An Acute Bout of Endurance Exercise Does Not Prevent the Inhibitory Effect of Caffeine on Glucose Tolerance the following Morning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Approvals
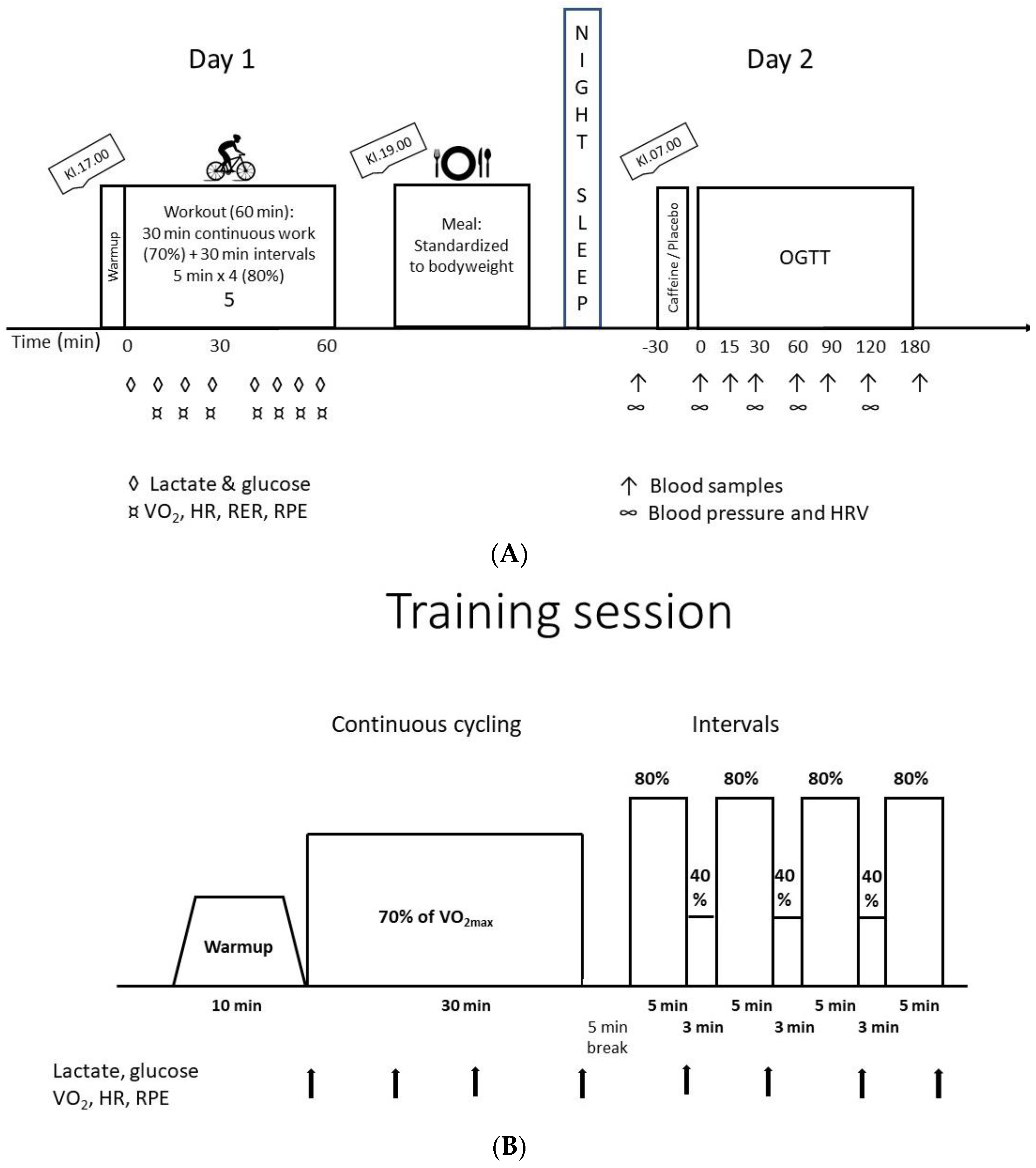
2.2. Design/Experimental Procedures
2.3. Pre-Tests: Incremental Test and VO2max Test
2.4. Measurement of VO2, CO2, Lactate and Glucoses during the Bike Tests
2.5. Registration of Meals, Physical Activity and Caffeine Intake
2.6. Standardized Exercise
2.7. Standardized Meal
2.8. Oral Glucose Tolerance Test (OGTT)
2.9. Blood Pressure, Heart Rate and Heart Rate Variability
2.10. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loftfield, E.; Freedman, N.D.; Dodd, K.W.; Vogtmann, E.; Xiao, Q.; Sinha, R.; Graubard, B.I. Coffee Drinking Is Widespread in the United States, but Usual Intake Varies by Key Demographic and Lifestyle Factors. J. Nutr. 2016, 146, 1762–1768. [Google Scholar] [CrossRef]
- Reyes, C.M.; Cornelis, M.C. Caffeine in the Diet: Country-Level Consumption and Guidelines. Nutrients 2018, 10, 1772. [Google Scholar] [CrossRef]
- Stadheim, H.K.; Kvamme, B.; Olsen, R.; Drevon, C.A.; Ivy, J.L.; Jensen, J. Caffeine Increases Performance in Cross-country Double-Poling Time Trial Exercise. Med. Sci. Sport. Exerc. 2013, 45, 2175–2183. [Google Scholar] [CrossRef]
- Stadheim, H.K.; Stensrud, T.; Brage, S.; Jensen, J. Caffeine Increases Exercise Performance, Maximal Oxygen Uptake, and Oxygen Deficit in Elite Male Endurance Athletes. Med. Sci. Sport. Exerc. 2021, 53, 2264–2273. [Google Scholar] [CrossRef] [PubMed]
- Moisey, L.L.; Kacker, S.; Bickerton, A.C.; Robinson, L.E.; Graham, T.E. Caffeinated coffee consumption impairs blood glucose homeostasis in response to high and low glycemic index meals in healthy men. Am. J. Clin. Nutr. 2008, 87, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Moisey, L.L.; Robinson, L.E.; Graham, T.E. Consumption of caffeinated coffee and a high carbohydrate meal affects postprandial metabolism of a subsequent oral glucose tolerance test in young, healthy males. Br. J. Nutr. 2010, 103, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.; Graham, T.E. Performance effects and metabolic consequences of caffeine and caffeinated energy drink consumption on glucose disposal. Nutr. Rev. 2014, 72 (Suppl. S1), 121–136. [Google Scholar] [CrossRef]
- Hingst, J.R.; Bruhn, L.; Hansen, M.B.; Rosschou, M.F.; Birk, J.B.; Fentz, J.; Foretz, M.; Viollet, B.; Sakamoto, K.; Faergeman, N.J.; et al. Exercise-induced molecular mechanisms promoting glycogen supercompensation in human skeletal muscle. Mol. Metab. 2018, 16, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Jelstad, S.; Ditta, V.T.; Johansen, E.I.; Jensen, J.R. Eight sessions of endurance training decrease fasting glucose and improve glucose tolerance in middle-aged overweight males. Arch. Physiol. Biochem. 2019, 127, 12–19. [Google Scholar] [CrossRef]
- Langleite, T.M.; Jensen, J.; Norheim, F.; Gulseth, H.L.; Tangen, D.S.; Kolnes, K.J.; Heck, A.; Storas, T.; Grothe, G.; Dahl, M.A.; et al. Insulin sensitivity, body composition and adipose depots following 12 w combined endurance and strength training in dysglycemic and normo-glycemic sedentary men. Arch. Physiol. Biochem. 2016, 122, 167–179. [Google Scholar] [CrossRef]
- Sandvei, M.; Jeppesen, P.B.; Støen, L.; Litleskare, S.; Johansen, E.; Stensrud, T.; Enoksen, E.; Hautala, A.; Martinmaki, K.; Kinnunen, H.; et al. Sprint interval running increases insulin sensitivity in young healthy subjects. Arch. Physiol. Biochem. 2012, 118, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Bättig, K.; Holmén, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar] [PubMed]
- Petrie, H.J.; Chown, S.E.; Belfie, L.M.; Duncan, A.M.; McLaren, D.H.; Conquer, J.A.; Graham, T.E. Caffeine ingestion increases the insulin response to an oral-glucose-tolerance test in obese men before and after weight loss. Am. J. Clin. Nutr. 2004, 80, 22–28. [Google Scholar] [CrossRef]
- Thong, F.S.; Graham, T.E. Caffeine-induced impairment of glucose tolerance is abolished by beta-adrenergic receptor blockade in humans. J. Appl. Physiol. 2002, 92, 2347–2352. [Google Scholar] [CrossRef]
- Battram, D.S.; Graham, T.E.; Dela, F. Caffeine’s impairment of insulin-mediated glucose disposal cannot be solely attributed to adrenaline in humans. J. Physiol. 2007, 583, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Thong, F.S.; Derave, W.; Kiens, B.; Graham, T.E.; Urso, B.; Wojtaszewski, J.F.; Hansen, B.F.; Richter, E.A. Caffeine-induced impairment of insulin action but not insulin signaling in human skeletal muscle is reduced by exercise. Diabetes 2002, 51, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, M.-S.; Allen, B.; Mazzetti, G.; Sullivan, P.J.; Graham, T.E. Caffeine ingestion impairs insulin sensitivity in a dose-dependent manner in both men and women. Appl. Physiol. Nutr. Metab. 2013, 38, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.E.; Sathasivam, P.; Rowland, M.; Marko, N.; Greer, F.; Battram, D. Caffeine ingestion elevates plasma insulin response in humans during an oral glucose tolerance test. Can. J. Physiol. Pharmacol. 2001, 79, 559–565. [Google Scholar] [CrossRef]
- Foukas, L.C.; Daniele, N.; Ktori, C.; Anderson, K.E.; Jensen, J.; Shepherd, P.R. Direct effects of caffeine and theophylline on p110 delta and other phosphoinositide 3-kinases. Differential effects on lipid kinase and protein kinase activities. J. Biol. Chem. 2002, 277, 37124–37130. [Google Scholar] [CrossRef] [PubMed]
- Rush, J.W.; Spriet, L.L. Skeletal muscle glycogen phosphorylase a kinetics: Effects of adenine nucleotides and caffeine. J. Appl. Physiol. 2001, 91, 2071–2078. [Google Scholar] [CrossRef]
- Kolnes, A.J.; Ingvaldsen, A.; Bolling, A.; Stuenaes, J.T.; Kreft, M.; Zorec, R.; Shepherd, P.R.; Jensen, J. Caffeine and theophylline block insulin-stimulated glucose uptake and PKB phosphorylation in rat skeletal muscles. Acta Physiol. 2010, 200, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Foukas, L.C.; Beeton, C.A.; Jensen, J.; Phillips, W.A.; Shepherd, P.R. Regulation of Phosphoinositide 3-Kinase by Its Intrinsic Serine Kinase Activity In Vivo. Mol. Cell. Biol. 2004, 24, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Stadheim, H.K.; Nossum, E.M.; Olsen, R.; Spencer, M.; Jensen, J. Caffeine improves performance in double poling during acute exposure to 2000-m altitude. J. Appl. Physiol. 2015, 119, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Aslesen, R.; Engebretsen, E.M.L.; Franch, J.; Jensen, J. Glucose uptake and metabolic stress in rat muscles stimulated electrically with different protocols. J. Appl. Physiol. 2001, 91, 1237–1244. [Google Scholar] [CrossRef]
- Lin, F.C.; Bolling, A.; Stuenæs, J.T.; Cumming, K.T.; Ingvaldsen, A.; Lai, Y.-C.; Ivy, J.L.; Jensen, J. Effect of insulin and contraction on glycogen synthase phosphorylation and kinetic properties in epitrochlearis muscles from lean and obese Zucker rats. Am. J. Physiol. Physiol. 2012, 302, C1539–C1547. [Google Scholar] [CrossRef]
- Ruzzin, J.; Jensen, J. Contraction activates glucose uptake and glycogen synthase normally in muscles from dexame-thasone-treated rats. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E241–E250. [Google Scholar] [CrossRef]
- Whitehead, J.P.; Soos, M.A.; Aslesen, R.; O’Rahilly, S.; Jensen, J. Contraction inhibits insulin-stimulated insulin receptor substrate-1/2-associated phosphoinositide 3-kinase activity, but not PKB activation or glucose uptake in rat muscle. Biochem. J. 2000, 349, 775–781. [Google Scholar] [CrossRef]
- Franch, J.; Aslesen, R.; Jensen, J. Regulation of glycogen synthesis in rat skeletal muscle after glycogen depleting contractile activity: Effects of adrenaline on glycogen synthesis and activation of glycogen synthase and glycogen phosphorylase. Biochem. J. 1999, 344, 231–235. [Google Scholar] [CrossRef]
- Hingst, J.R.; Onslev, J.D.; Holm, S.; Kjøbsted, R.; Frøsig, C.; Kido, K.; Steenberg, D.E.; Larsen, M.R.; Kristensen, J.M.; Carl, C.S.; et al. Insulin Sensitization Following a Single Exercise Bout Is Uncoupled to Glycogen in Human Skeletal Muscle: A Meta-analysis of 13 Single-Center Human Studies. Diabetes 2022, 71, 2237–2250. [Google Scholar] [CrossRef]
- Kjøbsted, R.; Munk-Hansen, N.; Birk, J.B.; Foretz, M.; Viollet, B.; Björnholm, M.; Zierath, J.R.; Treebak, J.T.; Wojtaszewski, J.F. Enhanced Muscle Insulin Sensitivity After Contraction/Exercise Is Mediated by AMPK. Diabetes 2017, 66, 598–612. [Google Scholar] [CrossRef]
- Valsdottir, T.D.; Henriksen, C.; Odden, N.; Nellemann, B.; Jeppesen, P.B.; Hisdal, J.; Westerberg, A.C.; Jensen, J. Effect of a Low-Carbohydrate High-Fat Diet and a Single Bout of Exercise on Glucose Tolerance, Lipid Profile and Endothelial Function in Normal Weight Young Healthy Females. Front. Physiol. 2019, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
- Venables, M.C.; Shaw, C.S.; Jeukendrup, A.E.; Wagenmakers, A.J.M. Effect of acute exercise on glucose tolerance following post-exercise feeding. Eur. J. Appl. Physiol. 2007, 100, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Hindsø, M.; Kuhlman, A.B.; Dohlmann, T.L.; Lund, M.T.; Hartmann, B.; Holst, J.J.; Larsen, S.; Helge, J.W. Effect of 6 weeks of very low-volume high-intensity interval training on oral glucose-stimulated incretin hormone response. Eur. J. Sport Sci. 2022, 22, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Steenberg, D.E.; Jørgensen, N.B.; Birk, J.B.; Sjøberg, K.A.; Kiens, B.; Richter, E.A.; Wojtaszewski, J.F. Exercise training reduces the insulin-sensitizing effect of a single bout of exercise in human skeletal muscle. J. Physiol. 2019, 597, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Flockhart, M.; Nilsson, L.C.; Tais, S.; Ekblom, B.; Apró, W.; Larsen, F.J. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. 2021, 33, 957–970.e956. [Google Scholar] [CrossRef]
- Battram, D.S.; Shearer, J.; Robinson, D.; Graham, T.E. Caffeine ingestion does not impede the resynthesis of proglycogen and macroglycogen after prolonged exercise and carbohydrate supplementation in humans. J. Appl. Physiol. 2004, 96, 943–950. [Google Scholar] [CrossRef]
- Pedersen, D.J.; Lessard, S.J.; Coffey, V.G.; Churchley, E.G.; Wootton, A.M.; Ng, T.; Watt, M.J.; Hawley, J.A. High rates of muscle glycogen resynthesis after exhaustive exercise when carbohydrate is coingested with caffeine. J. Appl. Physiol. 2008, 105, 7–13. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S.; Kempf, K. Coffee and Lower Risk of Type 2 Diabetes: Arguments for a Causal Relationship. Nutrients 2021, 13, 1144. [Google Scholar] [CrossRef]
- Kolnes, K.J.; Petersen, M.H.; Lien-Iversen, T.; Højlund, K.; Jensen, J. Effect of Exercise Training on Fat Loss-Energetic Perspectives and the Role of Improved Adipose Tissue Function and Body Fat Distribution. Front. Physiol. 2021, 12, 737709. [Google Scholar] [CrossRef]
- Turnbull, D.; Rodricks, J.V.; Mariano, G.F.; Chowdhury, F. Caffeine and cardiovascular health. Regul. Toxicol. Pharmacol. 2017, 89, 165–185. [Google Scholar] [CrossRef]
- Rustad, P.I.; Sailer, M.; Cumming, K.T.; Jeppesen, P.B.; Kolnes, K.J.; Sollie, O.; Franch, J.; Ivy, J.L.; Daniel, H.; Jensen, J. Intake of Protein Plus Carbohydrate during the First Two Hours after Exhaustive Cycling Improves Performance the following Day. PLoS ONE 2016, 11, e0153229. [Google Scholar] [CrossRef]
- Borg, G.A. Perceived exertion. Exerc. Sport. Sci. Rev. 1974, 2, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Hills, A.P.; Emokhtar, N.; Byrne, N.M. Assessment of Physical Activity and Energy Expenditure: An Overview of Objective Measures. Front. Nutr. 2014, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Battram, D.S.; Arthur, R.; Weekes, A.; Graham, T.E. The Glucose Intolerance Induced by Caffeinated Coffee Ingestion Is Less Pronounced than That Due to Alkaloid Caffeine in Men. J. Nutr. 2006, 136, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.E.; Savani, S.; Battram, D.S.; McLaren, D.H.; Sathasivam, P.; Graham, T.E. Caffeine Ingestion Before an Oral Glucose Tolerance Test Impairs Blood Glucose Management in Men with Type 2 Diabetes. J. Nutr. 2004, 134, 2528–2533. [Google Scholar] [CrossRef]
- Greer, F.; Hudson, R.; Ross, R.; Graham, T. Caffeine ingestion decreases glucose disposal during a hyperinsuline-mic-euglycemic clamp in sedentary humans. Diabetes 2001, 50, 2349–2354. [Google Scholar] [CrossRef]
- Lee, S.; Hudson, R.; Kilpatrick, K.; Graham, T.E.; Ross, R. Caffeine Ingestion Is Associated with Reductions in Glucose Uptake Independent of Obesity and Type 2 Diabetes Before and After Exercise Training. Diabetes Care 2005, 28, 566–572. [Google Scholar] [CrossRef]
- Battram, D.S.; Graham, T.E.; Richter, E.A.; Dela, F. The effect of caffeine on glucose kinetics in humans-influence of adrenaline. J. Physiol. 2005, 569, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Franch, J.; Knudsen, J.; Ellis, B.A.; Pedersen, P.K.; Cooney, G.J.; Jensen, J. Acyl-CoA binding protein expression is fibre type specific and elevated in muscles from obese insulin-resistant Zucker rat. Diabetes 2002, 51, 449–454. [Google Scholar] [CrossRef]
- Pehmøller, C.; Brandt, N.; Birk, J.B.; Høeg, L.D.; Sjøberg, K.A.; Goodyear, L.J.; Kiens, B.; Richter, E.A.; Wojtaszewski, J.F. Exercise Alleviates Lipid-Induced Insulin Resistance in Human Skeletal Muscle–Signaling Interaction at the Level of TBC1 Domain Family Member 4. Diabetes 2012, 61, 2743–2752. [Google Scholar] [CrossRef]
- Collado-Mateo, D.; Lavín-Pérez, A.M.; Merellano-Navarro, E.; Coso, J.D. Effect of Acute Caffeine Intake on the Fat Oxi-dation Rate during Exercise: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 3603. [Google Scholar] [CrossRef]
- Banks, N.F.; Tomko, P.M.; Colquhoun, R.J.; Muddle, T.W.D.; Emerson, S.R.; Jenkins, N.D.M. Genetic Polymorphisms in ADO-RA2A and CYP1A2 Influence Caffeine’s Effect on Postprandial Glycaemia. Sci. Rep. 2019, 9, 10532. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, J.F.; Martins, F.; Rodrigues, T.; Matafome, P.; Ribeiro, M.J.; Olea, E.; Conde, S.V. A2 Adenosine Receptors Mediate Whole-Body Insulin Sensitivity in a Prediabetes Animal Model: Primary Effects on Skeletal Muscle. Front. Endocrinol. 2020, 11, 262. [Google Scholar] [CrossRef]
- Mikines, K.J.; Sonne, B.; Farrell, P.A.; Tronier, B.; Galbo, H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am. J. Physiol. Metab. 1988, 254, E248–E259. [Google Scholar] [CrossRef] [PubMed]
- Beelen, M.; Kranenburg, J.; Senden, J.M.; Kuipers, H.; Loon, L.J. Impact of caffeine and protein on postexercise muscle glycogen synthesis. Med. Sci. Sport. Exerc. 2012, 44, 692–700. [Google Scholar] [CrossRef]
- Stadheim, H.K.; Spencer, M.; Olsen, R.; Jensen, J. Caffeine and performance over consecutive days of simulated competi-tion. Med. Sci. Sport. Exerc. 2014, 46, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef]
- Loftfield, E.; Cornelis, M.C.; Caporaso, N.; Yu, K.; Sinha, R.; Freedman, N. Association of Coffee Drinking with Mortality by Genetic Variation in Caffeine Metabolism: Findings From the UK Biobank. JAMA Intern. Med. 2018, 178, 1086–1097. [Google Scholar] [CrossRef]
- van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef]
- Farah, A. Coffee Constituents. In Coffee: Emerging Health Effects and Disease Prevention; Chu, Y.F., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 21–58. [Google Scholar]
- Rauch, H.G.L.; Gibson, A.S.C.; Lambert, E.; Noakes, T.D. A signalling role for muscle glycogen in the regulation of pace during prolonged exercise. Br. J. Sport. Med. 2005, 39, 34–38. [Google Scholar] [CrossRef]
- Thomas, R.M.; Algrain, H.A.; Ryan, E.J.; Popojas, A.; Carrigan, P.; Abdulrahman, A.; Carrillo, A. Influence of a CYP1A2 polymorphism on post-exercise heart rate variability in response to caffeine intake: A double-blind, placebo-controlled trial. Ir. J. Med. Sci. 2016, 186, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann-Viehoff, F.; Thayer, J.; Koenig, J.; Herrmann, C.; Weber, C.S.; Deter, H.-C. Short-term effects of espresso coffee on heart rate variability and blood pressure in habitual and non-habitual coffee consumers–A randomized crossover study. Nutr. Neurosci. 2016, 19, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Yeragani, V.K.; Krishnan, S.; Engels, H.J.; Gretebeck, R. Effects of caffeine on linear and nonlinear measures of heart rate variability before and after exercise. Depress. Anxiety 2005, 21, 130–134. [Google Scholar] [CrossRef]
- Sondermeijer, H.P.; van Marle, A.G.; Kamen, P.; Krum, H. Acute effects of caffeine on heart rate variability. Am. J. Cardiol. 2002, 90, 906–907. [Google Scholar] [CrossRef] [PubMed]
- Weissman, A.; Lowenstein, L.; Peleg, A.; Thaler, I.; Zimmer, E.Z. Power Spectral Analysis of Heart Rate Variability During the 100-g Oral Glucose Tolerance Test in Pregnant Women. Diabetes Care 2006, 29, 571–574. [Google Scholar] [CrossRef]
- Furlan, R.; Piazza, S.; Dell’Orto, S.; Gentile, E.; Cerutti, S.; Pagani, M.; Malliani, A. Early and late effects of exercise and athletic training on neural mechanisms controlling heart rate. Cardiovasc. Res. 1993, 27, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Hautala, A.; Tulppo, M.; Mäkikallio, T.H.; Laukkanen, R.; Nissilä, S.; Huikuri, H.V. Changes in cardiac autonomic regulation after prolonged maximal exercise. Clin. Physiol. Funct. Imaging 2001, 21, 238–245. [Google Scholar] [CrossRef]
- Stanley, J.; Peake, J.M.; Buchheit, M. Cardiac Parasympathetic Reactivation Following Exercise: Implications for Training Prescription. Sport. Med. 2013, 43, 1259–1277. [Google Scholar] [CrossRef]

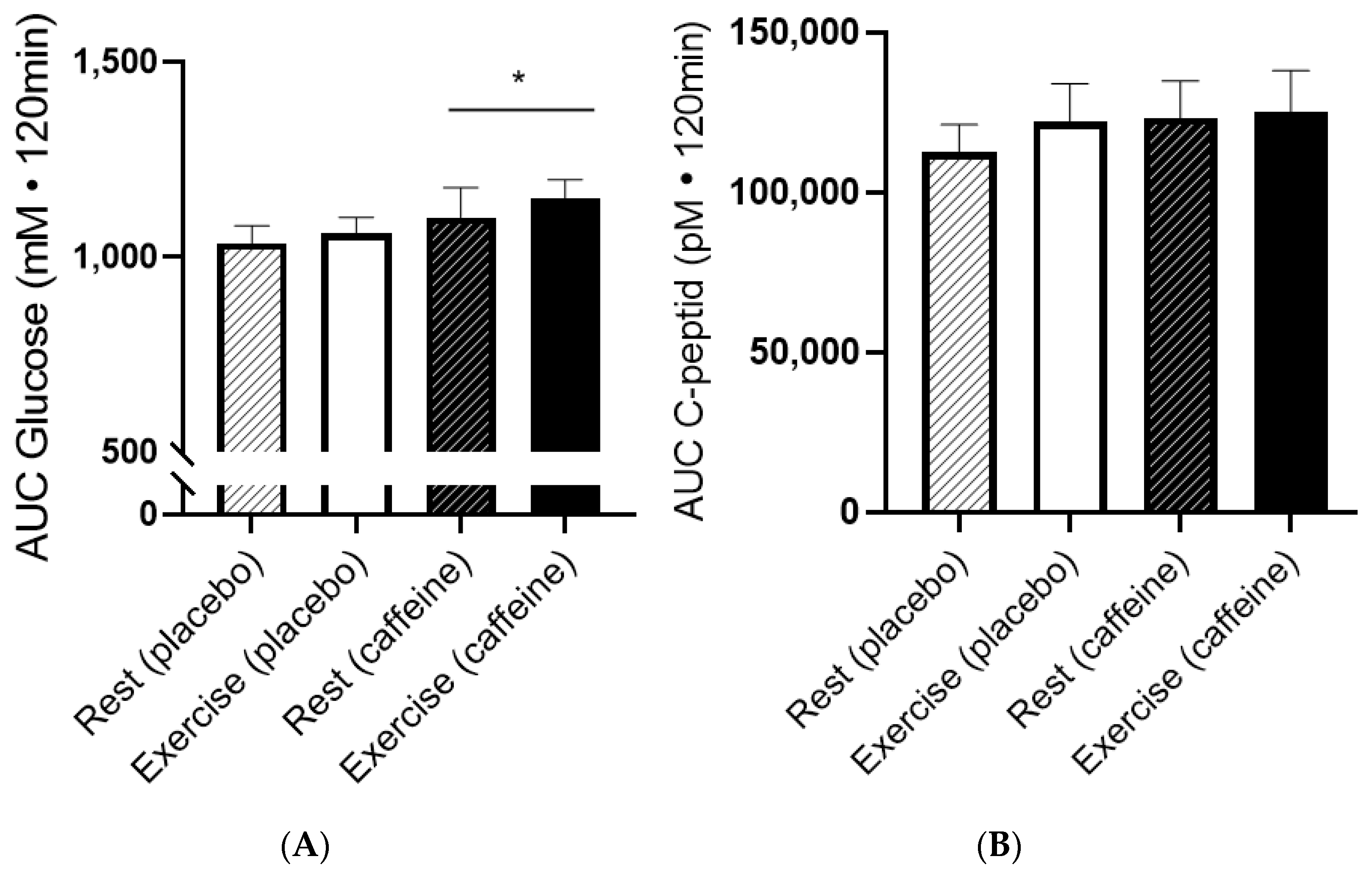

| Rest/PLA | Rest/CAF | Ex/PLA | Ex/CAF | |
|---|---|---|---|---|
| Fasted glucose (mM) | 5.0 ± 0.1 | 5.3 ± 0.1 | 5.2 ± 0.1 | 5.1 ± 0.1 |
| Glucose PLA/CAF (mM) * | 5.4 ± 0.1 | 5.4 ± 0.1 | 5.3 ± 0.1 | 5.6 ± 0.1 |
| C-peptide (pM) | 334 ± 18 | 300 ± 10 | 351 ± 28 | 301 ± 11 |
| Rest + Placebo | Rest + Caffeine | Training + Placebo | Training + Caffeine | |
|---|---|---|---|---|
| Time −30 min | ||||
| Sitting | ||||
| HF [bpm] | 61 ± 2 | 63 ± 2 | 65 ± 1 | 65 ± 1 |
| RRI [ms] | 1019.8 ± 29.2 | 979.5 ± 25.2 | 946.8 ± 16.3 | 951.3 ± 19.2 |
| SDNN [ms] | 82.97 ± 5.63 | 70.21 ± 4.56 | 76.02 ± 3.93 | 74.07 ± 5.54 |
| LF/HF | 4.84 ± 0.40 | 6.16 ± 1.03 | 6.56 ± 0.36 | 4.53 ± 0.39 |
| Standing | ||||
| HF [bpm] | 73 ± 2 | 77 ± 1 | 76 ± 1 | 77 ± 1 |
| RRI [ms] | 745.7 ± 48.9 | 790.6 ± 12.9 | 792.4 ± 9.3 | 785.3 ± 12.0 |
| SDNN [ms] | 68.08 ± 4.88 | 50.90 ± 1.58 | 56.36 ± 2.84 | 51.03 ± 3.74 |
| LF/HF ratio | 8.26 ± 1.10 | 12.51 ± 1.57 | 11.60 ± 1.41 | 9.22 ± 0.83 |
| Time 0 | ||||
| Sitting | ||||
| HF | 57 ± 1 | 59 ± 2 | 61 ± 1 | 62 ± 2 |
| RRI [ms] | 1063.9 ± 20.1 | 1040.3 ± 29.,9 | 999.5 ±16.8 | 991.55 ± 31.5 |
| SDNN [ms] | 81.69 ± 3.86 | 84.59 ± 4.18 | 83.99 ± 5.63 | 84.25 ± 7.02 |
| LF/HF ratio | 3.93 ± 0.39 | 3.50 ± 0.27 | 3.95 ± 0.23 | 4.29 ± 0.62 |
| Standing | ||||
| HF | 69 ± 1 | 68 ± 1 | 71 ± 1 | 70 ± 2 |
| RRI [ms] | 883.5 ± 15.2 | 893.9 ± 19.1 | 833.6 ±14.9 | 884.9 ± 23.5 |
| SDNN [ms] | 81.30 ± 4.35 | 75.91 ± 2.54 | 65.70 ± 3.13 | 78.26 ± 5.17 |
| LF/HF ratio | 4.46 ± 0.31 | 4.44 ± 0.35 | 4.69 ± 0.27 | 4.85 ± 0.51 |
| Time 30 min | ||||
| Sitting | ||||
| HF | 59 ± 2 | 61 ± 2 | 63 ± 1 | 61 ± 1 |
| RRI [ms] | 1053.1 ± 30.7 | 1001.7 ± 26.1 | 964.9 ±14.3 | 998.61 ± 18.1 |
| SDNN [ms] | 80.40 ± 3.78 | 89.61 ± 7.07 | 88.79 ± 5.45 | 84.13 ± 4.23 |
| LF/HF ratio | 5.07 ± 0.68 | 4.52 ± 0.52 | 3.88 ± 0.20 | 3.72 ± 0.43 |
| Standing | ||||
| HF | 70 ± 2 | 71 ± 2 | 73 ± 1 | 70 ± 1 |
| RRI [ms] | 881.2 ± 22.1 | 848.6 ± 17.1 | 831.2 ± 12.1 | 867.6 ± 13.2 |
| SDNN [ms] | 64.59 ± 4.37 | 84.33 ± 6.56 | 69.23 ± 4.17 | 69.02 ± 2.45 |
| LF/HF ratio | 5.43 ± 0.53 | 5.63 ± 0.34 | 5.41 ± 0.48 | 7.34 ± 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenne, K.T.; Clauss, M.; Schäfer Olstad, D.; Johansen, E.I.; Jensen, J. An Acute Bout of Endurance Exercise Does Not Prevent the Inhibitory Effect of Caffeine on Glucose Tolerance the following Morning. Nutrients 2023, 15, 1941. https://doi.org/10.3390/nu15081941
Fenne KT, Clauss M, Schäfer Olstad D, Johansen EI, Jensen J. An Acute Bout of Endurance Exercise Does Not Prevent the Inhibitory Effect of Caffeine on Glucose Tolerance the following Morning. Nutrients. 2023; 15(8):1941. https://doi.org/10.3390/nu15081941
Chicago/Turabian StyleFenne, Karoline T., Matthieu Clauss, Daniela Schäfer Olstad, Egil I. Johansen, and Jørgen Jensen. 2023. "An Acute Bout of Endurance Exercise Does Not Prevent the Inhibitory Effect of Caffeine on Glucose Tolerance the following Morning" Nutrients 15, no. 8: 1941. https://doi.org/10.3390/nu15081941
APA StyleFenne, K. T., Clauss, M., Schäfer Olstad, D., Johansen, E. I., & Jensen, J. (2023). An Acute Bout of Endurance Exercise Does Not Prevent the Inhibitory Effect of Caffeine on Glucose Tolerance the following Morning. Nutrients, 15(8), 1941. https://doi.org/10.3390/nu15081941








