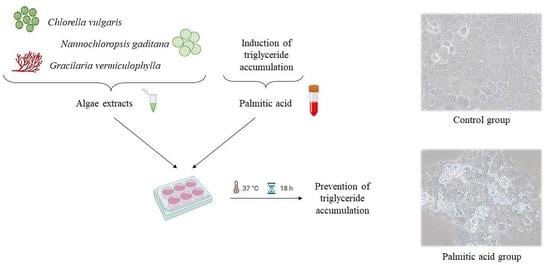
Anti-Steatotic Effects of Chlorella vulgaris, Nannochloropsis gaditana and Gracilaria vermiculophylla Algae Extracts in AML-12 Hepatocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algae Extract Preparation
2.2. Algae Extract Composition
2.3. Characterization of the Protein Fraction
2.4. Cell Culture and Treatment
2.5. Cell Viability Assay
2.6. Determination of Triglyceride Content
2.7. Detection of Alanine Aminotransferase (ALT/GPT) Levels in Cell Culture Medium
2.8. Analysis of Gene Expression by Real-Time PCR
2.9. Analysis of Protein Expression by Western Blot
2.10. Statistical Analysis
3. Results
3.1. Composition of Algae Extracts
3.2. Cell Viability
3.3. Effects on Triglyceride Accumulation
3.4. Detection of ALT/GPT Level in Cell Culture Medium
3.5. Effects on Genes and Proteins Involved in Triglyceride Metabolism
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell. Mol. Life Sci. 2019, 76, 99–128. [Google Scholar] [CrossRef]
- Drescher, H.K.; Weiskirchen, S.; Weiskirchen, R. Current Status in Testing for Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH). Cells 2019, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Kimura, T.; Fujimori, N.; Nagaya, T.; Komatsu, M.; Tanaka, E. Current status, problems, and perspectives of non-alcoholic fatty liver disease research. World J. Gastroenterol. 2019, 25, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J.; Panel, I.C. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Makri, E.; Goulas, A.; Polyzos, S.A. Epidemiology, Pathogenesis, Diagnosis and Emerging Treatment of Nonalcoholic Fatty Liver Disease. Arch. Med. Res. 2021, 52, 25–37. [Google Scholar] [CrossRef]
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. UK 2015, 2015, 61–91. [Google Scholar] [CrossRef] [PubMed]
- Biris-Dorhoi, E.S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A Sustainable Source of Chemical Compounds with Biological Activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Saadaoui, I.; Rasheed, R.; Abdulrahman, N.; Bounnit, T.; Cherif, M.; Al Jabri, H.; Mraiche, F. Algae-Derived Bioactive Compounds with Anti-Lung Cancer Potential. Mar. Drugs 2020, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- González-Arceo, M.; Gómez-Zorita, S.; Aguirre, L.; Portillo, M.P. Effect of Microalgae and Macroalgae Extracts on Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 2017. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; González-Arceo, M.; Trepiana, J.; Eseberri, I.; Fernández-Quintela, A.; Milton-Laskibar, I.; Aguirre, L.; González, M.; Portillo, M.P. Anti-Obesity Effects of Macroalgae. Nutrients 2020, 12, 2378. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Trepiana, J.; González-Arceo, M.; Aguirre, L.; Milton-Laskibar, I.; González, M.; Eseberri, I.; Fernández-Quintela, A.; Portillo, M.P. Anti-Obesity Effects of Microalgae. Int. J. Mol. Sci. 2019, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Attjioui, M.; Ryan, S.; Ristic, A.K.; Higgins, T.; Goñi, O.; Gibney, E.R.; Tierney, J.; O’Connell, S. Comparison of edible brown algae extracts for the inhibition of intestinal carbohydrate digestive enzymes involved in glucose release from the diet. J. Nutr. Sci. 2021, 10, e5. [Google Scholar] [CrossRef] [PubMed]
- Safi, C.; Frances, C.; Ursu, A.V.; Laroche, C.; Pouzet, C.; Vaca-Garcia, C.; Pontalier, P.-Y. Understanding the effect of cell disruption methods on the diffusion of Chlorella vulgaris proteins and pigments in the aqueous phase. Algal Res. 2015, 8, 61–68. [Google Scholar] [CrossRef]
- Safi, C.; Charton, M.; Pignolet, O.; Silvestre, F.; Vaca-Garcia, C.; Pontalier, P.-Y. Influence of microalgae cell wall characteristics on protein extractability and determination of nitrogen-to-protein conversion factors. J. Appl. Phycol. 2013, 25, 523–529. [Google Scholar] [CrossRef]
- Cunniff, P. Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 1995. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar] [CrossRef]
- Trepiana, J.; Krisa, S.; Renouf, E.; Portillo, M.P. Resveratrol Metabolites Are Able to Reduce Steatosis in Cultured Hepatocytes. Pharmaceuticals 2020, 13, 285. [Google Scholar] [CrossRef]
- Gillies, R.J.; Didier, N.; Denton, M. Determination of cell number in monolayer cultures. Anal. Biochem. 1986, 159, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Baldrick, F.R.; McFadden, K.; Ibars, M.; Sung, C.; Moffatt, T.; Megarry, K.; Thomas, K.; Mitchell, P.; Wallace, J.M.W.; Pourshahidi, L.K.; et al. Impact of a (poly)phenol-rich extract from the brown algae Ascophyllum nodosum on DNA damage and antioxidant activity in an overweight or obese population: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Cui, Y.; Fan, X.; Qi, P.; Liu, C.; Zhou, X.; Zhang, X. Anti-obesity effects of Spirulina platensis protein hydrolysate by modulating brain-liver axis in high-fat diet fed mice. PLoS ONE 2019, 14, e0218543. [Google Scholar] [CrossRef]
- Fan, X.; Cui, Y.; Zhang, R.; Zhang, X. Purification and identification of anti-obesity peptides derived from Spirulina platensis. J. Funct. Foods 2018, 47, 350–360. [Google Scholar] [CrossRef]
- Paterson, S.; Gómez-Cortés, P.; de la Fuente, M.A.; Hernández-Ledesma, B. Bioactivity and Digestibility of Microalgae Tetraselmis sp. and Nannochloropsis sp. as Basis of Their Potential as Novel Functional Foods. Nutrients 2023, 15, 477. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-H.; Chiu, C.-Y.; Lu, T.-J.; Liu, S.-H.; Chiang, M.-T. The Anti-Obesity Effect of Polysaccharide-Rich Red Algae (Gelidium amansii) Hot-Water Extracts in High-Fat Diet-Induced Obese Hamsters. Mar. Drugs 2019, 17, 532. [Google Scholar] [CrossRef]
- Kolsi, R.B.A.; Jardak, N.; Hajkacem, F.; Chaaben, R.; Jribi, I.; Feki, A.E.; Rebai, T.; Jamoussi, K.; Fki, L.; Belghith, H.; et al. Anti-obesity effect and protection of liver-kidney functions by Codium fragile sulphated polysaccharide on high fat diet induced obese rats. Int. J. Biol. Macromol. 2017, 102, 119–129. [Google Scholar] [CrossRef]
- Rashed, Z.E.; Grasselli, E.; Khalifeh, H.; Canesi, L.; Demori, I. Brown-Algae Polysaccharides as Active Constituents against Nonalcoholic Fatty Liver Disease. Planta Med. 2022, 88, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ursu, A.V.; Marcati, A.; Sayd, T.; Sante-Lhoutellier, V.; Djelveh, G.; Michaud, P. Extraction, fractionation and functional properties of proteins from the microalgae Chlorella vulgaris. Bioresour. Technol. 2014, 157, 134–139. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Chang, C.; Chen, J.; Cao, F.; Zhao, J.; Zheng, Y.; Zhu, J. Physicochemical and functional properties of proteins extracted from three microalgal species. Food Hydrocoll. 2019, 96, 510–517. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Sáez, M.I.; Martínez, T.F.; Acién, F.G.; Alarcón, F.J. Differential hydrolysis of proteins of four microalgae by the digestive enzymes of gilthead sea bream and Senegalese sole. Algal Res. 2019, 37, 145–153. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Fernández-Quintela, A.; Aguirre, L.; Macarulla, M.T.; Rimando, A.M.; Portillo, M.P. Pterostilbene improves glycaemic control in rats fed an obesogenic diet: Involvement of skeletal muscle and liver. Food Funct. 2015, 6, 1968–1976. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Jung, U.J.; Choi, M.S. Differential effects of low-dose resveratrol on adiposity and hepatic steatosis in diet-induced obese mice. Br. J. Nutr. 2012, 108, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Reinado, R.; Bermudez-Sauco, M.; Escobar-Niño, A.; Cantoral, J.M.; Fernández-Acero, F.J. Development of the “Applied Proteomics” Concept for Biotechnology Applications in Microalgae: Example of the Proteome Data in. Mar. Drugs 2021, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Reinado, R.; Escobar-Niño, A.; Fajardo, C.; Morano, I.M.; Amil-Ruiz, F.; Martinez-Rodríguez, G.; Fuentes-Almagro, C.; Capilla, V.; Tomás-Cobos, L.; Soriano-Romaní, L.; et al. Development of New Antiproliferative Compound against Human Tumor Cells from the Marine Microalgae Nannochloropsis gaditana by Applied Proteomics. Int. J. Mol. Sci. 2021, 22, 96. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Harwanto, D.; Tirtawijaya, G.; Negara, B.F.S.P.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Fucosterol of Marine Macroalgae: Bioactivity, Safety and Toxicity on Organism. Mar. Drugs 2021, 19, 545. [Google Scholar] [CrossRef]







| SYBR Green RT-PCR | |||
| Gene | Gene Accession | Sense Primer 5′-3′ | Antisense Primer 5′-3′ |
| Acadl | NM_007381.4 | TGG GGA CTT GCT CTC AAC A | GGC CTG TGC AAT TGG AGT |
| Actb | NM_007393.5 | ACG AGG CCC AGA GCA AGA G | GGT GTG GTG CCA GAT CTT CTC |
| Atgl | NM_025802.3 | GAG CTT CGC GTC ACC AAC | CAC ATC TCT CGG AGG ACC A |
| Cpt1a | NM_013495.2 | CGG TTC AAG AAT GGC ATC ATC | TCA CAC CCA CCA CCA CGA T |
| Cs | NM_026444.4 | GCC TCT GCA TGG ACT AGC AAA | TTG CCG ACT TCC TTC TGT AGC T |
| Fasn | NM_007988.3 | AGC CCC TCA AGT GCA CAG T | TGC CAA TGT GTT TTC CCT G |
| Tfam | NM_009360.4 | AAG CTT ATC CAT GAC AGC TAA AGG | GGC TGG CTC ACC ACA GTT |
| Ucp2 | NM_011671.5 | TAC TCT CCT GAA AGC CAA CCT C | CAA TGA CGG TGG TGC AGA AG |
| Taqman RT-PCR | |||
| Gene | Gene Accession | Assay ID | |
| Acc | NM_133360.2 | Mm01304285_m1 | |
| Actb | NM_007393.5 | Mm02619580_g1 | |
| Dgat2 | NM_026384.3 | Mm00499536_m1 | |
| Protein (%) | Fat (%) | Ash (%) | Carbohydrates (%) | |
|---|---|---|---|---|
| Chlorella vulgaris | 48.0 ± 0.2 | 11.8 ± 2.0 | 20.5 ± 0.6 | 21.1 ± 0.6 |
| Nannochloropsis gaditana | 42.0 ± 9.2 | 5.6 ± 0.1 | 42.6 ± 4.2 | 9.9 ± 4.4 |
| Gracilaria vermiculophylla | 41.4 ± 0.7 | 3.5 ± 0.1 | 22.1 ± 3.2 | 33.4 ± 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Arceo, M.; Trepiana, J.; Aguirre, L.; Ibarruri, J.; Martínez-Sanz, M.; Cebrián, M.; Recio, I.; Portillo, M.P.; Gómez-Zorita, S. Anti-Steatotic Effects of Chlorella vulgaris, Nannochloropsis gaditana and Gracilaria vermiculophylla Algae Extracts in AML-12 Hepatocytes. Nutrients 2023, 15, 1960. https://doi.org/10.3390/nu15081960
González-Arceo M, Trepiana J, Aguirre L, Ibarruri J, Martínez-Sanz M, Cebrián M, Recio I, Portillo MP, Gómez-Zorita S. Anti-Steatotic Effects of Chlorella vulgaris, Nannochloropsis gaditana and Gracilaria vermiculophylla Algae Extracts in AML-12 Hepatocytes. Nutrients. 2023; 15(8):1960. https://doi.org/10.3390/nu15081960
Chicago/Turabian StyleGonzález-Arceo, Maitane, Jenifer Trepiana, Leixuri Aguirre, Jone Ibarruri, Marta Martínez-Sanz, Marta Cebrián, Isidra Recio, María P. Portillo, and Saioa Gómez-Zorita. 2023. "Anti-Steatotic Effects of Chlorella vulgaris, Nannochloropsis gaditana and Gracilaria vermiculophylla Algae Extracts in AML-12 Hepatocytes" Nutrients 15, no. 8: 1960. https://doi.org/10.3390/nu15081960
APA StyleGonzález-Arceo, M., Trepiana, J., Aguirre, L., Ibarruri, J., Martínez-Sanz, M., Cebrián, M., Recio, I., Portillo, M. P., & Gómez-Zorita, S. (2023). Anti-Steatotic Effects of Chlorella vulgaris, Nannochloropsis gaditana and Gracilaria vermiculophylla Algae Extracts in AML-12 Hepatocytes. Nutrients, 15(8), 1960. https://doi.org/10.3390/nu15081960