Safety and Tolerability of Whole Soybean Products: A Dose-Escalating Clinical Trial in Older Adults with Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Study Procedures
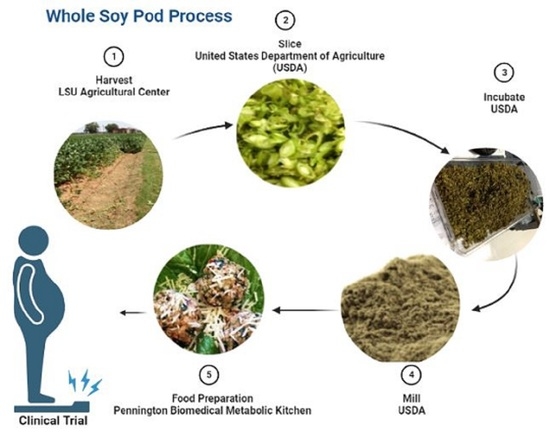
2.4. Whole Green Soybean Flour (WGS)
2.5. Diet Intervention
2.6. Food Tolerability
2.7. Adverse Events
2.8. Statistical Analysis
2.9. Study to Evaluate Nutrition and Glyceollin Content of LSS-G
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef]
- Guo, X.F.; Li, J.M.; Tang, J.; Li, D. Effects of resveratrol supplementation on risk factors of non-communicable diseases: A meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2018, 58, 3016–3029. [Google Scholar] [CrossRef]
- Kim, H.J.; Lim, J.S.; Kim, W.K.; Kim, J.S. Soyabean glyceollins: Biological effects and relevance to human health. Proc. Nutr. Soc. 2012, 71, 166–174. [Google Scholar] [CrossRef]
- Boue, S.; Fortgang, I.; Levy, R.J., Jr.; Bhatnagar, D.; Burow, M.; Fahey, G.; Heiman, M.L. A novel gastrointestinal microbiome modulator from soy pods reduces absorption of dietary fat in mice. Obesity 2016, 24, 87–95. [Google Scholar] [CrossRef]
- Boue, S.M.; Isakova, I.A.; Burow, M.E.; Cao, H.; Bhatnagar, D.; Sarver, J.G.; Shinde, K.V.; Erhardt, P.W.; Heiman, M.L. Glyceollins, soy isoflavone phytoalexins, improve oral glucose disposal by stimulating glucose uptake. J. Agric. Food Chem. 2012, 60, 6376–6382. [Google Scholar] [CrossRef]
- Park, S.; Ahn, I.S.; Kim, J.H.; Lee, M.R.; Kim, J.S.; Kim, H.J. Glyceollins, one of the phytoalexins derived from soybeans under fungal stress, enhance insulin sensitivity and exert insulinotropic actions. J. Agric. Food Chem. 2010, 58, 1551–1557. [Google Scholar] [CrossRef]
- Slavin, J. Nutritional benefits of soy protein and soy fiber. J. Am. Diet. Assoc. 1991, 91, 816–819. [Google Scholar] [CrossRef]
- Asbaghi, O.; Ashtary-Larky, D.; Mousa, A.; Kelishadi, M.R.; Moosavian, S.P. The Effects of Soy Products on Cardiovascular Risk Factors in Patients with Type 2 Diabetes: A Systematic Review and Meta-analysis of Clinical Trials. Adv. Nutr. 2021, 13, 455–473. [Google Scholar] [CrossRef]
- Munro, I.C.; Harwood, M.; Hlywka, J.J.; Stephen, A.M.; Doull, J.; Flamm, W.G.; Adlercreutz, H. Soy isoflavones: A safety review. Nutr. Rev. 2003, 61, 1–33. [Google Scholar] [CrossRef]
- Fitzpatrick, L.A. Soy isoflavones: Hope or hype? Maturitas 2008, 61, 132–140. [Google Scholar] [CrossRef]
- Wuttke, W.; Jarry, H.; Seidlova-Wuttke, D. Isoflavones—Safe food additives or dangerous drugs? Ageing Res. Rev. 2007, 6, 150–188. [Google Scholar] [CrossRef]
- Barreto, N.M.B.; Sandora, D.; Braz, B.F.; Santelli, R.E.; de Oliveira Silva, F.; Monteiro, M.; Perrone, D. Biscuits Prepared with Enzymatically-Processed Soybean Meal Are Rich in Isoflavone Aglycones, Sensorially Well-Accepted and Stable during Storage for Six Months. Molecules 2022, 27, 7975. [Google Scholar] [CrossRef]
- Grabitske, H.A.; Slavin, J.L. Gastrointestinal effects of low-digestible carbohydrates. Crit. Rev. Food Sci. Nutr. 2009, 49, 327–360. [Google Scholar] [CrossRef]
- USDA. What We Eat in America, 2013–2016. Available online: https://data.nal.usda.gov/dataset/what-we-eat-america-wweia-database (accessed on 2 September 2022).
- National Academies Press. Dietary Reference Intakes for Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids (Macronutrients). Available online: http://nap.edu/10490 (accessed on 28 December 2022).
- Foster, D.M.; Vicini, P. Non-compartmental and compartmental approaches to pharmacokinetic data analysis. In Principles of Clinical Pharmacology, 3rd ed.; Atkinson, A.J., Jr., Huang, S.-M., Lertora, J.J.L., Markey, S.P., Eds.; Academic Press: Oxford, UK, 2012; pp. 97–116. [Google Scholar]
- Ivy, S.P.; Siu, L.L.; Garrett-Mayer, E.; Rubinstein, L. Approaches to phase 1 clinical trial design focused on safety, efficiency, and selected patient populations: A report from the clinical trial design task force of the national cancer institute investigational drug steering committee. Clin. Cancer Res. 2010, 16, 1726–1736. [Google Scholar] [CrossRef]
- Hernot, D.C.; Boileau, T.W.; Bauer, L.L.; Middelbos, I.S.; Murphy, M.R.; Swanson, K.S.; Fahey, G.C., Jr. In vitro fermentation profiles, gas production rates, and microbiota modulation as affected by certain fructans, galactooligosaccharides, and polydextrose. J. Agric. Food Chem. 2009, 57, 1354–1361. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Arlington, TX, USA, 2019; Volume 1. [Google Scholar]
- Bovenschen, H.J.; Janssen, M.J.; van Oijen, M.G.; Laheij, R.J.; van Rossum, L.G.; Jansen, J.B. Evaluation of a gastrointestinal symptoms questionnaire. Dig. Dis. Sci. 2006, 51, 1509–1515. [Google Scholar] [CrossRef]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.L.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and Pharmacokinetics of Naringenin: A Randomized, Controlled, Single Ascending Dose, Clinical Trial. Diabetes Obes. Metab. 2019, in press. [CrossRef]
- FDA. Good Review Practice: Clinical Review of Investigational New Drug Applications. Available online: https://www.fda.gov/media/87621/download (accessed on 12 October 2022).
- United States Department of Agriculture, FSIS Environmental Safety and Health Group: Health Hazard Information Sheet: Peroxyacetic Acid (PAA). Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2020-08/Peroxyacetic-Acid.pdf (accessed on 28 December 2022).
- Akhlaghi, M.; Zare, M.; Nouripour, F. Effect of Soy and Soy Isoflavones on Obesity-Related Anthropometric Measures: A Systematic Review and Meta-analysis of Randomized Controlled Clinical Trials. Adv. Nutr. 2017, 8, 705–717. [Google Scholar] [CrossRef]
- Hermansen, K.; Sondergaard, M.; Hoie, L.; Carstensen, M.; Brock, B. Beneficial effects of a soy-based dietary supplement on lipid levels and cardiovascular risk markers in type 2 diabetic subjects. Diabetes Care 2001, 24, 228–233. [Google Scholar] [CrossRef]
- van Nielen, M.; Feskens, E.J.; Rietman, A.; Siebelink, E.; Mensink, M. Partly replacing meat protein with soy protein alters insulin resistance and blood lipids in postmenopausal women with abdominal obesity. J. Nutr. 2014, 144, 1423–1429. [Google Scholar] [CrossRef]
- Meyer, B.J.; Larkin, T.A.; Owen, A.J.; Astheimer, L.B.; Tapsell, L.C.; Howe, P.R. Limited lipid-lowering effects of regular consumption of whole soybean foods. Ann. Nutr. Metab. 2004, 48, 67–78. [Google Scholar] [CrossRef]
- Wiseman, H.; Casey, K.; Bowey, E.A.; Duffy, R.; Davies, M.; Rowland, I.R.; Lloyd, A.S.; Murray, A.; Thompson, R.; Clarke, D.B. Influence of 10 wk of soy consumption on plasma concentrations and excretion of isoflavonoids and on gut microflora metabolism in healthy adults. Am. J. Clin. Nutr. 2004, 80, 692–699. [Google Scholar] [CrossRef]
- Acharjee, S.; Zhou, J.R.; Elajami, T.K.; Welty, F.K. Effect of soy nuts and equol status on blood pressure, lipids and inflammation in postmenopausal women stratified by metabolic syndrome status. Metabolism 2015, 64, 236–243. [Google Scholar] [CrossRef]
- Reverri, E.J.; LaSalle, C.D.; Franke, A.A.; Steinberg, F.M. Soy provides modest benefits on endothelial function without affecting inflammatory biomarkers in adults at cardiometabolic risk. Mol. Nutr. Food Res. 2015, 59, 323–333. [Google Scholar] [CrossRef]
- Welty, F.K.; Lee, K.S.; Lew, N.S.; Zhou, J.R. Effect of soy nuts on blood pressure and lipid levels in hypertensive, prehypertensive, and normotensive postmenopausal women. Arch. Intern. Med. 2007, 167, 1060–1067. [Google Scholar] [CrossRef]
- Padhi, E.M.; Blewett, H.J.; Duncan, A.M.; Guzman, R.P.; Hawke, A.; Seetharaman, K.; Tsao, R.; Wolever, T.M.; Ramdath, D.D. Whole Soy Flour Incorporated into a Muffin and Consumed at 2 Doses of Soy Protein Does Not Lower LDL Cholesterol in a Randomized, Double-Blind Controlled Trial of Hypercholesterolemic Adults. J. Nutr. 2015, 145, 2665–2674. [Google Scholar] [CrossRef]
- Matthan, N.R.; Jalbert, S.M.; Ausman, L.M.; Kuvin, J.T.; Karas, R.H.; Lichtenstein, A.H. Effect of soy protein from differently processed products on cardiovascular disease risk factors and vascular endothelial function in hypercholesterolemic subjects. Am. J. Clin. Nutr. 2007, 85, 960–966. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Kim, J.H.; Kim, J.S.; Kim, H.J. Glyceollin-containing fermented soybeans improve glucose homeostasis in diabetic mice. Nutrition 2012, 28, 204–211. [Google Scholar] [CrossRef]
- USDA. Food Data Central, Search Results Soybeans, Green, Raw. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169282/nutrients (accessed on 21 February 2022).
- Rebello, C.J.; Greenway, F.L.; Finley, J.W. A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities. Obes. Rev. 2014, 15, 392–407. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- Guo, M. Chapter 7—Soy Food Products and Their Health Benefits. In Functional Foods; Guo, M., Ed.; Woodhead Publishing: Cambridge, UK, 2009; pp. 237–277. [Google Scholar] [CrossRef]
- Arntfield, S.D. 7—Proteins from oil-producing plants. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 187–221. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef]
| Age a | 73 (2) |
| Body mass index (kg/m2) a | 34.0 (2.8) |
| Gender | |
| Females | 3 |
| Males | 4 |
| Race | |
| White | 5 |
| Black | 2 b |
| 1 | Lime pound cake |
| 2 | Banana bread |
| 3 | Corn bread |
| 4 | Spinach tomato quiche |
| 5 | Tomato soup |
| 6 | Chili with carrots and corn |
| 7 | Apple cinnamon muffin |
| 8 | Crackers |
| 9 | Herbed tomato bread |
| 10 | Berry banana shake |
| 11 | Vanilla peach shake |
| 12 | Mocha shake |
| 13 | Tropical lime shake |
| 14 | Blueberry shake |
| Event | Baseline | Week 1 | Week 2 | Week 3 |
|---|---|---|---|---|
| Bloating | ||||
| Mild | 1 | 1 | 0 | 1 |
| None | 6 | 6 | 7 | 6 |
| Total Participants | 7 | 7 | 7 | 7 |
| Abdominal Rumbling | ||||
| Mild | 1 | 2 | 1 | 1 |
| None | 6 | 5 | 6 | 6 |
| Total | 7 | 7 | 7 | 7 |
| Flatulence | ||||
| Mild | 4 | 2 | 5 | 5 |
| None | 3 | 5 | 2 | 2 |
| Total Participants | 7 | 7 | 7 | 7 |
| Abdominal Pain | ||||
| Mild | 0 | 0 | 0 | 1 |
| None | 7 | 7 | 7 | 6 |
| Total Participants | 7 | 7 | 7 | 7 |
| Stool Consistency | ||||
| 1 | 0 | 0 | 0 | 1 * |
| 3 | 2 | 2 | 0 | 0 |
| 4 | 3 | 3 | 5 | 2 |
| 5 | 2 | 2 | 2 | 4 |
| Total | 7 | 7 | 7 | 7 |
| Stool Frequency | ||||
| 1–2 | 1 | 2 | 1 | 1 |
| 3–4 | 3 | 4 | 5 | 3 |
| 5–6 | 3 | 1 | 1 | 3 |
| Total Participants | 7 | 7 | 7 | 7 |
| Biomarker | Baseline | Week 3 | p-Value |
|---|---|---|---|
| Chemistry-15 Panel | |||
| Glucose (mg/dL) | 94.57 ± 3.62 | 99.29 ± 3.62 | 0.28 |
| Creatinine (mg/dL) | 0.83 ± 0.08 | 0.91 ± 0.08 | 0.02 * |
| Potassium (mmol/L) | 4.23 ± 0.15 | 4.28 ± 0.15 | 0.69 |
| Uric acid (mg/dL) | 6.06 ± 0.53 | 5.97 ± 0.53 | 0.78 |
| Albumin (g/dL) | 4.20 ± 0.09 | 3.96 ± 0.09 | 0.01 * |
| Calcium (mg/dL) | 9.55 ± 0.10 | 9.21 ± 0.10 | <0.01 * |
| Magnesium (mg/dL) | 2.04 ± 0.06 | 2.03 ± 0.06 | 0.81 |
| Creatine phosphokinase (IU/L) | 186.71 ± 56.60 | 172.57 ± 56.60 | 0.49 |
| Alanine transaminase (IU/L) | 25.71 ± 3.67 | 26.0 ± 3.67 | 0.86 |
| Alkaline phosphatase (IU/L) | 72.14 ± 7.17 | 67.14 ± 7.17 | 0.19 |
| Iron (µg/dL) | 97.13 ± 9.54 | 84.14 ± 9.54 | 0.05 |
| Total cholesterol (mg/dL) | 173.57 ± 20.73 | 173.71 ± 20.73 | 0.98 |
| High density lipoprotein cholesterol (mg/dL) | 52.71 ± 3.34 | 48.67 ± 3.34 | <0.01 * |
| Low density lipoprotein cholesterol (mg/dL) | 99.49 ± 17.14 | 97.16 ± 17.14 | 0.66 |
| Triglycerides (mg/dL) | 106.86 ± 19.20 | 139.43 ± 19.20 | 0.04 * |
| Complete Blood Count | |||
| Hemoglobin (g/dL) | 13.17 ± 0.41 | 12.81 ± 0.41 | 0.20 |
| Hematocrit (%) | 39.59 ± 1.07 | 38.5 ± 1.07 | 0.16 |
| Mean cell volume (fL) | 87.46 ± 1.68 | 86.87 ± 1.68 | 0.30 |
| Platelet count (×103 cells/µL) | 242.14 ± 20.11 | 231.86 ± 20.11 | 0.30 |
| White blood cell count | 6.01 ± 0.50 | 5.64 ± 0.50 | 0.15 |
| Absolute Granulocytes (×103 cells/µL) | 3.34 ± 0.38 | 3.13 ± 0.38 | 0.16 |
| Neutrophil count (×103 cells/µL) | 3.09 ± 0.36 | 2.87 ± 0.36 | 0.16 |
| Eosinophil count (×103 cells/µL) | 0.23 ± 0.05 | 0.23 ± 0.05 | 1.00 |
| Composition | WGS | LSS-G | SBF * | FSBF * | ESBF * |
|---|---|---|---|---|---|
| Macronutrients/Ash/Fiber g/100 g | |||||
| Ash | 6.04 | 5.35 | 6.50 | 7.00 | 6.70 |
| Protein | 25.40 | 38.30 | 47.50 | 51.70 | 48.20 |
| Lipid | 14.00 | 16.60 | 2.20 | 1.90 | 1.50 |
| Total carbohydrate | 51.60 | 36.60 | 42.60 | 36.90 | 39.20 |
| Dietary fiber | 35.60 | 28.80 | 27.30 | 30.40 | 28.10 |
| Insoluble fiber | 32.50 | 23.70 | - | - | - |
| Soluble fiber | 3.10 | 5.05 | - | - | - |
| Minerals mg/100 g | |||||
| Copper | 0.85 | 0.73 | 0.83 | 0.91 | 0.83 |
| Manganese | 3.40 | 2.81 | 2.40 | 2.60 | 2.40 |
| Zinc | 3.38 | 4.49 | 4.00 | 4.50 | 4.00 |
| Iron | 6.17 | 6.78 | 9.10 | 6.70 | 6.00 |
| Sodium | 2.50 | 7.54 | 13.20 | 18.10 | 12.10 |
| Calcium | 549.00 | 284.00 | 260.30 | 286.10 | 267.00 |
| Magnesium | 438.00 | 277.00 | 269.70 | 287.60 | 263.00 |
| Phosphorus | 349.00 | 646.00 | 522.00 | 581.80 | 526.10 |
| Potassium | 2170.00 | 1880.00 | 2046.30 | 2202.60 | 2022.90 |
| Oligosaccharides g/100 g | |||||
| Sucrose | 1.90 | 5.15 | 4.30 | Not detected | 2.30 |
| Glucose | 0.22 | 0.12 | - | - | - |
| Fructose | 0.21 | <0.10 | - | - | - |
| Maltose | <0.10 | <0.10 | - | - | - |
| Lactose | <0.10 | <0.10 | - | - | - |
| Galactose | <0.10 | <0.10 | - | - | - |
| Raffinose | 0.26 | 0.16 | 0.82 | Not detected | 0.43 |
| Stachyose | 0.65 | 0.63 | 2.72 | Not detected | 1.59 |
| Energy kcal/100 g | 297.00 | 340.00 | - | - | - |
| Glyceollins µg/g | - | 266.91 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebello, C.J.; Boué, S.; Levy, R.J., Jr.; Puyau, R.; Beyl, R.A.; Greenway, F.L.; Heiman, M.L.; Keller, J.N.; Reynolds, C.F., III; Kirwan, J.P. Safety and Tolerability of Whole Soybean Products: A Dose-Escalating Clinical Trial in Older Adults with Obesity. Nutrients 2023, 15, 1920. https://doi.org/10.3390/nu15081920
Rebello CJ, Boué S, Levy RJ Jr., Puyau R, Beyl RA, Greenway FL, Heiman ML, Keller JN, Reynolds CF III, Kirwan JP. Safety and Tolerability of Whole Soybean Products: A Dose-Escalating Clinical Trial in Older Adults with Obesity. Nutrients. 2023; 15(8):1920. https://doi.org/10.3390/nu15081920
Chicago/Turabian StyleRebello, Candida J., Stephen Boué, Ronald J. Levy, Jr., Renée Puyau, Robbie A. Beyl, Frank L. Greenway, Mark L. Heiman, Jeffrey N. Keller, Charles F. Reynolds, III, and John P. Kirwan. 2023. "Safety and Tolerability of Whole Soybean Products: A Dose-Escalating Clinical Trial in Older Adults with Obesity" Nutrients 15, no. 8: 1920. https://doi.org/10.3390/nu15081920
APA StyleRebello, C. J., Boué, S., Levy, R. J., Jr., Puyau, R., Beyl, R. A., Greenway, F. L., Heiman, M. L., Keller, J. N., Reynolds, C. F., III, & Kirwan, J. P. (2023). Safety and Tolerability of Whole Soybean Products: A Dose-Escalating Clinical Trial in Older Adults with Obesity. Nutrients, 15(8), 1920. https://doi.org/10.3390/nu15081920