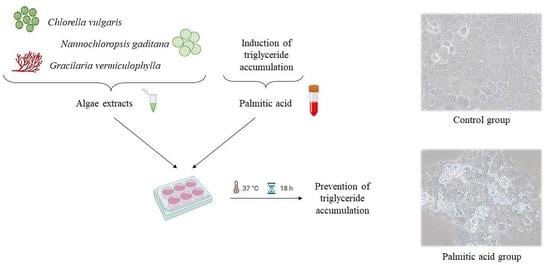
Anti-Steatotic Effects of Chlorella vulgaris, Nannochloropsis gaditana and Gracilaria vermiculophylla Algae Extracts in AML-12 Hepatocytes
Abstract
Share and Cite
González-Arceo, M.; Trepiana, J.; Aguirre, L.; Ibarruri, J.; Martínez-Sanz, M.; Cebrián, M.; Recio, I.; Portillo, M.P.; Gómez-Zorita, S. Anti-Steatotic Effects of Chlorella vulgaris, Nannochloropsis gaditana and Gracilaria vermiculophylla Algae Extracts in AML-12 Hepatocytes. Nutrients 2023, 15, 1960. https://doi.org/10.3390/nu15081960
González-Arceo M, Trepiana J, Aguirre L, Ibarruri J, Martínez-Sanz M, Cebrián M, Recio I, Portillo MP, Gómez-Zorita S. Anti-Steatotic Effects of Chlorella vulgaris, Nannochloropsis gaditana and Gracilaria vermiculophylla Algae Extracts in AML-12 Hepatocytes. Nutrients. 2023; 15(8):1960. https://doi.org/10.3390/nu15081960
Chicago/Turabian StyleGonzález-Arceo, Maitane, Jenifer Trepiana, Leixuri Aguirre, Jone Ibarruri, Marta Martínez-Sanz, Marta Cebrián, Isidra Recio, María P. Portillo, and Saioa Gómez-Zorita. 2023. "Anti-Steatotic Effects of Chlorella vulgaris, Nannochloropsis gaditana and Gracilaria vermiculophylla Algae Extracts in AML-12 Hepatocytes" Nutrients 15, no. 8: 1960. https://doi.org/10.3390/nu15081960
APA StyleGonzález-Arceo, M., Trepiana, J., Aguirre, L., Ibarruri, J., Martínez-Sanz, M., Cebrián, M., Recio, I., Portillo, M. P., & Gómez-Zorita, S. (2023). Anti-Steatotic Effects of Chlorella vulgaris, Nannochloropsis gaditana and Gracilaria vermiculophylla Algae Extracts in AML-12 Hepatocytes. Nutrients, 15(8), 1960. https://doi.org/10.3390/nu15081960