Some Immune Parameters of Term Newborns at Birth Are Associated with the Concentration of Iron, Copper and Magnesium in Maternal Serum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Determination in Neonatal Cord Blood Serum of IgG Antibodies as a Promoter of Immunity
- -
- Adequate concentration of IgG in the UCS, when it was 636–1606 mg/dL (which indicated the correct IgG-related immune functions of newborns);
- -
- Insufficient concentration of IgG in the UCS, when it was <636 mg/dL (which indicated impaired IgG-related immune functions of newborns).
2.3. Determination in Neonatal Cord Blood Serum of Lf-ANCA Auto-Antibodies as an Inhibitor of Immunity
- -
- Adequate concentration of Lf-ANCA in the UCS, when it was <10 U/mL (i.e., the concentration of Lf-ANCA was too low to impair the immune functions of newborns);
- -
- Excessive concentration of Lf-ANCA in the UCS, when it was ≥10 U/mL (i.e., the concentration of Lf-ANCA was high enough to impair the immune functions of newborns).
2.4. Determination in Maternal Serum of Selected Macro- and Microelements
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
4. Discussion
4.1. Iron
4.2. Copper
4.3. Magnesium
4.4. Zinc
4.5. Calcium
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, H.; Acharjee, A.; Pearce, H.; Quraishi, M.N.; Powell, R.; Rossiter, A.; Beggs, A.; Ewer, A.; Moss, P.; Toldi, G. Breastfeeding promotes early neonatal regulatory T-cell expansion and immune tolerance of non-inherited maternal antigens. Allergy 2021, 76, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune function and micronutrient requirements change over the life course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettengill, M.A.; van Haren, S.D.; Levy, O. Soluble mediators regulating immunity in early life. Front. Immunol. 2014, 5, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmeira, P.; Quinello, C.; Silveira-Lessa, A.; Zago, C.A.; Carneiro-Sampaio, M. IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012, 2012, 985646. [Google Scholar] [CrossRef] [PubMed]
- Fouda, G.G.; Martinez, D.R.; Swamy, G.K.; Permar, S.R. The impact of IgG transplacental transfer on early life immunity. ImmunoHorizons 2018, 2, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, H.W.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Silveira Lessa, A.L.; Krebs, V.L.; Brasil, T.B.; Pontes, G.N.; Carneiro-Sampaio, M.; Palmeira, P. Preterm and term neonates transplacentally acquire IgG antibodies specific to LPS from Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 2011, 62, 236–243. [Google Scholar] [CrossRef] [Green Version]
- van den Berg, J.P.; Westerbeek, E.A.; Berbers, G.A.; van Gageldonk, P.G.; van der Klis, F.R.; van Elburg, R.M. Transplacental transport of IgG antibodies specific for pertussis, diphtheria, tetanus, Heamofhilus influenzae type b, and neisseria meningitides serogroup C is lower in preterm compared with term infants. Pediatr. Infect. Dis. J. 2010, 29, 801–805. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chen, H.L.; Yen, C.C.; Lee, P.Y.; Tsai, H.C.; Lin, M.F.; Chen, C.M. Bovine lactoferrin inhibits lung cancer growth through suppression of both inflammation and expression of vascular endothelial growth factor. J. Diary Sci. 2013, 96, 2095–2106. [Google Scholar] [CrossRef] [Green Version]
- Legrand, D. Overview of lactoferrin as a natural immune modulator. J. Pediatr. 2016, 173, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Kyriakidia, K.S.; Tsianosa, V.E.; Karvounisa, E.; Christodouloub, D.K.; Katsanosb, K.H.; Tsianos, E.V. Neutrophil anti-neutrophil cytoplasmic autoantibody proteins: Bactericidal increasing protein, lactoferrin, cathepsin, and elastase as serological markers of infl ammatory bowel and other diseases. Ann. Gastroenterol. 2016, 29, 258–267. [Google Scholar] [CrossRef]
- Rak, K.; Bronkowska, M. Immunologiczne znaczenie siary. Hygeia Public Health 2014, 49, 249–254. [Google Scholar]
- Shida, H.; Nakazawa, D.; Tateyama, Y.; Miyoshi, A.; Kusunoki, Y.; Hattanda, F.; Masuda, S.; Tomaru, U.; Kawakami, T.; Atsumi, T.; et al. The presence of anti-lactoferrin antibodies in a subgroup of eosinophilic granulomatosis with polyangiitis patients and their possible contribution to enhancement of neutrophil extracellular trap formation. Front. Immunol. 2016, 7, 636. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Zhang, Y.; Peng, W.; Chen, J.; Li, H.; Ming, F. Detection of anti-lactoferrin antibodies and anti-myeloperoxidase antibodies in autoimmune hepatitis: A retrospective study. J. Immunoassay Immunochem. 2014, 35, 388–397. [Google Scholar] [CrossRef]
- Caccavo, D.; Rigon, A.; Picardi, A.; Galluzzo, S.; Vadacca, M.; Ferri, G.M.; Amoroso, A.; Afeltra, A. Anti-lactoferrin antibodies in systemic lupus erythematosus: Isotypes and clinical correlates. Clin. Rheumatol. 2005, 24, 381–387. [Google Scholar] [CrossRef]
- Boxer, L.A.; Coates, T.D.; Haak, R.A.; Wolach, J.B.; Hoffstein, S.; Baehner, R.L. Lactoferrin deficiency associated with altered granulocyte function. N. Engl. J. Med. 1982, 307, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.A.; Andersson, B.; Carlsson, B.; Dahlgren, U.; Porras, O.; Söderström, T.; Svanborg Edén, C.; Mellander, L. Defence of mucous membranes by antibodies, receptor analogues and non-specific host factors. Infection 1985, 13 (Suppl. S2), 166–170. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Oda, H.; Yamauchi, K.; Abe, F. Lactoferrin for prevention of common viral infections. J. Infect. Chemother. 2014, 20, 666–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.S.; Hasan, S.S.; Kow, C.S.; Merchant, H.A. Lactoferrin reduces the risk of respiratory tract infections: A meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2021, 45, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Reyes-Hernández, C.G.; López de Pablo, A.; González, M.C.; Arribas, S.M. Implication of oxidative stress in fetal programming of cardiovascular disease. Front. Physiol. 2018, 9, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone, S.; Tataranno, M.L.; Santacroce, A.; Bracciali, C.; Riccitelli, M.; Alagna, M.G.; Longini, M.; Belvisi, E.; Bazzini, F.; Buonocore, G. Fetal programming, maternal nutrition, and oxidative stress hypothesis. J. Pediatr. Biochem. 2016, 6, 96–102. [Google Scholar]
- Fernandez-Twinn, D.S.; Constância, M.; Ozanne, S.E. Intergenerational epigenetic inheritance in models of developmental programming of adult disease. Semin. Cell Dev. Biol. 2015, 43, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.H.; O’Connor, T.G.; Roth, C.; Susser, E.; Bjorke-Monsen, A.-L. The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front. Neurosci. 2013, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Jennewein, M.F.; Abu-Raya, B.; Jiang, Y.; Alter, G.; Marchant, A. Transfer of maternal immunity and programming of the newborn immune system. Semin. Immunopathol. 2017, 39, 605–613. [Google Scholar] [CrossRef]
- Macpherson, A.J.; de Agüero, M.G.; Ganal-Vonarburg, S.C. How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol. 2017, 17, 508–517. [Google Scholar] [CrossRef]
- Amarasekera, M.; Prescott, S.L.; Palmer, D.J. Nutrition in early life, immune-programming and allergies: The role of epigenetics. Asian Pac. J. Allergy Immunol. 2013, 31, 175–182. [Google Scholar]
- Palmer, A.C. Nutritionally mediated programming of the developing immune system. Adv. Nutr. 2011, 2, 377–395. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.D.; Berkley, J.A.; Warner, J.O. Perinatal nutrition and immunity to infection. Pediatr. Allergy Immunol. 2010, 21, 564–576. [Google Scholar] [CrossRef] [Green Version]
- Prentice, S. They are what you eat: Can nutritional factors during gestation and early infancy modulate the neonatal immune response? Front. Immunol. 2017, 8, 1641. [Google Scholar] [CrossRef] [PubMed]
- Darnton-Hill, I.; Mkparu, U.C. Micronutrients in pregnancy in low- and middLe-income countries. Nutrients 2015, 7, 1744–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middLe-income countries. Lancet 2013, 2, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Torheim, L.E.; Ferguson, E.L.; Penrose, K.; Arimond, M. Women in resource-poor settings are at risk of inadequate intakes of multiple micronutrients. J. Nutr. 2010, 140, 2051–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parisi, F.; Laoreti, A.; Cetin, I. Multiple micronutrient need in pregnancy in industrialized countries. Ann. Nutr. Metab. 2014, 65, 13–21. [Google Scholar] [CrossRef]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef] [Green Version]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [Green Version]
- Scholing, J.M.; Olthof, M.R.; Jonker, F.A.M.; Vrijkotte, T.G.M. Association between pre-pregnancy weight status and maternal micronutrient status in early pregnancy. Public Health Nutr. 2018, 21, 2046–2055. [Google Scholar] [CrossRef] [Green Version]
- Livock, M.; Anderson, P.J.; Lewis, S.; Bowden, S.; Muggli, E.; Halliday, J. Maternal micronutrient consumption periconceptionally and during pregnancy: A prospective cohort study. Public Health Nutr. 2016, 20, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Hatzopoulou, K.; Filis, V.; Grammatikopoulou, M.G.; Kotzamanidis, C.; Tsigga, M. Greek pregnant women demonstrate inadequate micronutrient intake despite supplement use. J. Diet. Suppl. 2014, 11, 155–165. [Google Scholar] [CrossRef]
- Khayat, S.; Fanaei, H.; Ghanbarzehi, A. Minerals in Pregnancy and Lactation: A Review Article. JCDR 2017, 11, QE01–QE05. [Google Scholar] [CrossRef] [PubMed]
- Tuck, M.K.; Chan, D.W.; Chia, D.; Godwin, A.K.; Grizzle, W.E.; Krueger, K.E.; Rom, W.; Sanda, M.; Sorbara, L.; Stass, S.; et al. Standard operating procedures for serum and plasma collection: Early detection research network consensus statement Standard operating procedure integration working group. J. Proteome Res. 2009, 8, 113–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolliff, C.R.; Cost, K.M.; Stivrins, P.C.; Grossman, P.P.; Nolte, C.R.; Franco, S.M.; Fijan, K.J.; Fletcher, L.L.; Shriner, H.C. Reference intervals for serum IgG, IgA, IgM, C3, and C4 as determined by rate nephelometry. Clin. Chem. 1982, 28, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Rak, K.; Łoźna, K.; Styczyńska, M.; Bobak, Ł.; Bronkowska, M. Oxidative stress at birth is associated with the concentration of iron and copper in maternal serum. Nutrients 2021, 13, 1491. [Google Scholar] [CrossRef] [PubMed]
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and laboratory studies: A reference table for clinicians. Obstet. Gynecol. 2009, 114, 1326–1331. [Google Scholar] [CrossRef]
- Stanisz, A. The Accessible Course of Statistics with Use the STATISTICA PL for Medicine Examples; Basic Statistics; StatSoft Polska: Kraków, Poland, 2006; Volume 1. [Google Scholar]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Breymann, C. Iron deficiency anemia in pregnancy. Semin. Hematol. 2015, 52, 339–347. [Google Scholar] [CrossRef]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Contribution of selected vitamins and trace elements to immune function. Ann. Nutr. Metab. 2007, 51, 301–323. [Google Scholar] [CrossRef] [Green Version]
- Ganz, T.; Nemeth, E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 2015, 15, 500–510. [Google Scholar] [CrossRef] [Green Version]
- Ward, R.J.; Crichton, R.R.; Taylor, D.L.; Della Corte, L.; Srai, S.K.; Dexter, D.T. Iron and the immune system. J. Neural Transm. 2011, 118, 315–328. [Google Scholar] [CrossRef]
- Khan, F.A.; Fisher, M.A.; Khakoo, R.A. Association of hemochromatosis with infectious diseases: Expanding spectrum. Int. J. Infect. Dis. 2007, 11, 482–487. [Google Scholar] [CrossRef] [Green Version]
- Dewey, K.G.; Domellof, M.; Cohen, R.J.; Landa Rivera, L.; Hernell, O.; Lonnerdal, B. Iron supplementation affects growth and morbidity of breast-fed infants: Results of a randomized trial in Sweden and Honduras. J. Nutr. 2002, 132, 3249–3255. [Google Scholar] [CrossRef] [Green Version]
- Soofi, S.; Cousens, S.; Iqbal, S.P.; Akhund, T.; Khan, J.; Ahmed, I.; Zaidi, A.K.; Bhutta, Z.A. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: A cluster-randomised trial. Lancet 2013, 382, 29–40. [Google Scholar] [CrossRef]
- Sazawal, S.; Black, R.E.; Ramsan, M.; Chwaya, H.M.; Stoltzfus, R.J.; Dutta, A.; Dhingra, U.; Kabole, I.; Deb, S.; Othman, M.K.; et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: Community-based, randomised, placebo-controlled trial. Lancet 2006, 367, 133–143. [Google Scholar] [CrossRef]
- Pasricha, S.R.; Hayes, E.; Kalumba, K.; Biggs, B.A. Effect of daily iron supplementation on health in children aged 4–23 months: A systematic review and meta-analysis of randomised controlled trials. Lancet Glob. Health 2013, 1, 77–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kortman, G.A.; Boleij, A.; Swinkels, D.W.; Tjalsma, H. Iron availability increases the pathogenic potential of Salmonella typhimurium and other enteric pathogens at the intestinal epithelial interface. PLoS ONE 2012, 7, 29968. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, T.; Han, H.; Yang, Z. Iron, oxidative stress and gestational diabetes. Nutrients 2014, 6, 3968–3980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beard, J.L. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr. 2001, 131, 568–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, D.M. Clinical syndromes of copper deficiency. In Nutritional Anemia. Scientific Principles, Clinical Practice, and Public Health; Means, R.T., Jr., Ed.; Cambridge University Press: Cambridge, UK, 2019; pp. 111–132. [Google Scholar]
- Al-Jameil, N.; Tabassum, H.; Al-Mayouf, H.; Aljohar, H.I.; Alenzi, N.D.; Hijazy, S.M.; Khan, F.A. Analysis of serum trace elementscopper, manganese and zinc in preeclamptic pregnant women by inductively coupled plasma optical emission spectrometry: A prospective case controlled study in Riyadh, Saudi Arabia. Int. J. Clin. Exp. Pathol. 2014, 7, 1900–1910. [Google Scholar]
- Sarwar, M.S.; Ahmed, S.; Ullah, M.S.; Kabir, H.; Rahman, G.K.; Hasnat, A.; Islam, M.S. Comparative study of serum zinc, copper, manganese, and iron in preeclamptic pregnant women. Biol. Trace Elem. Res. 2013, 154, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Roy, A.; Biswas, S. Comparative study of copper, zinc, ferritin, calcium and magnesium levels in pregnancy induced hypertension and normotensive primigravida mothers. Int. J. Res. Med. Sci. 2016, 4, 1879–1883. [Google Scholar] [CrossRef]
- Buamah, P.K.; Russell, M.; Milford-Ward, A.; Taylor, P.; Roberts, D.F. Serum copper concentration significantly less in abnormal pregnancies. Clin. Chem. 1984, 30, 1676–1677. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.J.; Gong, B.; Xu, F.Y.; Luo, Y. Four trace elements in pregnant women and their relationships with adverse pregnancy outcomes. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4690–4697. [Google Scholar] [PubMed]
- Li, Z.; Liang, C.; Huang, K.; Yan, S.; Tao, R.; Sheng, J.; Pan, W.; Xia, X.; Tao, Y.; Xiang, H.; et al. Umbilical Serum Copper Status and Neonatal Birth Outcomes: A Prospective Cohort Study. Biol. Trace Elem. Res. 2018, 183, 200–208. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef] [PubMed]
- Uriu-Adams, J.Y.; Scherr, R.E.; Lanoue, L.; Keen, C.L. Influence of copper on early development: Prenatal and postnatal considerations. Biofactors 2010, 36, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Hawk, S.N.; Lanoue, L.; Keen, C.L.; Kwik-Uribe, C.L.; Rucker, R.B.; Uriu-Adams, J.Y. Copper-deficient rat embryos are characterized by low superoxide dismutase activity and elevated superoxide anions. Biol. Reprod. 2003, 68, 896–903. [Google Scholar] [CrossRef] [Green Version]
- Pryde, P.G.; Mittendorf, R. Contemporary usage of obstetric magnesium sulfate: Indication, contradiction, and relevance of dose. Obstet. Gynecol. 2009, 114, 669–673. [Google Scholar] [CrossRef]
- Sherwin, C.M.; Balch, A.; Campbell, S.C.; Fredrickson, J.; Clark, E.A.; Varner, M.; Stockmann, C.; Korgenski, E.K.; Bonkowsky, J.L.; Spigarelli, M.G. Maternal magnesium sulphate exposure predicts neonatal magnesium blood concentrations. Basic Clin. Pharmacol. Toxicol. 2014, 114, 318–322. [Google Scholar] [CrossRef] [Green Version]
- Narasimhulu, D.; Brown, A.; Egbert, N.M.; Rojas, M.; Haberman, S.; Bhutada, A.; Minkoff, H.; Rastogi, S. Maternal magnesium therapy, neonatal serum magnesium concentration and immediate neonatal outcomes. J. Perinatol. 2017, 37, 1297–1303. [Google Scholar] [CrossRef]
- Ambadkar, A.; Prasad, M.; Chauhan, A.R. Neonatal effects of maternal magnesium sulphate in late preterm and term pregnancies. J. Obstet. Gynaecol. India 2019, 69, 25–30. [Google Scholar] [CrossRef]
- Das, M.; Chaudhuri, P.; Mondal, B.C.; Mitra, S.; Bandyopadhyay, D.; Pramanik, S. Assessment of serum magnesium levels and its outcome in neonates of eclamptic mothers treated with low-dose magnesium sulfate regimen. Indian J. Pharmacol. 2015, 47, 502–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbassi-Ghanavati, M.; Alexander, J.; McIntire, D.; Savani, R.C.; Leveno, K.J. Neonatal effects of magnesium sulfate given to the mother. Am. J. Perinatol. 2012, 29, 795–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittendorf, R.; Dambrosia, J.; Pryde, P.G.; Lee, K.S.; Gianopoulos, J.G.; Besinger, R.E.; Tomich, P.G. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. Am. J. Obstet. Gynecol. 2002, 186, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Grisen, A.; Greenberg, M.B.; El-Sayed, Y.Y.; Lee, H.; Carvalho, B.; Lyell, D.J. Magnesium sulphate exposure and neonatal intensive care unit admission at term. J. Perinatol. 2015, 35, 181–185. [Google Scholar] [CrossRef]
- Greenberg, M.B.; Penn, A.A.; Thomas, L.J.; El-Sayed, Y.Y.; Caughey, A.B.; Lyell, D.J. Neonatal medical admission in a term and late-preterm cohort exposed to magnesium sulfate. Am. J. Obstet. Gynecol. 2011, 204, 515.e1–515.e7. [Google Scholar] [CrossRef]
- Greenberg, M.B.; Penn, A.A.; Whitaker, K.R.; Kogut, E.A.; El-Sayed, Y.Y.; Caughey, A.B.; Lyell, D.J. Effect of magnesium sulphate exposure in term neonates. J. Perinatol. 2013, 33, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; El-Dib, M.; Ahmad, T.; Aly, H. Baseline serum magnesium concentrations and neurodevelopmental outcomes of extremely low birth weight premature infants. Early Hum. 2013, 89, 239–242. [Google Scholar] [CrossRef]
- Bonaventura, P.; Benedetti, G.; Albarede, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Beach, R.S.; Gershwin, M.E.; Hurley, L.S. Gestational zinc deprivation in mice: Persistence of immunodeficiency for three generations. Science 1982, 218, 469–471. [Google Scholar] [CrossRef]
- Beach, R.S.; Gershwin, M.E.; Hurley, L.S. Persistent immunological consequences of gestation zinc deprivation. Am. J. Clin. Nutr. 1983, 38, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, L.; Li, C.; Hu, X.; Wang, X.; Huang, Q.; Zhou, G. Dietary zinc deficiency impairs humoral and cellular immune response to BCG and ESAT-6/CFP-10 vaccination in offspring and adult rats. Tuberculosis 2016, 97, 86–96. [Google Scholar] [CrossRef]
- Osendarp, S.J.; West, C.E.; Black, R.E.; Maternal Zinc Supplementation Study Group. The need for maternal zinc supplementation in developing countries: An unresolved issue. J. Nutr. 2003, 1333, 817–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.M.; Hossain, M.B.; Monirujjaman, M.; Islam, S.; Huda, M.N.; Kabir, Y.; Raqib, R.; Lönnerdal, B.L. Maternal zinc supplementation improves hepatitis B antibody response in infants but decreases plasma zinc level. Eur. J. Nutr. 2016, 55, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Osendarp, S.J.; Fuch, G.J.; van Raaij, J.M.A.; Mahmud, H.; Tofail, F.; Black, R.E.; Prabhakar, H.; Santosham, M. The effect of zinc supplementation during pregnancy on immune response to Hib and BCG vaccines in Bangladesh. J. Trop. Pediatr. 2006, 52, 316–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannotti, L.L.; Zavaleta, N.; León, Z.; Huasquiche, C.; Shankar, A.H.; Caulfield, L.E. Maternal zinc supple-mentation reduces diarrheal morbidity in Peruvian infants. J. Pediatr. 2010, 156, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Wieringa, F.T.; Dijkhuizen, M.A.; Muhilal; Meer, J.W. Maternal micronutrient supplementation with zinc and beta-carotene affects morbidity and immune function of infants during the first 6 months of life. Eur. J. Clin. Nutr. 2010, 64, 1072–1079. [Google Scholar] [CrossRef]
- Osendarp, S.J.; van Raaij, J.M.; Darmstadt, G.L.; Baqui, A.H.; Hautvast, J.G.; Fuchs, G.J. Zinc supplementation during pregnancy and effects on growth and morbidity in low birth weight infants: A randomized placebo controlled trial. Lancet 2001, 357, 1080–1085. [Google Scholar] [CrossRef]
- Sharkar, M.T.; Jou, M.Y.; Hossain, M.B.; Lönnerdal, B.L.; Stephensen, C.B.; Ragib, R. Prenatal zinc supplementation of zinc-adequate rats adversely affects immunity in offspring. J. Nutr. 2011, 141, 1559–1564. [Google Scholar] [CrossRef] [Green Version]
- Ragib, R.; Hossain, M.B.; Kelleher, S.L.; Stephensen, C.B.; Lönnerdal, B.L. Zinc supplementation of pregnant rats with adequate zinc nutriture suppresses immune functions in their offspring. J. Nutr. 2007, 137, 1037–1042. [Google Scholar] [CrossRef] [Green Version]
- Shankar, A.H.; Prasad, A.S. Zinc and immune functions: The biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998, 68, 447S–463S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, M.; Qiu, Q.; Pang, X.; Liang, X.; Li, L.; Cui, F.; Wang, F.; Zhang, G.; Li, H.; Wang, L.; et al. Immune response in infants after universal high-dose hepatitis B vaccination: A community-based study in Beijing, China. Vaccine 2015, 33, 5878–5883. [Google Scholar] [CrossRef] [PubMed]
- Mosha, D.; Liu, E.; Hertzmark, E.; Chan, G.; Sudfeld, C.; Masanja, H.; Fawzi, W. Dietary iron and calcium intakes during pregnancy are associated with lower risk of prematurity, stillbirth and neonatal mortality among women in Tanzania. Public Health Nutr. 2016, 20, 678–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
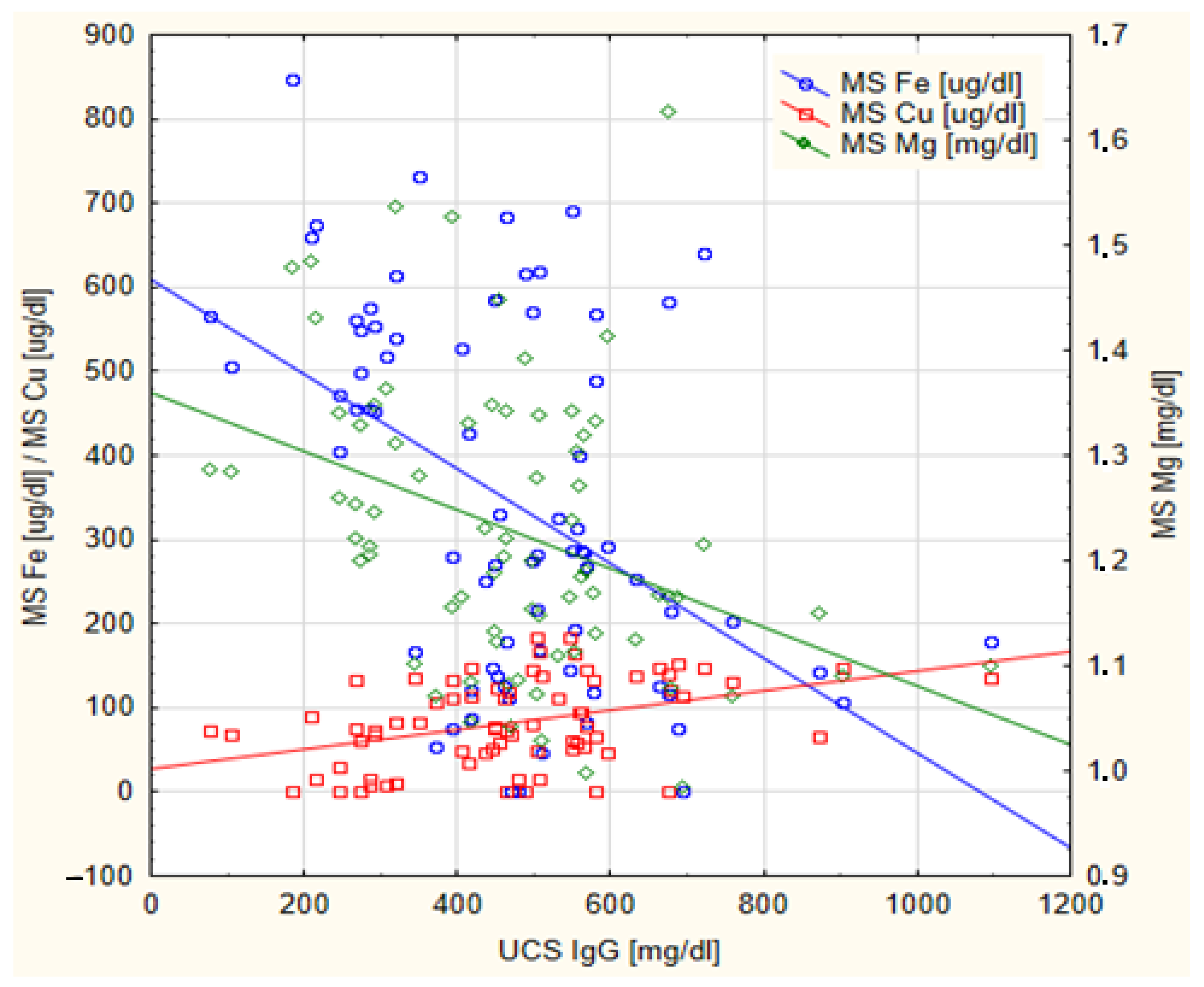
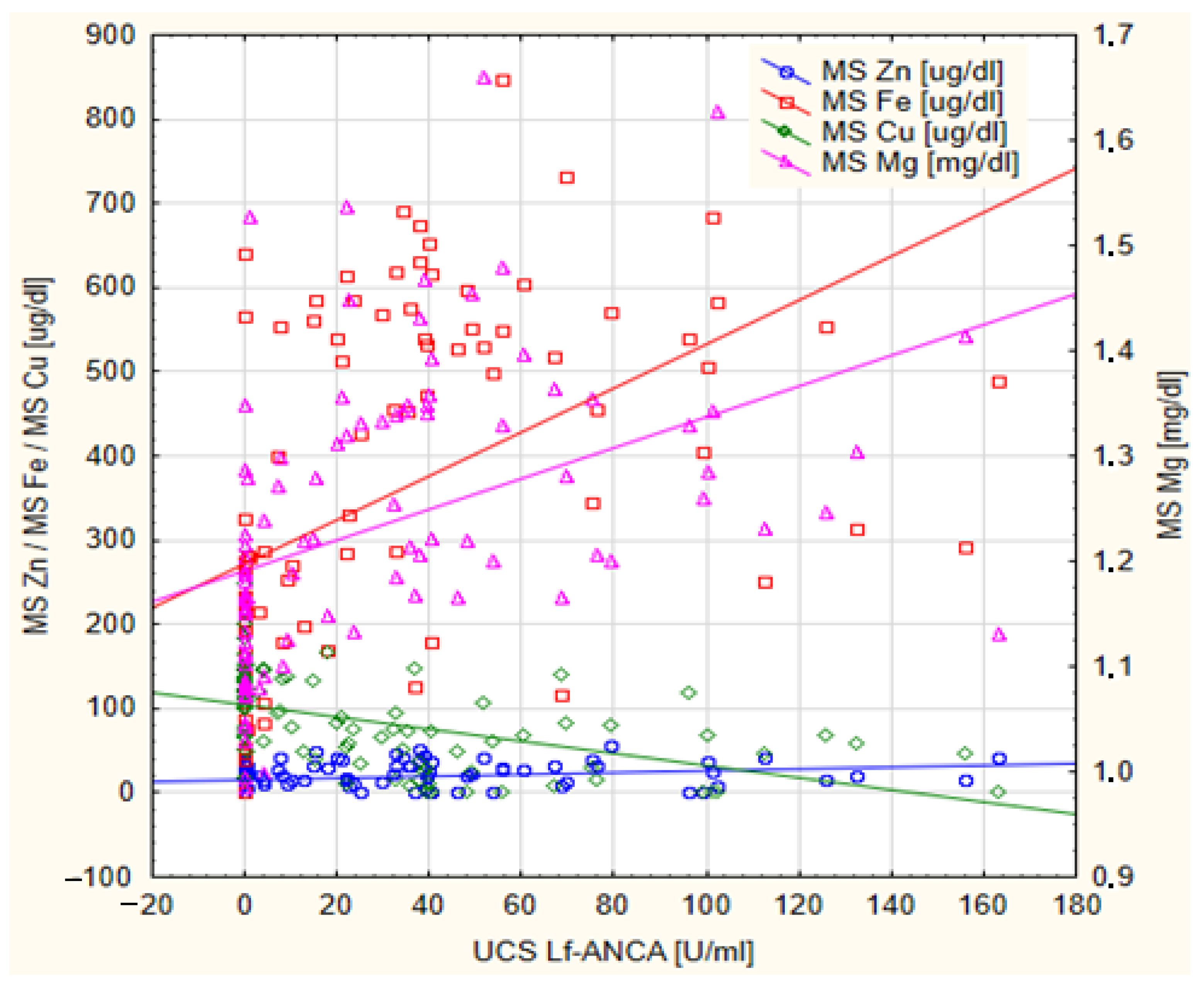
| N | Me | IQR | Min–Max | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Mother’s age [years] | 98 1 | 33.0 | 30.0–35.0 | 19.0–45.0 |
| Pre-pregnancy BMI [kg/m2] | 98 | 22.1 | 20.1–25.1 | 18.0–32.9 |
| MS Mg [mg/dL] 2 | 98 | 1.22 | 1.13–1.33 | 0.99–1.66 |
| MS Ca [mg/dL] | 98 | 17.68 | 15.93–18.96 | 10.18–26.94 |
| MS Zn [µg/dL] | 98 | 18.30 | 1.00–32.10 | 0.001–56.30 |
| MS Fe [µg/dL] | 98 | 331.0 | 167.0–555.0 | 0.001–1603.0 |
| MS Cu [µg/dL] | 98 | 75.85 | 38.00–122.5 | 0.000–248.10 |
| Neonatal characteristics | ||||
| Gestational age [weeks] | 98 | 39.0 | 38.0–39.0 | 37.0–41.0 |
| Birth weight [g] | 98 | 3430.0 | 3150.0–3730.0 | 2510.0–4980.00 |
| UCS IgG [mg/dL] | 77 2 | 480.0 | 370.0–568.0 | 78.0–1477.0 |
| UCS Lf-ANCA [U/mL] | 98 | 17.70 | 0.001–47.80 | 0.001–163.10 |
| Examined Elements in the MS | Insufficient | Adequate | Excessive |
|---|---|---|---|
| Mg [mg/dL] | <1.1 | 1.1–2.2 | >2.2 |
| Ca [mg/dL] | <8.2 | 8.2–9.7 | >9.7 |
| Zn [µg/dL] | <50 | 50–77 | >77 |
| Fe [µg/dL] | <30 | 30–193 | >193 |
| Cu [µg/dL] | <130 | 130–240 | >240 |
| Examined Elements in the MS | UCS IgG [mg/dL] (N = 77) | UCS Lf-ANCA [U/mL] (N = 98) | ||
|---|---|---|---|---|
| Rho | p | Rho | p | |
| Mg [mg/dL] | −0.354 | 0.002 * | 0.589 | <0.001 * |
| Ca [mg/dL] | 0.060 | 0.606 | 0.214 | 0.034 * |
| Zn [ug/dL] | −0.198 | 0.084 | 0.358 | <0.001 * |
| Fe [ug/dL] | −0.368 | <0.001 * | 0.635 | <0.001 * |
| Cu [ug/dL] | 0.361 | 0.001 * | −0.613 | <0.001 * |
| Examined Elements in the MS 3 | UCS IgG [mg/dL] 1 | MW-Z | p | UCS Lf-ANCA [U/mL] 2 | MW-Z | p | ||
|---|---|---|---|---|---|---|---|---|
| Adequate (N = 13) | Insufficient (N = 64) | Adequate (N = 45) | Excessive (N = 53) | |||||
| Me (IQR) | Me (IQR) | Me (IQR) | Me (IQR) | |||||
| Mg [mg/dL] | 1.15 (1.09–1.17) | 1.23 (1.15–1.33) | 1.992 | 0.046 * | 1.13 (1.08–1.19) | 1.31 (1.22–1.36) | −6.195 | <0.001 * |
| Ca [mg/dL] | 16.58 (14.49–20.07) | 17.57 (15.96–18.96) | 0.360 | 0.719 | 16.73 (13.13–18.67) | 17.88 (17.13–19.00) | −2.032 | 0.042 * |
| Zn [ug/dL] | 10.50 (6.20–20.90) | 17.65 (0.50–32.80) | 1.550 | 0.121 | 10.20 (0.00–21.85) | 24.60 (12.10–36.40) | −3.663 | <0.001 * |
| Fe [ug/dL] | 178.0 (117.0–253.0) | 366.0 (174.5–564.0) | 1.992 | 0.046 * | 146.50 (84.50–270.00) | 540.00 (427.0–596.0) | −6.549 | <0.000 * |
| Cu [ug/dL] | 134.90 (114.0–147.80) | 71.05 (39.90–112.65) | −2.584 | 0.010 * | 120.30 (97.80–145.10) | 49.60 (11.40–73.70) | 6.120 | <0.001 * |
| Examined Elements in the MS | UCS IgG [mg/dL] | MW-Z | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Insufficient | Adequate | Excessive | |||||||||
| N | Me | IQR | N | Me | IQR | N | Me | IQR | |||
| Mg [mg/dL] | 13 | 507.00 | 463.00–686.50 | 64 | 462.50 | 313.00–559.50 | 0 | --- | --- | −1.766 | 0.077 |
| Ca [mg/dL] | 0 | --- | --- | 0 | --- | --- | 77 | 469.00 | 351.00–564.00 | --- | --- |
| Zn [µg/dL] | 76 | 468.50 | 348.00–566.00 | 1 | --- | --- | 0 | --- | --- | --- | --- |
| Fe [µg/dL] | 3 | --- | --- | 22 | 509.00 | 445.00–662.00 | 52 | 451.50 | 289.00–557.50 | 2.223 | 0.026 * |
| Cu [µg/dL] | 57 | 450.00 | 307.00–548.00 | 20 | 560.00 | 499.50–681.50 | 0 | --- | --- | −3.166 | 0.002 * |
| Examined Elements in the MS | UCS Lf-ANCA [U/mL] | MW-Z | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Insufficient | Adequate | Excessive | |||||||||
| N | Me | IQR | N | Me | IQR | N | Me | IQR | |||
| Mg [mg/dL] | 16 | 0.00 | 0.00–0.00 | 82 | 30.70 | 0.00–55.50 | 0 | --- | --- | −4.136 | <0.001 * |
| Ca [mg/dL] | 0 | --- | --- | 0 | --- | --- | 98 | 18.75 | 0.00–47.80 | --- | --- |
| Zn [µg/dL] | 95 | 21.45 | 0.00–48.90 | 3 | --- | --- | 0 | --- | --- | --- | --- |
| Fe [µg/dL] | 2 | --- | --- | 29 | 0.00 | 0.00–4.30 | 67 | 36.95 | 12.70–64.40 | −5.007 | <0.001 * |
| Cu [µg/dL] | 75 | 32.80 | 0.00–55.50 | 22 | 0.00 | 0.00–7.95 | 1 | --- | --- | 3.687 | <0.001 * |
| Examined Elements in the MS | UCS IgG [mg/dL] | KW-H | p * | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||||||||||
| N | Me | IQR | N | Me | IQR | N | Me | IQR | N | Me | IQR | |||
| Ca [mg/dL] | 20 | 460.0 | 345.0–576.0 | 19 | 467.0 | 272.0–548.0 | 19 | 462.0 | 292.0–549.0 | 19 | 487.0 | 413.0–597.0 | 3.250 | 0.354 |
| Zn [µg/dL] | 20 | 461.5 | 409.5–541.0 | 19 | 547.0 | 403.0–632.0 | 19 | 505.0 | 418.5–624.0 | 19 | 356.0 | 267.0–503.0 | 7.946 | 0.047 1 |
| Examined Elements in the MS | UCS Lf-ANCA [U/mL] | KW-H | p | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||||||||||
| N | Me | IQR | N | Me | IQR | N | Me | IQR | N | Me | IQR | |||
| Ca [mg/dL] | 23 | 0.00 ∆ | 0.00–23.70 | 25 | 34.30 ∆ | 9.00–55.50 | 25 | 19.80 | 0.80–47.80 | 25 | 24.80 | 0.00–40.60 | 11.628 | 0.009 * |
| Zn [µg/dL] | 25 | 0.00 †◊ | 0.00–24.80 | 24 | 22.03 | 4.18–51.10 | 25 | 35.10 † | 3.25–55.50 | 24 | 36.00 ◊ | 19.8–51.70 | 15.034 | 0.002 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rak, K.; Styczyńska, M.; Godyla-Jabłoński, M.; Bronkowska, M. Some Immune Parameters of Term Newborns at Birth Are Associated with the Concentration of Iron, Copper and Magnesium in Maternal Serum. Nutrients 2023, 15, 1908. https://doi.org/10.3390/nu15081908
Rak K, Styczyńska M, Godyla-Jabłoński M, Bronkowska M. Some Immune Parameters of Term Newborns at Birth Are Associated with the Concentration of Iron, Copper and Magnesium in Maternal Serum. Nutrients. 2023; 15(8):1908. https://doi.org/10.3390/nu15081908
Chicago/Turabian StyleRak, Karolina, Marzena Styczyńska, Michaela Godyla-Jabłoński, and Monika Bronkowska. 2023. "Some Immune Parameters of Term Newborns at Birth Are Associated with the Concentration of Iron, Copper and Magnesium in Maternal Serum" Nutrients 15, no. 8: 1908. https://doi.org/10.3390/nu15081908





