Switching from Sugar- to Artificially-Sweetened Beverages: A 12-Week Trial
Abstract
:1. Introduction
2. Method
2.1. Ethics and Protocol Registration
2.2. Participants
2.3. Design
2.4. Primary and Secondary Outcomes
2.5. Procedure
2.6. Statistical Analysis
3. Results
3.1. Recruitment
3.2. Primary Outcomes: Logical Memory
3.3. COWAT
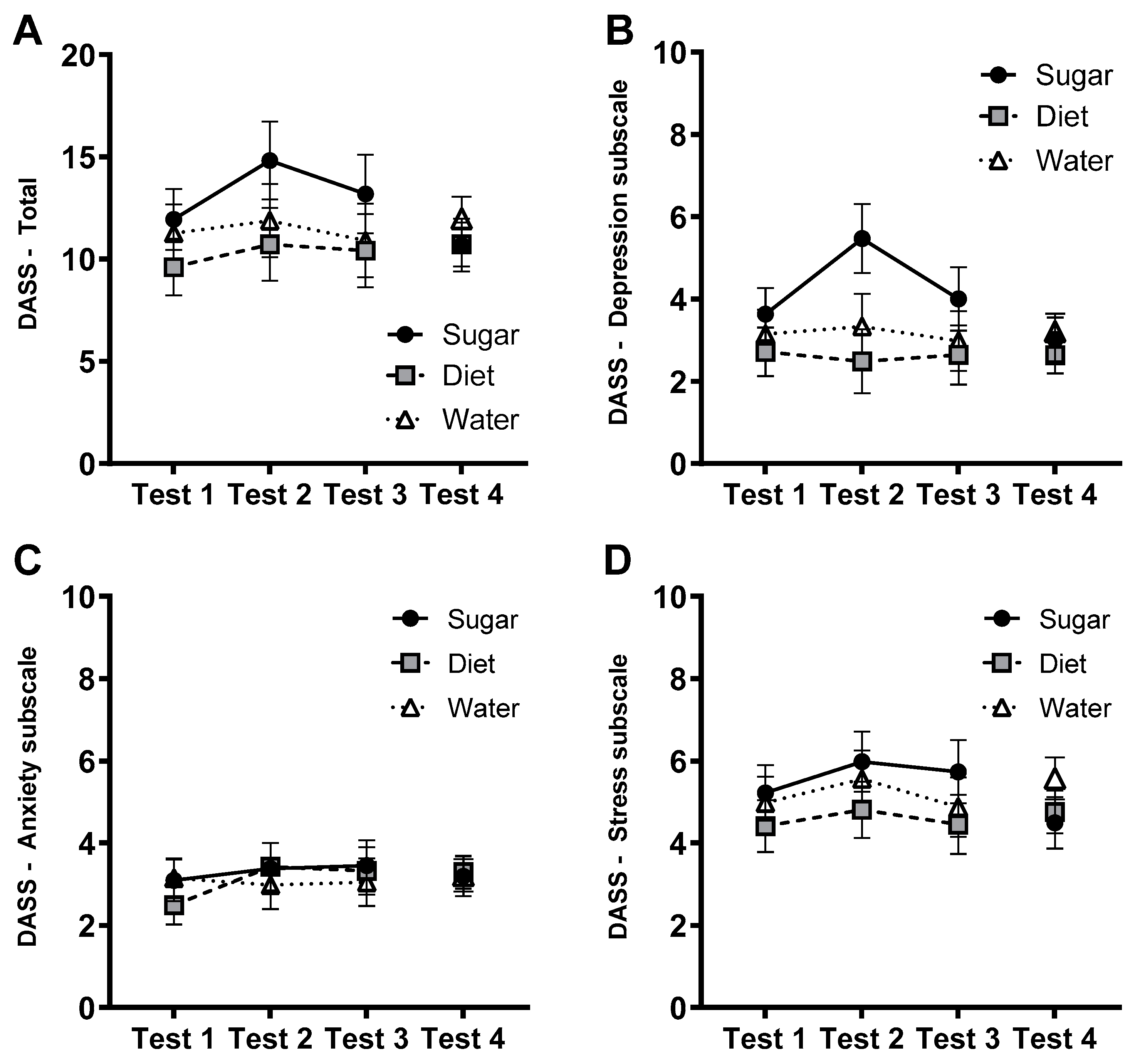
3.4. Affective Measures: DASS-21
3.5. Affective Measures: Impulsivity (BIS-11)
3.6. Primary Outcome: Waist Circumference to Height Ratio
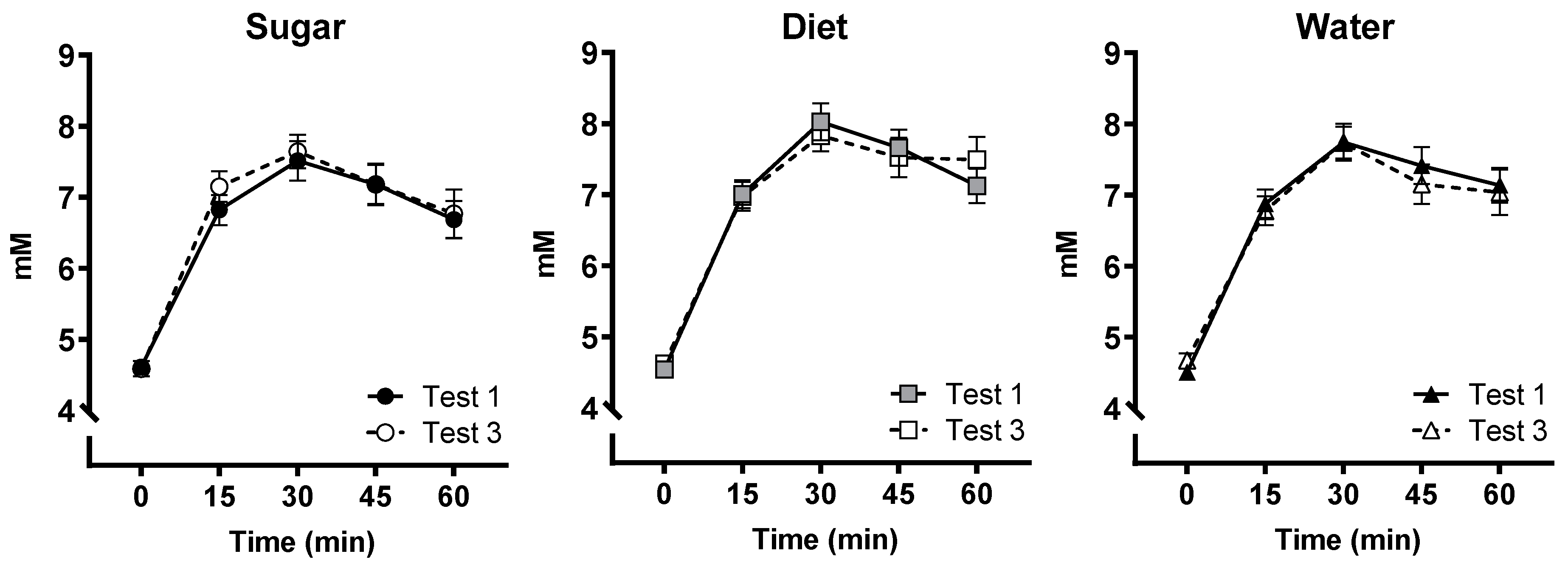
3.7. OGTT
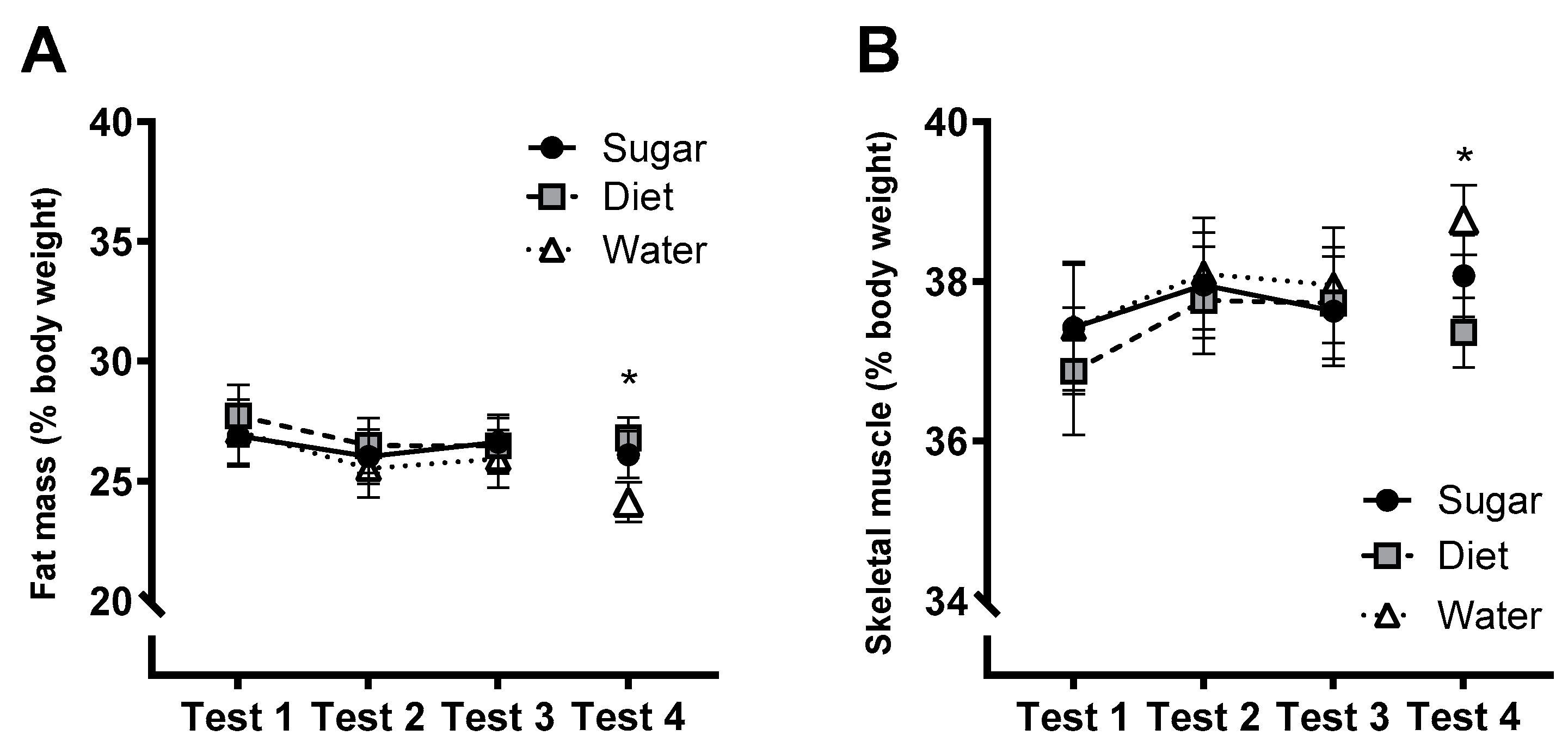
3.8. Metabolic and Physiological Measures
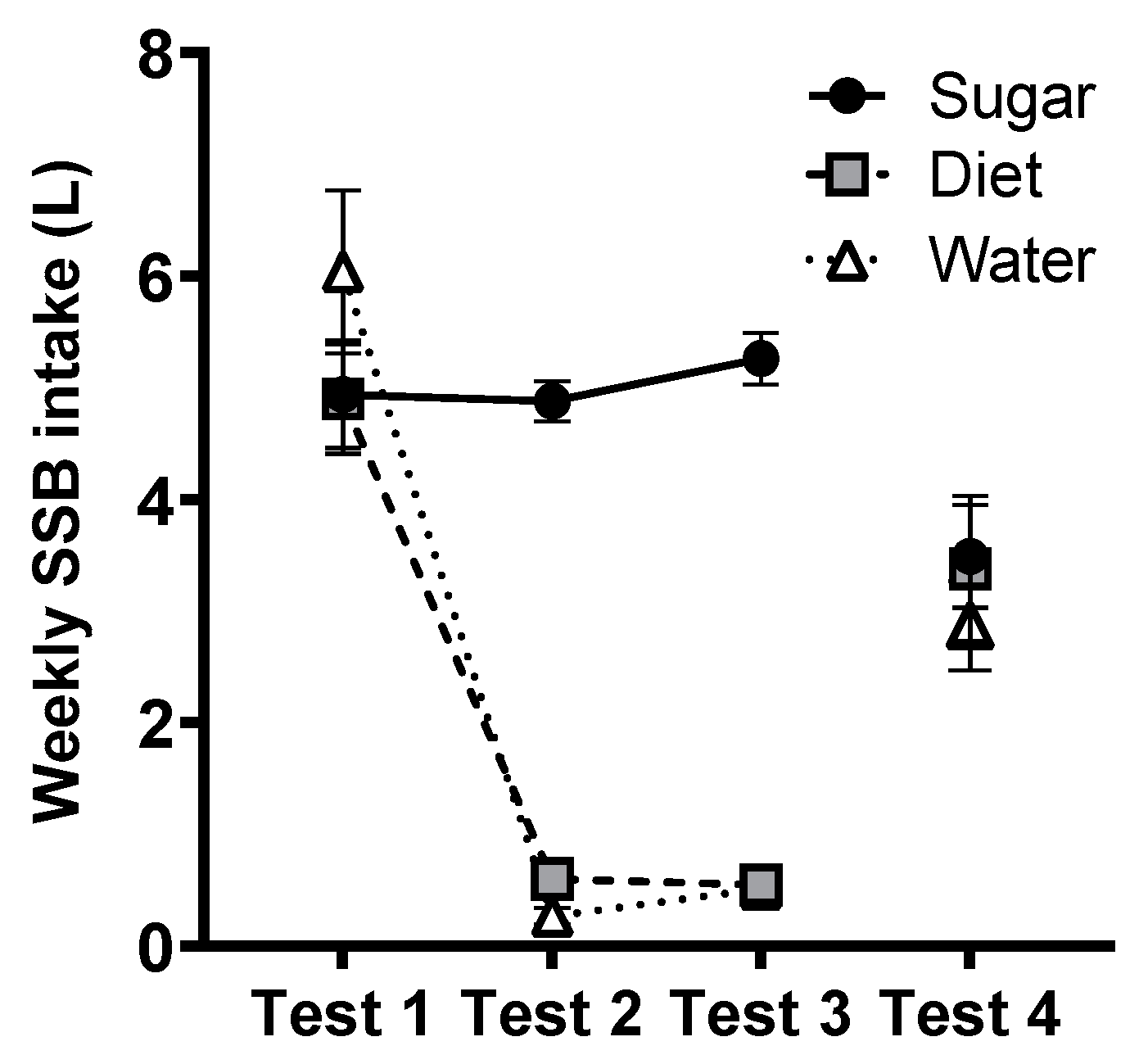
3.9. SSB Intake (BEVQ)
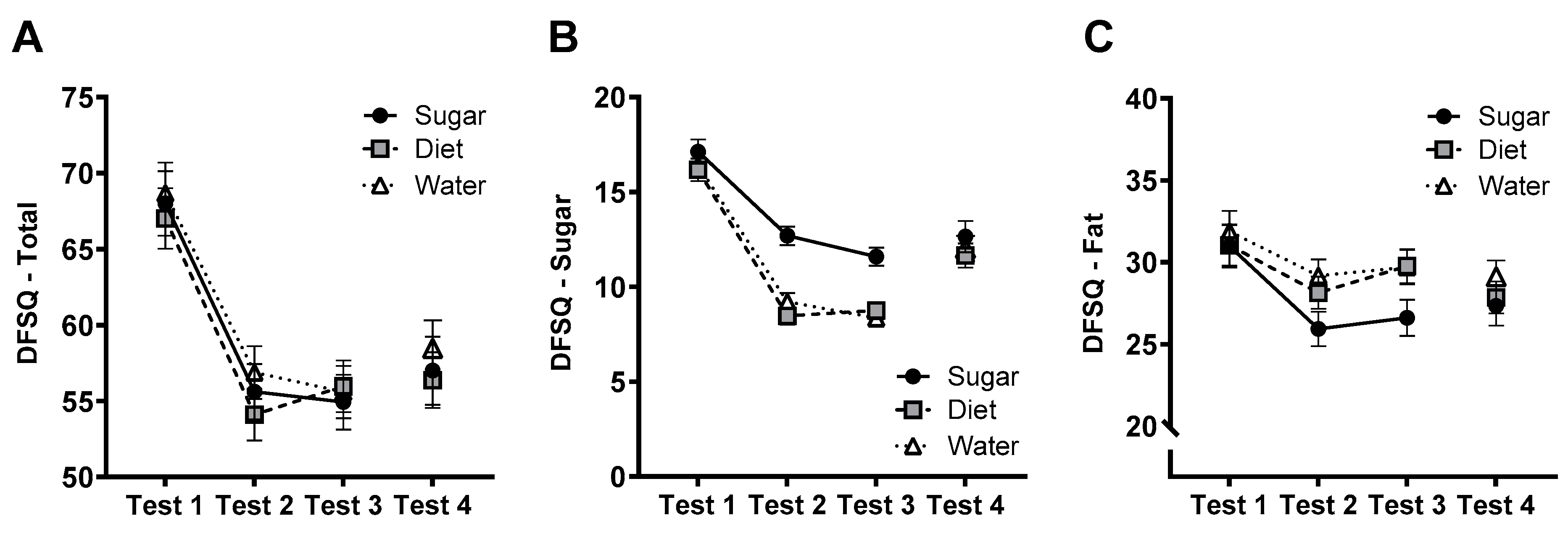
3.10. DFSQ
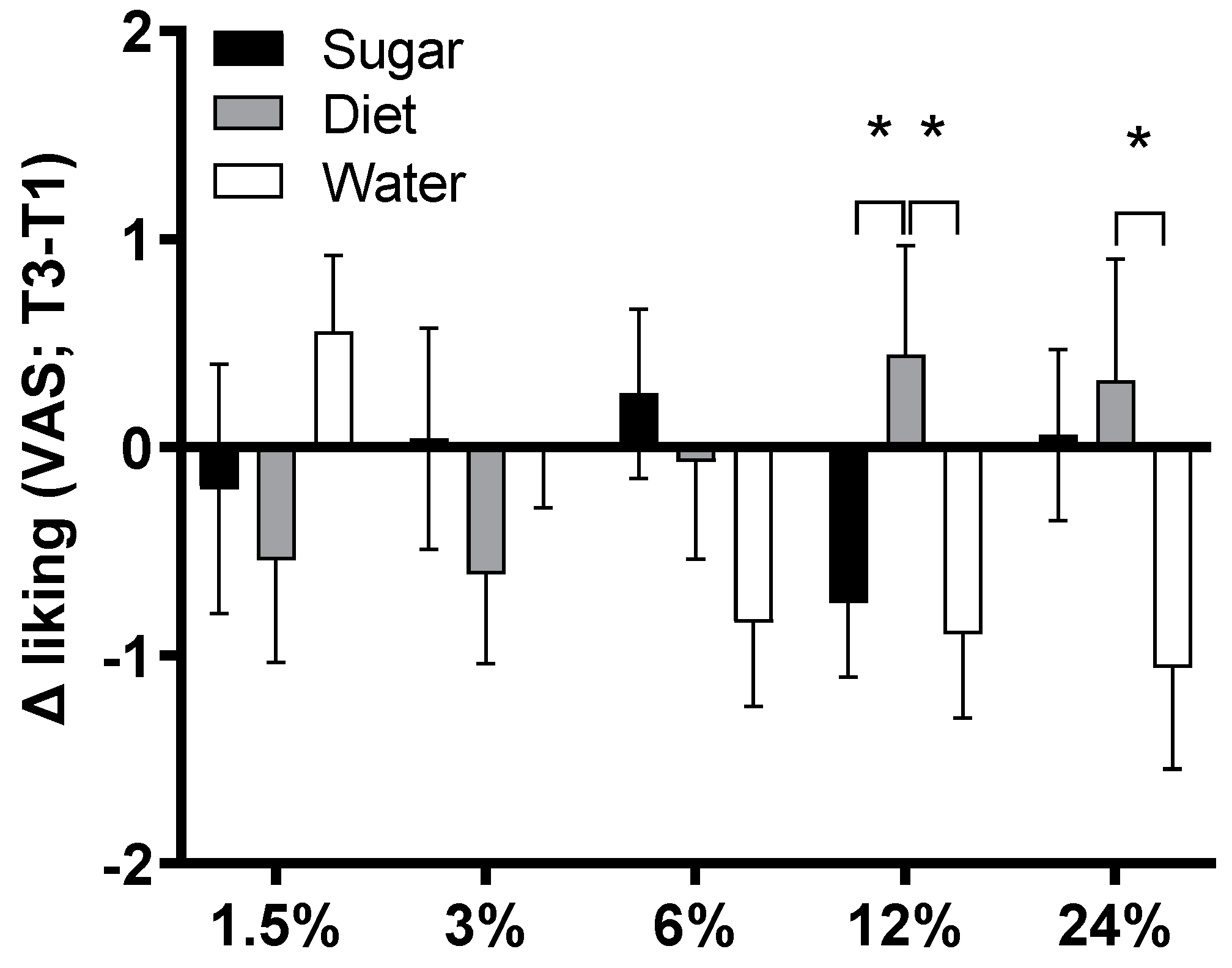
3.11. Sweet Taste Preference
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailey, R.L.; Fulgoni, I.I.I.V.L.; Cowan, A.E.; Gaine, P.C. Sources of added sugars in young children, adolescents, and adults with low and high intakes of added sugars. Nutrients 2018, 10, 102. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef]
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.M.; Dashti, H.S.; Ma, J.; Haslam, D.E.; Kiefte-de Jong, J.C.; Smith, C.E.; Tanaka, T.; Graff, M.; Lemaitre, R.N.; Rybin, D.; et al. Sugar-sweetened beverage intake associations with fasting glucose and insulin concentrations are not modified by selected genetic variants in a ChREBP-FGF21 pathway: A meta-analysis. Diabetologia 2018, 61, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Jarvis, S.E.; Tinajero, M.G.; Yu, J.; Chiavaroli, L.; Mejia, S.B.; Khan, T.A.; Tobias, D.K.; Willett, W.C.; Hu, F.B.; et al. Sugar-sweetened beverage consumption and weight gain in children and adults: A systematic review and meta-analysis of prospective cohort studies and randomized controlled trials. Am. J. Clin. Nutr. 2023, 117, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Cassady, B.A.; Considine, R.V.; Mattes, R.D. Beverage consumption, appetite, and energy intake: What did you expect? Am. J. Clin. Nutr. 2012, 95, 587–593. [Google Scholar] [CrossRef]
- Andreyeva, T.; Marple, K.; Marinello, S.; Moore, T.E.; Powell, L.M. Outcomes following taxation of sugar-sweetened beverages: A systematic review and meta-analysis. JAMA Netw. Open 2022, 5, e2215276. [Google Scholar] [CrossRef]
- Teng, A.M.; Jones, A.C.; Mizdrak, A.; Signal, L.; Genç, M.; Wilson, N. Impact of sugar-sweetened beverage taxes on purchases and dietary intake: Systematic review and meta-analysis. Obes. Rev. 2019, 20, 1187–1204. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Shi, M.; Zhou, Y.; Zhao, Y.; Xia, Y. Meta-analysis of sugar-sweetened beverage intake and the risk of cognitive disorders. J. Affect. Disord. 2022, 313, 177–185. [Google Scholar] [CrossRef]
- Muñoz-García, M.I.; Martínez-González, M.A.; Martín-Moreno, J.M.; Razquin, C.; Cervantes, S.; Guillén-Grima, F.; Toledo, E. Sugar-sweetened and artificially-sweetened beverages and changes in cognitive function in the SUN project. Nutr. Neurosci. 2020, 23, 946–954. [Google Scholar] [CrossRef]
- Kendig, M.D. Cognitive and behavioural effects of sugar consumption in rodents. A review. Appetite 2014, 80, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Abbott, K.N.; Arnott, C.K.; Westbrook, R.F.; Tran, D.M. The effect of high fat, high sugar, and combined high fat-high sugar diets on spatial learning and memory in rodents: A meta-analysis. Neurosci. Biobehav. Rev. 2019, 107, 399–421. [Google Scholar] [CrossRef]
- Taylor, Z.B.; Stevenson, R.J.; Ehrenfeld, L.; Francis, H.M. The impact of saturated fat, added sugar and their combination on human hippocampal integrity and function: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews 2021, 130, 91–106. [Google Scholar]
- Attuquayefio, T.; Stevenson, R.J.; Oaten, M.J.; Francis, H.M. A four-day Western-style dietary intervention causes reductions in hippocampal-dependent learning and memory and interoceptive sensitivity. PLoS ONE 2017, 12, e0172645. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Francis, H.M.; Attuquayefio, T.; Gupta, D.; Yeomans, M.R.; Oaten, M.J.; Davidson, T. Hippocampal-dependent appetitive control is impaired by experimental exposure to a Western-style diet. R. Soc. Open Sci. 2020, 7, 191338. [Google Scholar] [CrossRef]
- Kendig, M.D.; Fu, M.X.; Rehn, S.; Martire, S.I.; Boakes, R.A.; Rooney, K.B. Metabolic and cognitive improvement from switching to saccharin or water following chronic consumption by female rats of 10% sucrose solution. Physiol. Behav. 2018, 188, 162–172. [Google Scholar] [CrossRef]
- McLean, F.H.; Grant, C.; Morris, A.C.; Horgan, G.W.; Polanski, A.J.; Allan, K.; Campbell, F.M.; Langston, R.F.; Williams, L.M. Rapid and reversible impairment of episodic memory by a high-fat diet in mice. Sci. Rep. 2018, 8, 11976. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.M.; Westbrook, R.F. A high-fat high-sugar diet-induced impairment in place-recognition memory is reversible and training-dependent. Appetite 2017, 110, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Kendig, M.D.; Leigh, S.J.; Hasebe, K.; Kaakoush, N.O.; Westbrook, R.F.; Morris, M.J. Obesogenic diet cycling produces graded effects on cognition and microbiota composition in rats. Mol. Nutr. Food Res. 2023, in press. [CrossRef]
- McGlynn, N.D.; Khan, T.A.; Wang, L.; Zhang, R.; Chiavaroli, L.; Au-Yeung, F.; Lee, J.J.; Noronha, J.C.; Comelli, E.M.; Mejia, S.B.; et al. Association of low-and no-calorie sweetened beverages as a replacement for sugar-sweetened beverages with body weight and cardiometabolic risk: A systematic review and meta-analysis. JAMA Netw. Open 2022, 5, e222092. [Google Scholar] [CrossRef]
- Avery, A.; Bostock, L.; McCullough, F. A systematic review investigating interventions that can help reduce consumption of sugar-sweetened beverages in children leading to changes in body fatness. J. Hum. Nutr. Diet. 2015, 28, 52–64. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Feldman, H.A.; Chomitz, V.R.; Antonelli, T.A.; Gortmaker, S.L.; Osganian, S.K.; Ludwig, D.S. A randomized trial of sugar-sweetened beverages and adolescent body weight. N. Engl. J. Med. 2012, 367, 1407–1416. [Google Scholar] [CrossRef]
- Chen, L.; Appel, L.J.; Loria, C.; Lin, P.H.; Champagne, C.M.; Elmer, P.J.; Ard, J.D.; Mitchell, D.; Batch, B.C.; Svetkey, L.P.; et al. Reduction in consumption of sugar-sweetened beverages is associated with weight loss: The PREMIER trial. Am. J. Clin. Nutr. 2009, 89, 1299–1306. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Feldman, H.A.; Steltz, S.K.; Quinn, N.L.; Robinson, L.M.; Ludwig, D.S. Effects of sugar-Sweetened, artificially Sweetened, and Unsweetened beverages on cardiometabolic risk factors, body composition, and sweet taste preference: A randomized controlled trial. J. Am. Heart Assoc. 2020, 9, e015668. [Google Scholar] [CrossRef] [PubMed]
- Piernas, C.; Tate, D.F.; Wang, X.; Popkin, B.M. Does diet-beverage intake affect dietary consumption patterns? Results from the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am. J. Clin. Nutr. 2013, 97, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Appleton, K.M.; Tuorila, H.; Bertenshaw, E.J.; De Graaf, C.; Mela, D.J. Sweet taste exposure and the subsequent acceptance and preference for sweet taste in the diet: Systematic review of the published literature. Am. J. Clin. Nutr. 2018, 107, 405–419. [Google Scholar] [CrossRef]
- Francis, H.; Stevenson, R. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behav. Neurosci. 2011, 125, 943–955. [Google Scholar] [CrossRef]
- Maersk, M.; Belza, A.; Stødkilde-Jørgensen, H.; Ringgaard, S.; Chabanova, E.; Thomsen, H.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: A 6-mo randomized intervention study. Am. J. Clin. Nutr. 2012, 95, 283–289. [Google Scholar] [CrossRef]
- Ashwell, M.; Mayhew, L.; Richardson, J.; Rickayzen, B. Waist-to-height ratio is more predictive of years of life lost than body mass index. PLoS ONE 2014, 9, e103483. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Memory Scale-Revised; Psychological Corporation: New York, NY, USA, 1987. [Google Scholar]
- Sullivan, K. Alternate forms of prose passages for the assessment of auditory–verbal memory. Arch. Clin. Neuropsychol. 2005, 20, 745–753. [Google Scholar] [CrossRef]
- Schnabel, R. Overcoming the challenge of re-assessing logical memory. Clin. Neuropsychol. 2012, 26, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Benton, A.L. Problems of test construction in the field of aphasia. Cortex 1967, 3, 32–58. [Google Scholar] [CrossRef]
- Benton, A.L.; Hamsher, K.; Sivan, A.B. Multilingual Aphasia Examination; AJA Associates: Iowa City, IA, USA, 1994. [Google Scholar]
- Ross, T.P.; Furr, A.E.; Carter, S.E.; Weinberg, M. The psychometric equivalence of two alternate forms of the Controlled Oral Word Association Test. Clin. Neuropsychol. 2006, 20, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, J.G.; Benton, A.L.; Spreen, O. Word fluency and brain damage. Neuropsychologia 1967, 5, 135–140. [Google Scholar] [CrossRef]
- Hedrick, V.E.; Savla, J.; Comber, D.L.; Flack, K.D.; Estabrooks, P.A.; Nsiah-Kumi, P.A.; Ortmeier, S.; Davy, B.M. Development of a brief questionnaire to assess habitual beverage intake (BEVQ-15): Sugar-sweetened beverages and total beverage energy intake. J. Acad. Nutr. Diet. 2012, 112, 840–849. [Google Scholar] [CrossRef]
- Francis, H.; Stevenson, R. Validity and test–retest reliability of a short dietary questionnaire to assess intake of saturated fat and free sugars: A preliminary study. J. Hum. Nutr. Diet. 2013, 26, 234–242. [Google Scholar] [CrossRef]
- Barratt, E.S. Impulsiveness subtraits: Arousal and information processing. Motiv. Emot. Personal. 1985, 99, 137–146. [Google Scholar]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Henry, J.D.; Crawford, J.R. The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2005, 44, 227–239. [Google Scholar] [CrossRef]
- Lovibond, P.F.; Lovibond, S.H. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther. 1995, 33, 335–343. [Google Scholar] [CrossRef]
- García, C.R.; Piernas, C.; Martínez-Rodríguez, A.; Hernández-Morante, J.J. Effect of glucose and sucrose on cognition in healthy humans: A systematic review and meta-analysis of interventional studies. Nutr. Rev. 2021, 79, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Loonstra, A.S.; Tarlow, A.R.; Sellers, A.H. COWAT metanorms across age, education, and gender. Appl. Neuropsychol. 2001, 8, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Debras, C.; Chazelas, E.; Sellem, L.; Porcher, R.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaësse, C.; De Sa, A.; Lutchia, R.; et al. Artificial sweeteners and risk of cardiovascular diseases: Results from the prospective NutriNet-Santé cohort. BMJ 2022, 378, e071204. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, K. Is the Use of Artificial Sweeteners Beneficial for Patients with Diabetes Mellitus? The Advantages and Disadvantages of Artificial Sweeteners. Nutrients 2022, 14, 4446. [Google Scholar] [CrossRef]
- Suez, J.; Cohen, Y.; Valdés-Mas, R.; Mor, U.; Dori-Bachash, M.; Federici, S.; Zmora, N.; Leshem, A.; Heinemann, M.; Linevsky, R.; et al. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell 2022, 185, 3307–3328. [Google Scholar] [CrossRef]
- Crézé, C.; Notter-Bielser, M.L.; Knebel, J.F.; Campos, V.; Tappy, L.; Murray, M.; Toepel, U. The impact of replacing sugar-by artificially-sweetened beverages on brain and behavioral responses to food viewing–An exploratory study. Appetite 2018, 123, 160–168. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kendig, M.D.; Chow, J.Y.L.; Martire, S.I.; Rooney, K.B.; Boakes, R.A. Switching from Sugar- to Artificially-Sweetened Beverages: A 12-Week Trial. Nutrients 2023, 15, 2191. https://doi.org/10.3390/nu15092191
Kendig MD, Chow JYL, Martire SI, Rooney KB, Boakes RA. Switching from Sugar- to Artificially-Sweetened Beverages: A 12-Week Trial. Nutrients. 2023; 15(9):2191. https://doi.org/10.3390/nu15092191
Chicago/Turabian StyleKendig, Michael D., Julie Y. L. Chow, Sarah I. Martire, Kieron B. Rooney, and Robert A. Boakes. 2023. "Switching from Sugar- to Artificially-Sweetened Beverages: A 12-Week Trial" Nutrients 15, no. 9: 2191. https://doi.org/10.3390/nu15092191




