The Effects of Exercise on Appetite-Regulating Hormone Concentrations over a 36-h Fast in Healthy Young Adults: A Randomized Crossover Study
Abstract
:1. Introduction
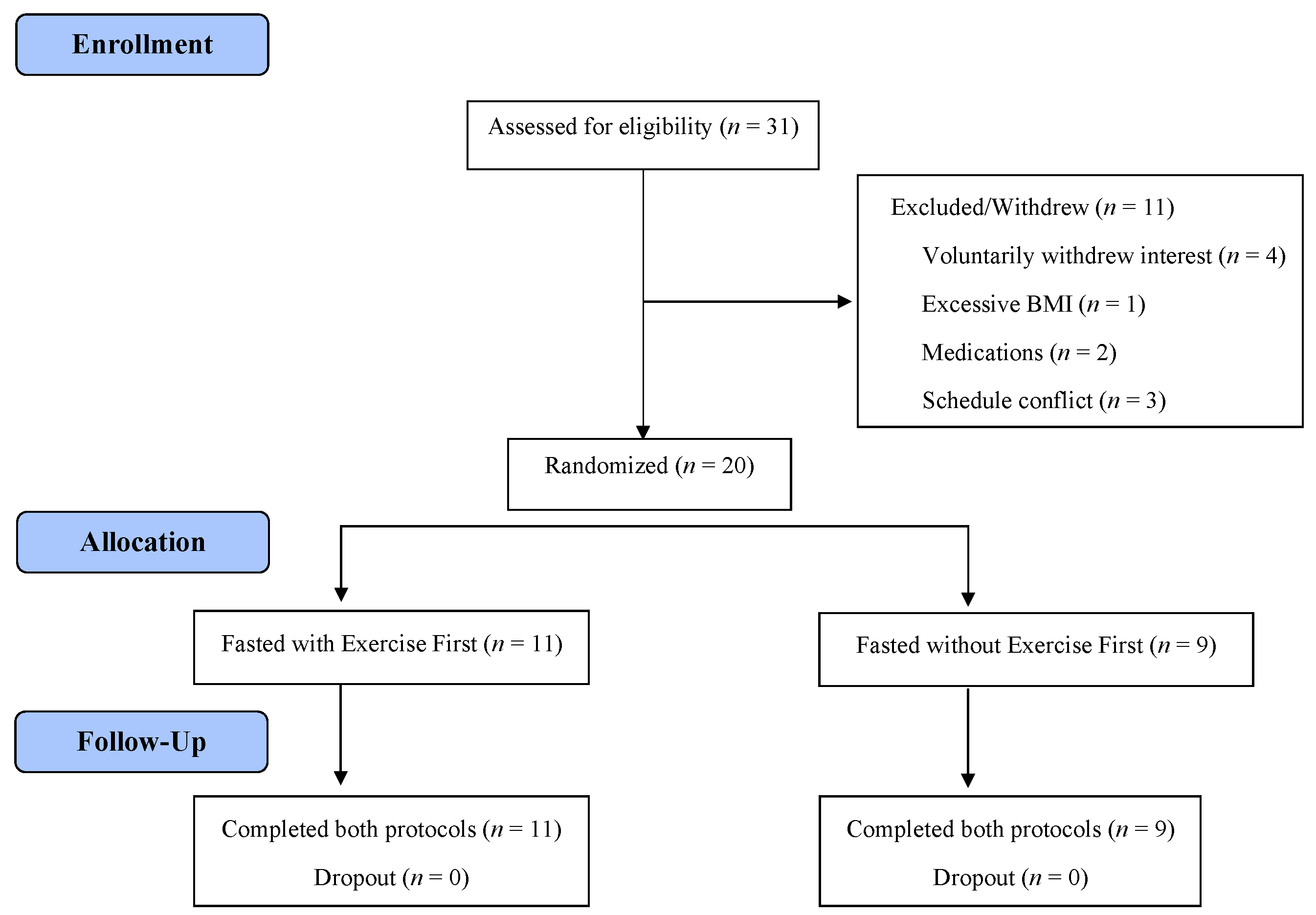
2. Materials and Methods
2.1. Participants
- Diagnosed with a metabolic disease;
- Diagnosed with an orthopedic impairment;
- Diagnosed with an eating disorder;
- Taking metabolism-altering medications [24];
- Consuming more than 60 mg of caffeine daily [25];
- Pregnant or lactating;
- Postmenopausal [26];
- underweight (BMI < 18.5 kg/m2) or obese (BMI > 30 kg/m2) [27];
- Practicing calorie or carbohydrate diets.
2.2. Measurements
2.2.1. Anthropometric Measurements
2.2.2. Plasma Hormone Levels
2.3. Procedures
2.3.1. Screening
2.3.2. Orientation
2.3.3. Standardized Meals
2.3.4. Treatment Sessions
2.3.5. Exercise Protocol
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, S.; Tung, N.; Casanova-Acebes, M.; Chang, C.; Cantoni, C.; Zhang, D.; Wirtz, T.H.; Naik, S.; Rose, S.A.; Brocker, C.N.; et al. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell 2019, 178, 1102–1114.e1117. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Saeed, F.; Arshad, M.U.; Afzaal, M.; Imran, A.; Ali, S.W.; Niaz, B.; Ahmad, A.; Imran, M. Impact of intermittent fasting on human health: An extended review of metabolic cascades. Int. J. Food Prop. 2018, 21, 2700–2713. [Google Scholar] [CrossRef]
- Blundell, J.; de Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; van der Knaap, H.; et al. Appetite control: Methodological aspects of the evaluation of foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef] [Green Version]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef]
- Zanchi, D.; Depoorter, A.; Egloff, L.; Haller, S.; Mählmann, L.; Lang, U.E.; Drewe, J.; Beglinger, C.; Schmidt, A.; Borgwardt, S. The impact of gut hormones on the neural circuit of appetite and satiety: A systematic review. Neurosci. Biobehav. Rev. 2017, 80, 457–475. [Google Scholar] [CrossRef]
- Figlewicz, D.P. Adiposity signals and food reward: Expanding the CNS roles of insulin and leptin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R882–R892. [Google Scholar] [CrossRef] [Green Version]
- Small, C.J.; Bloom, S.R. Gut hormones and the control of appetite. Trends Endocrinol. Metab. 2004, 15, 259–263. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20, 5–21. [Google Scholar] [CrossRef]
- Blundell, J.E.; Gibbons, C.; Caudwell, P.; Finlayson, G.; Hopkins, M. Appetite control and energy balance: Impact of exercise. Obes. Rev. 2015, 16 (Suppl. S1), 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, N.A.; Burley, V.J.; Blundell, J.E. Exercise-induced suppression of appetite: Effects on food intake and implications for energy balance. Eur. J. Clin. Nutr. 1994, 48, 715–724. [Google Scholar]
- Martins, C.; Morgan, L.M.; Bloom, S.R.; Robertson, M.D. Effects of exercise on gut peptides, energy intake and appetite. J. Endocrinol. 2007, 193, 251–258. [Google Scholar] [CrossRef]
- Solomon, T.P.J.; Haus, J.M.; Kelly, K.R.; Cook, M.D.; Filion, J.; Rocco, M.; Kashyap, S.R.; Watanabe, R.M.; Barkoukis, H.; Kirwan, J.P. A low–glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am. J. Clin. Nutr. 2010, 92, 1359–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIver, V.J.; Mattin, L.; Evans, G.H.; Yau, A.M.W. The effect of brisk walking in the fasted versus fed state on metabolic responses, gastrointestinal function, and appetite in healthy men. Int. J. Obes. 2019, 43, 1691–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deighton, K.; Zahra, J.C.; Stensel, D.J. Appetite, energy intake and resting metabolic responses to 60min treadmill running performed in a fasted versus a postprandial state. Appetite 2012, 58, 946–954. [Google Scholar] [CrossRef]
- Cheng, M.H.; Bushnell, D.; Cannon, D.T.; Kern, M. Appetite regulation via exercise prior or subsequent to high-fat meal consumption. Appetite 2009, 52, 193–198. [Google Scholar] [CrossRef]
- Hamilton, C.C.; Wiseman, S.B.; Copeland, J.L.; Bomhof, M.R. Influence of postexercise fasting on hunger and satiety in adults. Appl. Physiol. Nutr. Metab. 2020, 45, 1022–1030. [Google Scholar] [CrossRef]
- Deru, L.S.; Bikman, B.T.; Davidson, L.E.; Tucker, L.A.; Fellingham, G.; Bartholomew, C.L.; Yuan, H.L.; Bailey, B.W. The Effects of Exercise on β-Hydroxybutyrate Concentrations over a 36-h Fast: A Randomized Crossover Study. Med. Sci. Sports Exerc. 2021, 53, 1987–1998. [Google Scholar] [CrossRef]
- Ball, J.R. Effect of eating at various times on subsequent performances in swimming. N. Y. State J. Med. 1963, 63, 600–603. [Google Scholar] [CrossRef]
- Suresh, K. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J. Hum. Reprod. Sci. 2011, 4, 8–11. [Google Scholar] [CrossRef]
- Warburton, D.E.; Bredin, S.S.; Charlesworth, S.A.; Foulds, H.J.; McKenzie, D.C.; Shephard, R.J. Evidence-based risk recommendations for best practices in the training of qualified exercise professionals working with clinical populations. Appl. Physiol. Nutr. Metab. 2011, 36 (Suppl. S1), S232–S265. [Google Scholar] [CrossRef] [PubMed]
- Verhaegen, A.A.; Van Gaal, L.F. Drug-induced obesity and its metabolic consequences: A review with a focus on mechanisms and possible therapeutic options. J. Endocrinol. Investig. 2017, 40, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef] [Green Version]
- Morrow, P.G.; Marshall, W.P.; Kim, H.J.; Kalkhoff, R.K. Metabolic response to starvation. II. Effects of sex steroid administration to pre- and postmenopausal women. Metabolism 1981, 30, 274–278. [Google Scholar] [CrossRef]
- National Institutes of Health. Available online: https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmicalc.htm (accessed on 12 August 2022).
- Bailey, B.W.; Le Cheminant, G.; Hope, T.; Bell, M.; Tucker, L.A. A comparison of the agreement, internal consistency, and 2-day test stability of the InBody 720, GE iDXA, and BOD POD®gold standard for assessing body composition. Meas. Phys. Educ. Exerc. Sci. 2018, 22, 231–238. [Google Scholar] [CrossRef]
- Hall, K.D.; Sacks, G.; Chandramohan, D.; Chow, C.C.; Wang, Y.C.; Gortmaker, S.L.; Swinburn, B.A. Obesity 3 Quantification of the effect of energy imbalance on bodyweight. Lancet 2011, 378, 826–837. [Google Scholar] [CrossRef] [Green Version]
- Bailey, B.W.; Allen, M.D.; LeCheminant, J.D.; Tucker, L.A.; Errico, W.K.; Christensen, W.F.; Hill, M.D. Objectively Measured Sleep Patterns in Young Adult Women and the Relationship to Adiposity. Am. J. Heal. Promot. 2014, 29, 46–54. [Google Scholar] [CrossRef]
- Plumelle, D.; Lombard, E.; Nicolay, A.; Portugal, H. Influence of diet and sample collection time on 77 laboratory tests on healthy adults. Clin. Biochem. 2014, 47, 31–37. [Google Scholar] [CrossRef]
- Jetté, M.; Sidney, K.; Blümchen, G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin. Cardiol. 1990, 13, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Purdom, T.; Kravitz, L.; Dokladny, K.; Mermier, C. Understanding the factors that effect maximal fat oxidation. J. Int. Soc. Sports Nutr. 2018, 15, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solheim, T.J.; Keller, B.G.; Fountaine, C.J. VO2 Reserve vs. Heart Rate Reserve During Moderate Intensity Treadmill Exercise. Int. J. Exerc. Sci. 2014, 7, 311–317. [Google Scholar] [PubMed]
- Roy, S.; Mccrory, J. Validation of Maximal Heart Rate Prediction Equations Based on Sex and Physical Activity Status. Int. J. Exerc. Sci. 2015, 8, 318–330. [Google Scholar] [PubMed]
- Franklin, B.A.; Brinks, J.; Berra, K.; Lavie, C.J.; Gordon, N.F.; Sperling, L.S. Using Metabolic Equivalents in Clinical Practice. Am. J. Cardiol. 2018, 121, 382–387. [Google Scholar] [CrossRef] [Green Version]
- Patterson, R.E.; Laughlin, G.A.; LaCroix, A.Z.; Hartman, S.J.; Natarajan, L.; Senger, C.M.; Martínez, M.E.; Villaseñor, A.; Sears, D.D.; Marinac, C.R.; et al. Intermittent Fasting and Human Metabolic Health. J. Acad. Nutr. Diet. 2015, 115, 1203–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holliday, A.; Blannin, A.K. Very Low Volume Sprint Interval Exercise Suppresses Subjective Appetite, Lowers Acylated Ghrelin, and Elevates GLP-1 in Overweight Individuals: A Pilot Study. Nutrients 2017, 9, 362. [Google Scholar] [CrossRef] [Green Version]
- Ueda, S.-Y.; Yoshikawa, T.; Katsura, Y.; Usui, T.; Fujimoto, S. Comparable effects of moderate intensity exercise on changes in anorectic gut hormone levels and energy intake to high intensity exercise. J. Endocrinol. 2009, 203, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Hamasaki, H. Exercise and glucagon-like peptide-1: Does exercise potentiate the effect of treatment? World J. Diabetes 2018, 9, 138–140. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Hazell, T.J.; Islam, H.; Townsend, L.K.; Schmale, M.S.; Copeland, J.L. Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: Potential mechanisms. Appetite 2016, 98, 80–88. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Wasse, L.K.; Stensel, D.J.; Nimmo, M.A. Exercise and ghrelin. A narrative overview of research. Appetite 2013, 68, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Broom, D.R.; Stensel, D.J.; Bishop, N.C.; Burns, S.F.; Miyashita, M. Exercise-induced suppression of acylated ghrelin in humans. J. Appl. Physiol. 2007, 102, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, J.; Tahbaz, R.; Lippl, F.; Wagenpfeil, S.; Schusdziarra, V. Plasma ghrelin levels during exercise—Effects of intensity and duration. Regul. Pept. 2007, 143, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.F.; Broom, D.R.; Miyashita, M.; Mundy, C.; Stensel, D.J. A single session of treadmill running has no effect on plasma total ghrelin concentrations. J. Sports Sci. 2007, 25, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Golubeva, A.V.; Zhdanov, A.V.; Wallace, S.; Arboleya, S.; Papkovsky, D.B.; El Aidy, S.; Ross, P.; Roy, B.L.; Stanton, C.; et al. Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. FASEB J. 2019, 33, 13546–13559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin Is a Growth-Hormone-Releasing Acylated Peptide from Stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Shiiya, T.; Ueno, H.; Toshinai, K.; Kawagoe, T.; Naito, S.; Tobina, T.; Nishida, Y.; Shindo, M.; Kangawa, K.; Tanaka, H.; et al. Significant lowering of plasma ghrelin but not des-acyl ghrelin in response to acute exercise in men. Endocr. J. 2011, 58, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Halliday, T.M.; White, M.H.; Hild, A.K.; Conroy, M.B.; Melanson, E.L.; Cornier, M.A. Appetite and Energy Intake Regulation in Response to Acute Exercise. Med. Sci. Sports Exerc. 2021, 53, 2173–2181. [Google Scholar] [CrossRef]
- Kelly, K.R.; Brooks, L.M.; Solomon, T.P.J.; Kashyap, S.R.; O’Leary, V.B.; Kirwan, J.P. The glucose-dependent insulinotropic polypeptide and glucose-stimulated insulin response to exercise training and diet in obesity. Am. J. Physiol-Endoc. Metab. 2009, 296, E1269–E1274. [Google Scholar] [CrossRef] [Green Version]
- Bouassida, A.; Chamari, K.; Zaouali, M.; Feki, Y.; Zbidi, A.; Tabka, Z. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br. J. Sports Med. 2010, 44, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Hallworth, J.R.; Copeland, J.L.; Doan, J.; Hazell, T.J. The Effect of Exercise Intensity on Total PYY and GLP-1 in Healthy Females: A Pilot Study. J. Nutr. Metab. 2017, 2017, 4823102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, C.S.; Clark, J.; Wagenmakers, A.J. The Effect of Exercise and Nutrition on Intramuscular Fat Metabolism and Insulin Sensitivity. Annu. Rev. Nutr. 2010, 30, 13–34. [Google Scholar] [CrossRef]
- Richter, E.A. Is GLUT4 translocation the answer to exercise-stimulated muscle glucose uptake? Am. J. Physiol. Endocrinol. Metab. 2021, 320, E240–E243. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Rynders, C.A.; Weltman, J.Y.; Barrett, E.J.; Weltman, A. Exercise Intensity Modulates Glucose-Stimulated Insulin Secretion when Adjusted for Adipose, Liver and Skeletal Muscle Insulin Resistance. PLOS ONE 2016, 11, e0154063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guelfi, K.J.; Donges, C.E.; Duffield, R. Beneficial effects of 12 weeks of aerobic compared with resistance exercise training on perceived appetite in previously sedentary overweight and obese men. Metab. Clin. Exp. 2013, 62, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Zouhal, H.; Bagheri, R.; Triki, R.; Saeidi, A.; Wong, A.; Hackney, A.C.; Laher, I.; Suzuki, K.; Ben Abderrahman, A. Effects of Ramadan Intermittent Fasting on Gut Hormones and Body Composition in Males with Obesity. Int. J. Environ. Res. Public Health 2020, 17, 5600. [Google Scholar] [CrossRef] [PubMed]
- Hoddy, K.K.; Gibbons, C.; Kroeger, C.M.; Trepanowski, J.F.; Barnosky, A.; Bhutani, S.; Gabel, K.; Finlayson, G.; Varady, K.A. Changes in hunger and fullness in relation to gut peptides before and after 8 weeks of alternate day fasting. Clin. Nutr. 2016, 35, 1380–1385. [Google Scholar] [CrossRef]
- Espelund, U.; Hansen, T.K.; Højlund, K.; Beck-Nielsen, H.; Clausen, J.T.; Hansen, B.S.; Ørskov, H.; Jørgensen, J.O.L.; Frystyk, J. Fasting Unmasks a Strong Inverse Association between Ghrelin and Cortisol in Serum: Studies in Obese and Normal-Weight Subjects. J. Clin. Endocrinol. Metab. 2005, 90, 741–746. [Google Scholar] [CrossRef] [Green Version]
- Brennan, I.M.; Feltrin, K.L.; Nair, N.S.; Hausken, T.; Little, T.J.; Gentilcore, D.; Wishart, J.M.; Jones, K.L.; Horowitz, M.; Feinle-Bisset, C. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am. J. Physiol-Gast. 2009, 297, G602–G610. [Google Scholar] [CrossRef] [Green Version]

| Male (n = 11) | Female (n = 9) | Cumulative (n = 20) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 26.5 | 7.1 | 25.8 | 4.3 | 26.2 | 5.8 |
| BMI (kg/m2) | 24.7 | 3.1 | 22.7 | 3.6 | 23.8 | 3.4 |
| BF % | 18.8 | 6.3 | 27.8 | 4.5 | 22.9 | 7.1 |
| Visceral Adipose (g) | 443.6 | 275.5 | 133.5 | 116.6 | 304.0 | 265.9 |
| Exercise Time (min) | 49.3 | 5.9 | 54.7 | 7.8 | 51.7 | 7.2 |
| Ethnicity | n | % | n | % | n | % |
| Asian | 1 | 9 | 2 | 22 | 3 | 15 |
| Caucasian | 8 | 73 | 7 | 78 | 15 | 75 |
| Hawaiian/Pacific Islander | 2 | 18 | 0 | 0 | 2 | 10 |
| Hormone | Condition | 0 h * | 12 h | 24 h | 36 h | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| Insulin (pg/mL) | No Exercise | 2163.7 ± 1501.9 a | 1584.3 ± 1429.9 b | 1530.4 ± 1529.1 b | 1654.7 ± 1495.4 b | 0.79 | 0.4568 |
| Exercise | 2343.9 ± 1556.8 a | 1635.8 ± 1484.8 b | 1522.8 ± 1506.6 b | 1572.6 ± 1506.0 b | |||
| GIP (pg/mL) | No Exercise | 486.7 ± 231.8 a | 65.0 ± 27.6 b | 53.4 ± 28.6 b | 65.4 ±28.4 b | 2.82 | 0.0658 |
| Exercise | 550.7 ±242.4 a | 81.8 ± 30.9 b | 47.3 ± 19.9 c | 66.0 ± 26.5 d | |||
| GLP1 (pg/mL) | No Exercise | 454.1 ± 126.9 a | 307.5 ± 139.3 b | 381.1 ± 183.6 b | 563.4 ± 287.1 c† | 0.20 | 0.8226 |
| Exercise | 450.8 ± 143.4 a | 387.1 ± 158.8 b | 447.5 ± 185.2 | 672.9 ± 344.8 c | |||
| Ghrelin (pg/mL) | No Exercise | 38.9 ± 31.9 a | 45.9 ± 35.4 b† | 44.1 ± 38.5 b† | 28.4 ± 31.3 c | 3.22 | 0.0457 |
| Exercise | 38.0 ± 32.2 a | 31.8 ± 25.0 ab | 31.4 ± 26.8 ab | 28.2 ± 31.5 b | |||
| PP (pg/mL) | No Exercise | 251.0 ± 138.9 a | 37.7 ± 31.5 b | 140.4 ± 105.3 c | 84.4 ± 81.3 b | 0.09 | 0.9107 |
| Exercise | 248.1 ± 142.8 a | 40.2 ± 32.8 b | 149.7 ± 111.5 cd | 101.9 ± 114.4 d | |||
| PYY (pg/mL) | No Exercise | 274.2 ± 186.1 a | 249.6 ± 196.5 b | 233.5 ± 196.9 c | 242.6 ± 195.8 bc | 1.79 | 0.1750 |
| Exercise | 272.9 ± 180.7 a | 259.7 ± 190.1 b | 227.0 ± 201.7 c | 242.9 ± 203.5 d | |||
| Leptin (pg/mL) | No Exercise | 4015.2 ± 4488.0 a | 3542.3 ± 3820.6 b | 1382.0 ± 1426.8 c | 868.9 ± 1007.6 d | 0.00 | 0.9954 |
| Exercise | 4152.3 ± 4235.3 a | 3481.2 ± 3609.3 b | 1307.0 ± 1562.8 c | 844.8 ± 1011.3 d |
| Fasting and Exercise (pg/mL2) | Fasting without Exercise (pg/mL2) | Change Scores | F-Values | p-Values | |
|---|---|---|---|---|---|
| Ghrelin | −204.6 | 8.40 | 0.0105 | ||
| GIP | 535.9 | 0.34 | 0.5676 | ||
| GLP1 | 1868 | 4.82 | 0.0422 | ||
| Insulin | 1612.6 | 2.90 | 0.1070 | ||
| PP | 303 | 2.92 | 0.1055 | ||
| PYY | 36.9 | 1.22 | 0.2879 | ||
| Leptin | 89,348 ± 3471.94 | 86,018 ± 3473.66 | 3330.80 | 0.66 | 0.4287 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deru, L.S.; Chamberlain, C.J.; Lance, G.R.; Gipson, E.Z.; Bikman, B.T.; Davidson, L.E.; Tucker, L.A.; Coleman, J.L.; Bailey, B.W. The Effects of Exercise on Appetite-Regulating Hormone Concentrations over a 36-h Fast in Healthy Young Adults: A Randomized Crossover Study. Nutrients 2023, 15, 1911. https://doi.org/10.3390/nu15081911
Deru LS, Chamberlain CJ, Lance GR, Gipson EZ, Bikman BT, Davidson LE, Tucker LA, Coleman JL, Bailey BW. The Effects of Exercise on Appetite-Regulating Hormone Concentrations over a 36-h Fast in Healthy Young Adults: A Randomized Crossover Study. Nutrients. 2023; 15(8):1911. https://doi.org/10.3390/nu15081911
Chicago/Turabian StyleDeru, Landon S., Coleton J. Chamberlain, Garrett R. Lance, Elizabeth Z. Gipson, Benjamin T. Bikman, Lance E. Davidson, Larry A. Tucker, Jacob L. Coleman, and Bruce W. Bailey. 2023. "The Effects of Exercise on Appetite-Regulating Hormone Concentrations over a 36-h Fast in Healthy Young Adults: A Randomized Crossover Study" Nutrients 15, no. 8: 1911. https://doi.org/10.3390/nu15081911








