arriola Apelo, S.I.; Neuman, J.C.; Kasza, I.; Schmidt, B.A.; Cava, E.; Spelta, F.; Tosti, V.; Syed, F.A.; et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep. 2016, 16, 520–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, P.J.; Lapworth, A.L.; An, J.; Wang, L.; McGarrah, R.W.; Stevens, R.D.; Ilkayeva, O.; George, T.; Muehlbauer, M.J.; Bain, J.R.; et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab. 2016, 5, 538–551. [Google Scholar] [CrossRef]
- Xiao, F.; Yu, J.; Guo, Y.; Deng, J.; Li, K.; Du, Y.; Chen, S.; Zhu, J.; Sheng, H.; Guo, F. Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metabolism 2014, 63, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Mumphrey, M.B.; Townsend, R.L.; Morrison, C.D.; Munzberg, H.; Ye, J.; Berthoud, H.R. Body Composition, Food Intake, and Energy Expenditure in a Murine Model of Roux-en-Y Gastric Bypass Surgery. Obes. Surg. 2016, 26, 2173–2182. [Google Scholar] [CrossRef] [Green Version]
- Morrison, C.D.; Hao, Z.; Mumphrey, M.B.; Townsend, R.L.; Munzberg, H.; Ye, J.; Berthoud, H.R. Roux-en-Y gastric bypass surgery is effective in fibroblast growth factor-21 deficient mice. Mol. Metab. 2016, 5, 1006–1014. [Google Scholar] [CrossRef]
- Boland, B.B.; Mumphrey, M.B.; Hao, Z.; Townsend, R.L.; Gill, B.; Oldham, S.; Will, S.; Morrison, C.D.; Yu, S.; Munzberg, H.; et al. Combined loss of GLP-1R and Y2R does not alter progression of high-fat diet-induced obesity or response to RYGB surgery in mice. Mol. Metab. 2019, 25, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Zhao, Z.; Berthoud, H.R.; Ye, J. Development and verification of a mouse model for Roux-en-Y gastric bypass surgery with a small gastric pouch. PLoS ONE 2013, 8, e52922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckett, P.R. Spectrophotometric assay for measuring branched-chain amino acids. Methods Enzymol. 2000, 324, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Wicks, S.E.; Vandanmagsar, B.; Mendoza, T.M.; Bayless, D.S.; Salbaum, J.M.; Dearth, S.P.; Campagna, S.R.; Mynatt, R.L.; Noland, R.C. Extensive metabolic remodeling after limiting mitochondrial lipid burden is consistent with an improved metabolic health profile. J. Biol. Chem. 2019, 294, 12313–12327. [Google Scholar] [CrossRef] [Green Version]
- Clasquin, M.F.; Melamud, E.; Rabinowitz, J.D. LC-MS data processing with MAVEN: A metabolomic analysis and visualization engine. Curr. Protoc. Bioinform. 2012, 37, 14.11.1–14.11.23. [Google Scholar] [CrossRef] [Green Version]
- Melamud, E.; Vastag, L.; Rabinowitz, J.D. Metabolomic analysis and visualization engine for LC-MS data. Anal. Chem. 2010, 82, 9818–9826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magkos, F.; Bradley, D.; Schweitzer, G.G.; Finck, B.N.; Eagon, J.C.; Ilkayeva, O.; Newgard, C.B.; Klein, S. Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on branched-chain amino acid metabolism. Diabetes 2013, 62, 2757–2761. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Hutson, S.M.; Sweatt, A.J.; Lanoue, K.F. Branched-chain amino acid metabolism: Implications for establishing safe intakes. J. Nutr. 2005, 135, 1557S–1564S. [Google Scholar] [CrossRef] [Green Version]
- Herman, M.A.; She, P.; Peroni, O.D.; Lynch, C.J.; Kahn, B.B. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J. Biol. Chem. 2010, 285, 11348–11356. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.A.; Joshi, M.; Jeoung, N.H.; Obayashi, M. Overview of the molecular and biochemical basis of branched-chain amino acid catabolism. J. Nutr. 2005, 135, 1527S–1530S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batterham, R.L.; Cummings, D.E. Mechanisms of Diabetes Improvement Following Bariatric/Metabolic Surgery. Diabetes Care 2016, 39, 893–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purnell, J.Q.; Selzer, F.; Wahed, A.S.; Pender, J.; Pories, W.; Pomp, A.; Dakin, G.; Mitchell, J.; Garcia, L.; Staten, M.A.; et al. Type 2 Diabetes Remission Rates After Laparoscopic Gastric Bypass and Gastric Banding: Results of the Longitudinal Assessment of Bariatric Surgery Study. Diabetes Care 2016, 39, 1101–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Leccesi, L.; Nanni, G.; Pomp, A.; Castagneto, M.; Ghirlanda, G.; et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N. Engl. J. Med. 2012, 366, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Holst, J.J.; Madsbad, S.; Bojsen-Moller, K.N.; Svane, M.S.; Jorgensen, N.B.; Dirksen, C.; Martinussen, C. Mechanisms in bariatric surgery: Gut hormones, diabetes resolution, and weight loss. Surg. Obes. Relat. Dis. 2018, 14, 708–714. [Google Scholar] [CrossRef] [Green Version]
- Falken, Y.; Hellstrom, P.M.; Holst, J.J.; Naslund, E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: Role of gut peptides. J. Clin. Endocrinol. Metab. 2011, 96, 2227–2235. [Google Scholar] [CrossRef] [Green Version]
- Badman, M.K.; Pissios, P.; Kennedy, A.R.; Koukos, G.; Flier, J.S.; Maratos-Flier, E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007, 5, 426–437. [Google Scholar] [CrossRef] [Green Version]
- Liang, Q.; Zhong, L.; Zhang, J.; Wang, Y.; Bornstein, S.R.; Triggle, C.R.; Ding, H.; Lam, K.S.; Xu, A. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes 2014, 63, 4064–4075. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Stanislaus, S.; Chinookoswong, N.; Lau, Y.Y.; Hager, T.; Patel, J.; Ge, H.; Weiszmann, J.; Lu, S.C.; Graham, M.; et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models--association with liver and adipose tissue effects. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1105–E1114. [Google Scholar] [CrossRef] [Green Version]
- Coskun, T.; Bina, H.A.; Schneider, M.A.; Dunbar, J.D.; Hu, C.C.; Chen, Y.; Moller, D.E.; Kharitonenkov, A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008, 149, 6018–6027. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Wei, M.; Huang, X.; Cheng, Y.; Shao, Y.; Xia, P.; Zhong, M.; Liu, S.; Zhang, G.; et al. Improved FGF21 Sensitivity and Restored FGF21 Signaling Pathway in High-Fat Diet/Streptozotocin-Induced Diabetic Rats After Duodenal-Jejunal Bypass and Sleeve Gastrectomy. Front. Endocrinol. 2019, 10, 566. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Richardson, N.E.; Green, C.L.; Spicer, A.B.; Murphy, M.E.; Flores, V.; Jang, C.; Kasza, I.; Nikodemova, M.; Wakai, M.H.; et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab. 2021, 33, 905–922.e906. [Google Scholar] [CrossRef]
- Zhou, M.; Shao, J.; Wu, C.Y.; Shu, L.; Dong, W.; Liu, Y.; Chen, M.; Wynn, R.M.; Wang, J.; Gui, W.J.; et al. Targeting BCAA Catabolism to Treat Obesity-Associated Insulin Resistance. Diabetes 2019, 68, 1730–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Yin, H.; Guo, Y.; Fang, Y.; Yuan, F.; Chen, S.; Guo, F. A fifty percent leucine-restricted diet reduces fat mass and improves glucose regulation. Nutr. Metab. 2021, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Costa Junior, J.M.; Rosa, M.R.; Protzek, A.O.; de Paula, F.M.; Ferreira, S.M.; Rezende, L.F.; Vanzela, E.C.; Zoppi, C.C.; Silveira, L.R.; Kettelhut, I.C.; et al. Leucine supplementation does not affect protein turnover and impairs the beneficial effects of endurance training on glucose homeostasis in healthy mice. Amino. Acids. 2015, 47, 745–755. [Google Scholar] [CrossRef]
- White, P.J.; McGarrah, R.W.; Grimsrud, P.A.; Tso, S.C.; Yang, W.H.; Haldeman, J.M.; Grenier-Larouche, T.; An, J.; Lapworth, A.L.; Astapova, I.; et al. The BCKDH Kinase and Phosphatase Integrate BCAA and Lipid Metabolism via Regulation of ATP-Citrate Lyase. Cell Metab. 2018, 27, 1281–1293.e1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, G.F.; Carpentier, A.C.; Pereira, S.; Hahn, M.; Giacca, A. Direct and indirect control of hepatic glucose production by insulin. Cell Metab. 2021, 33, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Camastra, S.; Gastaldelli, A.; Mari, A.; Bonuccelli, S.; Scartabelli, G.; Frascerra, S.; Baldi, S.; Nannipieri, M.; Rebelos, E.; Anselmino, M.; et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia 2011, 54, 2093–2102. [Google Scholar] [CrossRef] [Green Version]
- Neinast, M.D.; Jang, C.; Hui, S.; Murashige, D.S.; Chu, Q.; Morscher, R.J.; Li, X.; Zhan, L.; White, E.; Anthony, T.G.; et al. Quantitative Analysis of the Whole-Body Metabolic Fate of Branched-Chain Amino Acids. Cell Metab. 2019, 29, 417–429.e414. [Google Scholar] [CrossRef] [Green Version]
- Vangipurapu, J.; Stancakova, A.; Smith, U.; Kuusisto, J.; Laakso, M. Nine Amino Acids Are Associated With Decreased Insulin Secretion and Elevated Glucose Levels in a 7.4-Year Follow-up Study of 5,181 Finnish Men. Diabetes 2019, 68, 1353–1358. [Google Scholar] [CrossRef]
- Boland, B.; Mumphrey, M.B.; Hao, Z.; Gill, B.; Townsend, R.L.; Yu, S.; Munzberg, H.; Morrison, C.D.; Trevaskis, J.L.; Berthoud, H.R. The PYY/Y2R-Deficient Mouse Responds Normally to High-Fat Diet and Gastric Bypass Surgery. Nutrients 2019, 11, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirro, V.; Roth, K.D.; Lin, Y.; Willency, J.A.; Milligan, P.L.; Wilson, J.M.; Ruotolo, G.; Haupt, A.; Newgard, C.B.; Duffin, K.L. Effects of Tirzepatide, a Dual GIP and GLP-1 RA, on Lipid and Metabolite Profiles in Subjects With Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2022, 107, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Samms, R.J.; Christe, M.E.; Collins, K.A.; Pirro, V.; Droz, B.A.; Holland, A.K.; Friedrich, J.L.; Wojnicki, S.; Konkol, D.L.; Cosgrove, R.; et al. GIPR agonism mediates weight-independent insulin sensitization by tirzepatide in obese mice. J. Clin. Investig. 2021, 131, 146353. [Google Scholar] [CrossRef]
- Clements, R.H.; Gonzalez, Q.H.; Long, C.I.; Wittert, G.; Laws, H.L. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am. Surg. 2004, 70, 1–4, discussion 4–5. [Google Scholar] [CrossRef] [PubMed]
- Goldfine, A.B.; Mun, E.C.; Devine, E.; Bernier, R.; Baz-Hecht, M.; Jones, D.B.; Schneider, B.E.; Holst, J.J.; Patti, M.E. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J. Clin. Endocrinol. Metab. 2007, 92, 4678–4685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laferrere, B.; Heshka, S.; Wang, K.; Khan, Y.; McGinty, J.; Teixeira, J.; Hart, A.B.; Olivan, B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007, 30, 1709–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, L.; Lam, K.S.L.; Xu, A. The therapeutic potential of FGF21 in metabolic diseases: From bench to clinic. Nat. Rev. Endocrinol. 2020, 16, 654–667. [Google Scholar] [CrossRef]
- Gong, Q.; Hu, Z.; Zhang, F.; Cui, A.; Chen, X.; Jiang, H.; Gao, J.; Han, Y.; Liang, Q.; Ye, D.; et al. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology 2016, 64, 425–438. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Cao, H.; Hou, Y.; Sun, G.; Li, D.; Wang, W. Liver Plays a Major Role in FGF-21 Mediated Glucose Homeostasis. Cell. Physiol. Biochem. 2018, 45, 1423–1433. [Google Scholar] [CrossRef]
- Badman, M.K.; Koester, A.; Flier, J.S.; Kharitonenkov, A.; Maratos-Flier, E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 2009, 150, 4931–4940. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, A.; Parlee, S.; Perez-Tilve, D.; Li, P.; Pan, J.; Mroz, P.A.; Kruse Hansen, A.M.; Andersen, B.; Finan, B.; Kharitonenkov, A.; et al. Molecular elements in FGF19 and FGF21 defining KLB/FGFR activity and specificity. Mol. Metab. 2018, 13, 45–55. [Google Scholar] [CrossRef]
- Fon Tacer, K.; Bookout, A.L.; Ding, X.; Kurosu, H.; John, G.B.; Wang, L.; Goetz, R.; Mohammadi, M.; Kuro-o, M.; Mangelsdorf, D.J.; et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 2010, 24, 2050–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hultman, K.; Scarlett, J.M.; Baquero, A.F.; Cornea, A.; Zhang, Y.; Salinas, C.B.G.; Brown, J.; Morton, G.J.; Whalen, E.J.; Grove, K.L.; et al. The central fibroblast growth factor receptor/beta klotho system: Comprehensive mapping in Mus musculus and comparisons to nonhuman primate and human samples using an automated in situ hybridization platform. J. Comp. Neurol. 2019, 527, 2069–2085. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Morgan, D.A.; Rahmouni, K.; Sonoda, J.; Fu, X.; Burgess, S.C.; Holland, W.L.; Kliewer, S.A.; Mangelsdorf, D.J. FGF19, FGF21, and an FGFR1/beta-Klotho-Activating Antibody Act on the Nervous System to Regulate Body Weight and Glycemia. Cell Metab. 2017, 26, 709–718.e703. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.M.; Matsen, M.E.; Mundinger, T.O.; Morton, G.J.; Stefanovski, D.; Bergman, R.N.; Kaiyala, K.J.; Taborsky, G.J., Jr.; Schwartz, M.W. Glucose intolerance induced by blockade of central FGF receptors is linked to an acute stress response. Mol. Metab. 2015, 4, 561–568. [Google Scholar] [CrossRef]
- Sarruf, D.A.; Thaler, J.P.; Morton, G.J.; German, J.; Fischer, J.D.; Ogimoto, K.; Schwartz, M.W. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 2010, 59, 1817–1824. [Google Scholar] [CrossRef] [Green Version]
- Shin, A.C.; Fasshauer, M.; Filatova, N.; Grundell, L.A.; Zielinski, E.; Zhou, J.Y.; Scherer, T.; Lindtner, C.; White, P.J.; Lapworth, A.L.; et al. Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Cell Metab. 2014, 20, 898–909. [Google Scholar] [CrossRef] [Green Version]
- Gannaban, R.B.; NamKoong, C.; Ruiz, H.H.; Choi, H.J.; Shin, A.C. Central Regulation of Branched-Chain Amino Acids Is Mediated by AgRP Neurons. Diabetes 2021, 70, 62–75. [Google Scholar] [CrossRef]
- Flippo, K.H.; Potthoff, M.J. Metabolic Messengers: FGF21. Nat. Metab. 2021, 3, 309–317. [Google Scholar] [CrossRef]
| Gene Name | Type | Sequence (5′→3′) |
|---|---|---|
| BCKDH | Forward | GGATGAGGAACAGGAGAAGG |
| Reverse | GGAGAAGAGGAGGCTTGG | |
| BCKDH Kinase | Forward | GACAGGTGGACTTAGATGGA |
| Reverse | CAAGAATGAGCAGAGCAGAG | |
| PPM1K | Forward | CCTGCTACTTCTCCACTTCA |
| Reverse | GCTCATCAATGCGGTTATCC | |
| BCAT2 | Forward | GCAGACCTCCAGATTCAGA |
| Reverse | TGTTATTCCACTCCACCATCA | |
| B2M | Forward | GAAGCCGAACATACTGAACTG |
| Reverse | CTGAAGGACATATCTGACATCTCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, H.; Kramer, A.; Mullins, C.A.; Mattern, M.; Gannaban, R.B.; Townsend, R.L.; Campagna, S.R.; Morrison, C.D.; Berthoud, H.-R.; Shin, A.C. Reduction of Plasma BCAAs following Roux-en-Y Gastric Bypass Surgery Is Primarily Mediated by FGF21. Nutrients 2023, 15, 1713. https://doi.org/10.3390/nu15071713
Shah H, Kramer A, Mullins CA, Mattern M, Gannaban RB, Townsend RL, Campagna SR, Morrison CD, Berthoud H-R, Shin AC. Reduction of Plasma BCAAs following Roux-en-Y Gastric Bypass Surgery Is Primarily Mediated by FGF21. Nutrients. 2023; 15(7):1713. https://doi.org/10.3390/nu15071713
Chicago/Turabian StyleShah, Harsh, Alyssa Kramer, Caitlyn A. Mullins, Marie Mattern, Ritchel B. Gannaban, R. Leigh Townsend, Shawn R. Campagna, Christopher D. Morrison, Hans-Rudolf Berthoud, and Andrew C. Shin. 2023. "Reduction of Plasma BCAAs following Roux-en-Y Gastric Bypass Surgery Is Primarily Mediated by FGF21" Nutrients 15, no. 7: 1713. https://doi.org/10.3390/nu15071713
APA StyleShah, H., Kramer, A., Mullins, C. A., Mattern, M., Gannaban, R. B., Townsend, R. L., Campagna, S. R., Morrison, C. D., Berthoud, H.-R., & Shin, A. C. (2023). Reduction of Plasma BCAAs following Roux-en-Y Gastric Bypass Surgery Is Primarily Mediated by FGF21. Nutrients, 15(7), 1713. https://doi.org/10.3390/nu15071713






