Malnutrition, Functional Decline, and Institutionalization in Older Adults after Hospital Discharge Following Community-Acquired Pneumonia
Abstract
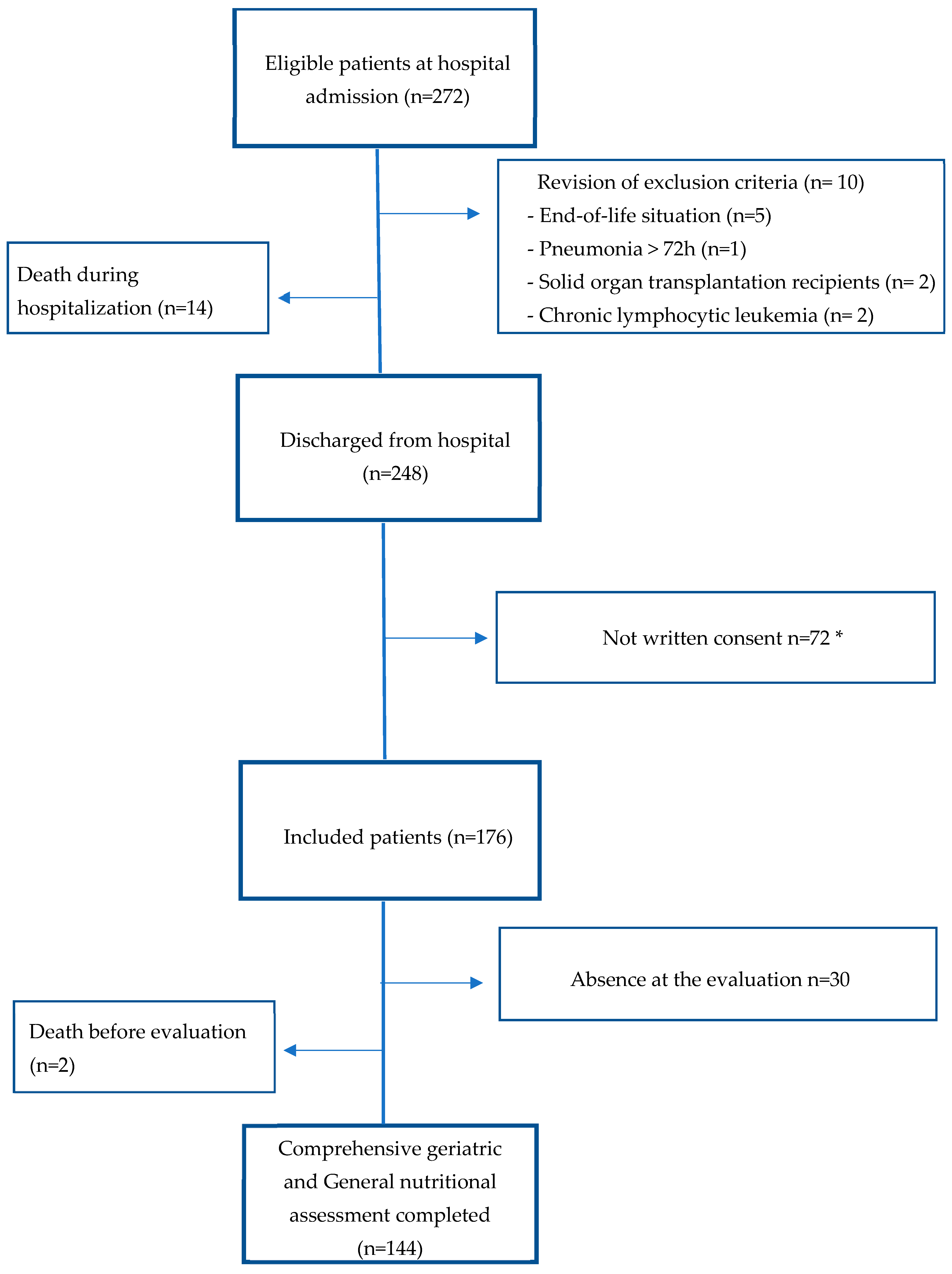
:1. Introduction
2. Methods and Patients
2.1. Study Design
2.2. Subjects
2.3. Data Collection and Measures
2.4. Statistical Analysis
3. Results
3.1. Functional Decline and Institutionalization after the CAP Episode
3.2. General Nutritional Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAP | Community-acquired pneumonia |
| FS | Functional status |
| BI | Barthel Index |
| PSI | Pneumonia Severity Index |
| CGA | Comprehensive geriatric assessment |
| GNA | General nutritional assessment |
| SPMSQ | Short Portable Mental Status Questionnaire |
| CCI | Charlson Comorbidity Index |
| ADL | Activities of daily living |
| BMI | Body mass index |
| MNA | Mini Nutritional Assessment |
References
- Almirall, J.; Serra-Prat, M.; Bolíbar, I.; Balasso, V. Risk Factors for Community-Acquired Pneumonia in Adults: A Systematic Review of Observational Studies. Respiration 2017, 94, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.B.; Hamer, D.H.; Meydani, S.N. Low zinc status: A new risk factor for pneumonia in the elderly? Nutr. Rev. 2010, 68, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Uno, C.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Ogawa, N.; Okamoto, T.; Hoyano, K.; Momosaki, R. Nutritional status change and activities of daily living in elderly pneumonia patients admitted to acute care hospital: A retrospective cohort study from the Japan Rehabilitation Nutrition Database. Nutrition 2020, 71, 110613. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hara, Y.; Horita, N.; Saigusa, Y.; Hirai, Y.; Kaneko, T. Declined Functional Status Prolonged Hospital Stay for Community-Acquired Pneumonia in Seniors. Clin. Interv. Aging 2020, 15, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Kosai, K.; Izumikawa, K.; Imamura, Y.; Tanaka, H.; Tsukamoto, M.; Kurihara, S.; Takazono, T.; Morinaga, Y.; Nakamura, S.; Miyazaki, T.; et al. Importance of Functional Assessment in the Management of Community-acquired and Healthcare-associated Pneumonia. Intern. Med. 2014, 53, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Torres, O.H.; Muñoz, J.; Ruiz, D.; Ris, J.; Gich, I.; Coma, E.; Gurguã, M.; Vázquez, G. Outcome Predictors of Pneumonia in Elderly Patients: Importance of Functional Assessment. J. Am. Geriatr. Soc. 2004, 52, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.; Verloo, H.; von Gunten, A.; del Rio Carral, M.; Meyer-Massetti, C.; Martins, M.M.; Wernli, B. Unplanned nursing home admission among discharged polymedicated older inpatients: A single-centre, registry-based study in Switzerland. BMJ Open 2022, 12, e057444. [Google Scholar] [CrossRef]
- Rapp, K.; Rothenbacher, D.; Magaziner, J.; Becker, C.; Benzinger, P.; König, H.-H.; Jaensch, A.; Büchele, G. Risk of Nursing Home Admission After Femoral Fracture Compared with Stroke, Myocardial Infarction, and Pneumonia. J. Am. Med. Dir. Assoc. 2015, 16, 715.e7–715.e12. [Google Scholar] [CrossRef]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults—Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Conzade, R.; Koenig, W.; Heier, M.; Schneider, A.; Grill, E.; Peters, A.; Thorand, B. Prevalence and Predictors of Subclinical Micronutrient Deficiency in German Older Adults: Results from the Population-Based KORA—Age Study. Nutrients 2017, 9, 1276. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Besora-Moreno, M.; Llauradó, E.; Tarro, L.; Solà, R. Social and Economic Factors and Malnutrition or the Risk of Malnutrition in the Elderly: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2020, 12, 737. [Google Scholar] [CrossRef] [PubMed]
- El Solh, A.; Pineda, L.; Bouquin, P.; Mankowski, C. Determinants of short and long term functional recovery after hospitalization for community-acquired pneumonia in the elderly: Role of inflammatory markers. BMC Geriatr. 2006, 6, 12–12. [Google Scholar] [CrossRef] [PubMed]
- Torres Bonafonte, O.H.; Gil Olivas, E.; Pérez Macho, E.; Pacho Pacho, C.; Mateo Roca, M.; Casademont Pou, J.; Ruiz Hidalgo, D. Predictors of drug-resistant pathogens in community-onset pneumonia: Are factors considered in health-care-associated pneumonia useful in the emergency department? Emergencias 2017, 29, 306–312. [Google Scholar] [PubMed]
- Fine, M.J.; Auble, T.E.; Yealy, D.M.; Hanusa, B.H.; Weissfeld, L.A.; Singer, D.E.; Coley, C.M.; Marrie, T.J.; Kapoor, W.N. A Prediction Rule to Identify Low-Risk Patients with Community-Acquired Pneumonia. N. Engl. J. Med. 1997, 336, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Pfeiffer, E. A Short Portable Mental Status Questionnaire for the Assessment of Organic Brain Deficit in Elderly Patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Stevens, P.E.; Levin, A. Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.-L. The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrients 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Andrew, M.K.; MacDonald, S.; Godin, J.; McElhaney, J.E.; LeBlanc, J.; Hatchette, T.F.; Bowie, W.; Katz, K.; McGeer, A.; Semret, M.; et al. Persistent Functional Decline Following Hospitalization with Influenza or Acute Respiratory Illness. J. Am. Geriatr. Soc. 2021, 69, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Serna-Higuita, L.M.; Martus, P.; Mirakaj, V.; Koeppen, M.; Zarbock, A.; Marx, G.; Putensen, C.; Rosenberger, P.; Haeberle, H.A. COVID-19 does not influence functional status after ARDS therapy. Crit. Care 2023, 27, 48. [Google Scholar] [CrossRef] [PubMed]
- Le Gentil, S.; Prampart, S.; Karakachoff, M.; Bureau, M.L.; Chapelet, G.; De Decker, L.; Rouaud, A.; Boureau, A.-S. Functional Decline in COVID-19 Older Survivors Compared to Other Pneumonia Patients, a Case Control Study. J. Nutr. Health Aging 2022, 26, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Kahn, K.L.; Keeler, E.B.; Sherwood, M.J.; Rogers, W.H.; Draper, D.; Bentow, S.S.; Reinisch, E.J.; Rubenstein, L.V.; Kosecoff, J.; Brook, R.H. Comparing Outcomes of Care Before and After Implementation of the DRG-Based Prospective Payment System. JAMA 1990, 264, 1984–1988. [Google Scholar] [CrossRef] [PubMed]
- Lozada, T.A.; Carrasco, P.G.; Codina, A.F. Impact on the risk of malnutrition and depression of a clinical trial with nutritional educational intervention in non-institutionalized elderly subjects receiving a telecare service in Terrassa (Spain). Nutr Hosp. 2021, 38, 260–266. [Google Scholar] [CrossRef]
- Leij-Halfwerk, S.; Verwijs, M.H.; van Houdt, S.; Borkent, J.W.; Guaitoli, P.R.; Pelgrim, T.; Heymans, M.W.; Power, L.; Visser, M.; Corish, C.A.; et al. Prevalence of protein-energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults ≥65 years: A systematic review and meta-analysis. Maturitas 2019, 126, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Amasene, M.; Besga, A.; Medrano, M.; Urquiza, M.; Rodriguez-Larrad, A.; Tobalina, I.; Barroso, J.; Irazusta, J.; Labayen, I. Nutritional status and physical performance using handgrip and SPPB tests in hospitalized older adults. Clin. Nutr. 2021, 40, 5547–5555. [Google Scholar] [CrossRef]
- Hamirudin, A.H.; Walton, K.; Charlton, K.; Carrie, A.; Tapsell, L.; Milosavljevic, M.; Pang, G.; Potter, J. Feasibility of home-based dietetic intervention to improve the nutritional status of older adults post-hospital discharge. Nutr. Diet. 2017, 74, 217–223. [Google Scholar] [CrossRef]
- Young, A.M.; Mudge, A.M.; Banks, M.D.; Rogers, L.; Allen, J.; Vogler, B.; Isenring, E. From hospital to home: Limited nutritional and functional recovery for older adults. J. Frailty Aging 2015, 4, 69–73. [Google Scholar] [CrossRef]
- Hori, S.; Yamamoto, Y.; Ushida, K.; Shirai, Y.; Shimizu, M.; Kato, Y.; Shimizu, A.; Momosaki, R. Impact of Frailty Risk on Oral Intake and Length of Hospital Stay in Older Patients with Pneumonia: A Historical Cohort Study. J. Clin. Med. 2022, 12, 77. [Google Scholar] [CrossRef]
- Shimizu, A.; Momosaki, R.; Kayashita, J.; Fujishima, I. Impact of Multiple Texture-Modified Diets on Oral Intake and Nutritional Status in Older Patients with Pneumonia: A Retrospective Cohort Study. Dysphagia 2020, 35, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Yanagita, Y.; Arizono, S.; Tawara, Y.; Oomagari, M.; Machiguchi, H.; Yokomura, K.; Katagiri, N.; Iida, Y. The severity of nutrition and pneumonia predicts survival in patients with aspiration pneumonia: A retrospective observational study. Clin. Respir. J. 2022, 16, 522–532. [Google Scholar] [CrossRef] [PubMed]
- De Blasio, F.; Scalfi, L.; Castellucci, B.; Sacco, A.M.; Berlingieri, G.M.; Capitelli, L.; Alicante, P.; Sanduzzi, A.; Bocchino, M. Poor Nutritional Status and Dynapenia Are Highly Prevalent in Post-Acute COVID-19. Front. Nutr. 2022, 9, 888485. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, L.F.C.; Barros, A.N.A.B.; Leite-Lais, L. Nutritional risk of vitamin D, vitamin C, zinc, and selenium deficiency on risk and clinical outcomes of COVID-19: A narrative review. Clin. Nutr. ESPEN 2022, 47, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Rink, L.; Ajsuvakova, O.P.; Aschner, M.; Gritsenko, V.A.; Alekseenko, S.I.; Svistunov, A.A.; Petrakis, D.; Spandidos, D.A.; Aaseth, J.; et al. Zinc and respiratory tract infections: Perspectives for COVID-19 (Review). Int. J. Mol. Med. 2020, 46, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Remmelts, H.H.F.; Spoorenberg, S.M.C.; Oosterheert, J.J.; Bos, W.J.W.; de Groot, M.C.H.; van de Garde, E.M.W. The role of vitamin D supplementation in the risk of developing pneumonia: Three independent case–control studies. Thorax 2013, 68, 990–996. [Google Scholar] [CrossRef]
- Padhani, Z.A.; Moazzam, Z.; Ashraf, A.; Bilal, H.; A Salam, R.; Das, J.K.; A Bhutta, Z. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst. Rev. 2020, 4, CD013134. [Google Scholar] [CrossRef]
- Díaz-Rizzolo, D.A.; Kostov, B.; Gomis, R.; Sisó-Almirall, A. Paradoxical suboptimal vitamin D levels in a Mediterranean area: A population-based study. Sci. Rep. 2022, 12, 19645. [Google Scholar] [CrossRef]
- Schüpbach, R.; Wegmüller, R.; Berguerand, C.; Bui, M.; Herteraeberli, I. Micronutrient status and intake in omnivores, vegetarians and vegans in Switzerland. Eur. J. Nutr. 2017, 56, 283–293. [Google Scholar] [CrossRef]
| Baseline Characteristics | N = 144 | Previous Institutionalization N = 13 | Previous Community Dwelling N = 131 | p |
|---|---|---|---|---|
| Male sex, n (%) | 80 (55.6) | 5 (38.4) | 75 (57.2) | 0.193 |
| Age, mean years (±SD) | 77.1 (7.9) | 81.5 (8.6) | 76.7 (7.7) | 0.046 |
| Smoking status, n (%) | 7 (4.9) | 0 | 7 (5) | 0.392 |
| Barthel Index, mean points (±SD) | 93.0 (17.1) | 78.0 (31.4) | 94.5 (14.3) | 0.064 |
| SARS-CoV-2 etiology, n (%) | 41 (28.4) | 1 (7.6) | 40 (30.5) | 0.082 |
| PSI, mean points (±SD) | 98.1 (25.9) | 111.2 (25.0) | 96.8 (25.7) | 0.025 |
| PSI without age, mean points (±SD) | 20.9 (23.5) | 29.6 (28.3) | 20.1 (22.9) | 0.166 |
| Intensive care admission, n (%) | 12 (8.3) | 1(7,6) | 11(8,3) | 0.930 |
| Length of stay, mean days (±SD) | 9.7 (7.8) | 9.9 (7.3) | 9.6 (7.9) | 0.810 |
| Charlson Comorbidity Index, mean points (±SD) | 1.5 (1.6) | 1.0 (1.8) | 1.0 (1.6) | 0.751 |
| Main comorbidities a: | ||||
| Chronic lung disease, n (%) | 42 (28.4) | 3 (23) | 38 (29) | 0.651 |
| Mild to moderate diabetes b, n (%) | 33 (22.3) | 1 (7.6) | 32 (24.4) | 0.171 |
| Congestive heart failure, n (%) | 24 (16.2) | 3 (23) | 20 (15.2) | 0.463 |
| Acute myocardial infarction, n (%) | 14 (9.5) | 1 (0.07) | 12 (9.1) | 0.896 |
| Cerebrovascular disease, n (%) | 14 (9.5) | 0 | 14 (10.6) | 0.215 |
| Peripheral vascular disease, n (%) | 10 (6.8) | 2 (15.3) | 8 (6.1) | 0.209 |
| Diabetes with chronic complications c, n (%) | 10 (6.8) | 1 (7.6) | 9 (6.8) | 0.911 |
| Malignant tumors, n (%) | 9 (6.1) | 1 (7.6) | 8 (6.1) | 0.822 |
| Dementia, n (%) | 8 (5.4) | 2 (15.3) | 6 (4.5) | 0.105 |
| Lymphoma, n (%) | 5 (3.4) | 0 | 5 (3.8) | 0.473 |
| Rheumatic disease, n (%) | 4 (2.7) | 1 (7.6) | 3 (2.2) | 0.258 |
| Peptic ulcer, n (%) | 3 (2) | 0 | 3 (2.2) | 0.581 |
| Solid metastatic tumor, n (%) | 2 (1.4) | 0 | 2 (1.5) | 0.654 |
| Mild liver disease, n (%) | 2 (1.4) | 0 | 2 (1.5) | 0.654 |
| Chronic kidney disease d, n (%) Polycythemia Vera | 2 (1.4) 2 (1.4) | 1 (7.6) 0 | 1 (0.7) 2 (1.5) | 0.042 0.654 |
| Comprehensive Geriatric Assessment | N = 144 | Institutionalization at 45 d after Discharge N = 28 | Community Dwelling at 45 d after Discharge N = 116 | p |
|---|---|---|---|---|
| Barthel Index, mean points (±SD) | 83.7 (22.7) | 54.0 (25.7) | 90.5 (14.7) | <0.001 |
| Independence for instrumental activities of daily living, mean points (±SD) | 4.4 (2.6) | 1.07 (1.1) | 5.11 (2.2) | <0.001 |
| Hospital readmission after episode of pneumonia, n (%) | 10 (6.9) | 2 (7.1) | 8 (6.8) | 0.963 |
| SPMSQ, mean points (±SD) | 1.2 (1.9) | 2.4 (6.3) | 1.0 (1.7) | <0.001 |
| General nutritional assessment at 45 days of discharge: | ||||
| BMI, kg/m2, mean points (±SD) a | 27.0 (5.4) | 25.0 (6.3) | 27.3 (5.2) | 0.016 |
| MNA questionnaire, mean points (±SD): | 20.51 (5.0) | 14.7 (4.7) | 21.6 (4.2) | <0.001 |
| Well-nourished (>24 points), n (%) | 53 (37) | 1 (3.6) | 52 (44.8) | <0.001 |
| Risk of malnutrition (17–24), n (%) | 61 (42) | 8 (28.6) | 53 (45.7) | <0.001 |
| Malnourished (<17 points), n (%) | 30 (21) | 19 (67.9) | 11 (9.5) | <0.001 |
| Hypoalbuminemia (<34.9 g/L), n (%) | 19 (13.2) | 9 (32.1) | 10 (8.6) | <0.001 |
| Lymphopenia (<1000 × 109/L), n (%) | 23 (16) | 4 (14.3) | 19 (16.4) | 0.786 |
| Chronic kidney disease b | 119 (82.6) | 23 (82.1) | 96 (82.7) | 0.938 |
| eGFR level 60–90 mL/min, n (%) | 82 (56.9) | 12 (42.8) | 70 (60.3) | 0.037 |
| eGFR level 30–60 mL/min, n (%) | 28 (19.4) | 6 (21.4) | 22 (18.9) | |
| eGFR level 15–30 mL/min, n (%) | 8 (5.6) | 4 (14.2) | 4 (3.4) | |
| eGFR level <15 mL/min, n (%) | 1 (0.7) | 1 (3.5) | 0 | |
| Albumin (g/L), mean points (±SD) | 38.8 (3.9) | 36.2 (4.2) | 39.0 (3.6) | <0.001 |
| CRP (mg/L), mean points (±SD) | 11.5 (22.5) | 24.54 (33.9) | 9.67 (19.2) | <0.001 |
| Sodium (mmol/L), mean points (±SD) | 139.8 (3.9) | 139.6 (3.9) | 139.9 (2.6) | 0.466 |
| Creatinine (pmol/L), mean points (±SD) | 90.8 (43.6) | 106.3 (70.0) | 87.0 (33.9) | 0.824 |
| Hemoglobin (g/dL), mean points (±SD) | 124.5 (18.0) | 120.1 (16.9) | 124.92 (18.7) | 0.149 |
| Functional Decline (>10 Points) N = 43 | No Functional Decline N = 101 | p | New Institutionalization N = 16 | Return to Community Dwelling N = 115 | p | |
|---|---|---|---|---|---|---|
| Male sex, n (%) | 9 (56.2) | 66 (57.3) | 0.931 | 9 (55.6) | 66 (57.3) | 0.931 |
| Age, mean points (±SD) | 82.1 (7.5) | 75.0 (7.1) | 0.001 | 80.0 (7.4) | 76.2 (7.6) | 0.063 |
| Smoking status, n (%) | 2 (4.6) | 5 (4.9) | 0.939 | 2 (12.5) | 5 (4.3) | 0.174 |
| Hospital admission after episode of CAP, n (%) | 6 (13.9) | 11 (10.8) | 0.602 | 2 (12.5) | 8 (6.8) | 0.434 |
| Previous Barthel Index, mean points (±SD) | 96.4 (15.5) | 91.6 (17.6) | 0.005 | 82.5 (25.6) | 96.2 (11.2) | 0.031 |
| SARS-CoV-2 etiology, n (%) | 5 (11.6) | 36 (35.6) | 0.003 | 0 | 40 (34.7) | 0.005 |
| PSI, mean points (±SD) | 113.4 (26.2) | 91.6 (23.0) | <0.001 | 103.94 (24.0) | 95.8 (25.9) | 0.112 |
| PSI without age, mean points (±SD) | 31.2 (26.5) | 16.5 (20.7) | <0.001 | 23.9 (21.8) | 19.5 (23.1) | 0.329 |
| Intensive care admission, n (%) | 5(11.6) | 7(6.9) | 0.351 | 3 (18.7) | 8 (6.9) | 0.111 |
| Length of stay, mean days (±SD) | 12.7 (8.5) | 8.4 (7.2) | <0.001 | 15.1 (9.0) | 8.9 (7.5) | 0.001 |
| Charlson Comorbidity Index, mean points (±SD) | 2.1 (1.8) | 1.2 (1.4) | <0.001 | 2.1 (2.2) | 1.4 (1.5) | 0.372 |
| Main comorbidities: | ||||||
| Chronic lung disease, n (%) | 13 (30.2) | 28 (27.7) | 0.760 | 4 (25) | 34 (29.5) | 0.706 |
| Diabetes (mild to severe), n (%) | 15 (34.8) | 27(26.7) | 0.325 | 6 (37.5) | 34 (29.5) | 0.518 |
| Cardiopathy a, n (%) | 19 (44.1) | 11 (10.8) | <0.001 | 4 (25) | 22 (19.1) | 0.518 |
| Chronic kidney disease b, n (%) | 1 (2.3) | 1 (0.9) | 0.531 | 1 (6.2) | 0 | 0.007 |
| Cerebrovascular disease, n (%) | 6 (13.9) | 8 (7.9) | 0.263 | 2 (12.5) | 12 (10.4) | 0.802 |
| Peripheral vascular disease, n (%) | 7 (16.2) | 3 (2.9) | 0.004 | 3 (18.7) | 5 (4.3) | 0.024 |
| Malignant tumors or solid metastatic tumors, n (%) | 4 (9.3) | 7 (6.9) | 0.624 | 2 (12.5) | 8 (6.9) | 0.434 |
| Dementia, n (%) | 4 (9.3) | 4 (3.9) | 0.200 | 1 (6.2) | 5 (6.2) | 0.733 |
| Vitamins | ||||
|---|---|---|---|---|
| Mean Value (±SD) | Deficiency Definitions | Deficiency n, (%) | Normal n, (%) | |
| Vitamin B1 (nmol/L) | 137 (41.1) | <66.5 nmol/L | 3/141 (2.1) | 138/141 (97.8) |
| Vitamin B2 (ng/mL) | 187.8 (38.6) | <125 ng/mL | 4/139 (2.8) | 135/139 (93.8) |
| Vitamin B6 (ng/mL) | 153.6 (544.7) | <125 ng/mL | 2/141 (1.4) | 139/141 (98.5) |
| Folate (nmol/L) | 16.8 (9.6) | <7 nmol/L | 11/144 (7.6) | 133/144 (92.3) |
| Vitamin B12 (pmol/L) | 343.1 (265.5) | <150 pmol/L | 5/143 (3.5) | 138/143 (96.5) |
| Vitamin C (mg/dL) | 0.55 (0.45) | <0.4 mg/dL | 65/136 (45.1) | 73/136 (54.0) |
| Vitamin D (nmol/L) * | 50.2 (30.9) | <50 nmol/L | 78/144 (54.1) | 26/144 (18.1) |
| Zinc (pmol/L) | 10.1 (2.3) | <8.4 pmol/L | 89/144 (61.8) | 55/144 (38.2) |
| Hypoalbuminemia or MNA < 17 N = 39/144 | Zinc | Folate | Vitamin C | Vitamin D * |
|---|---|---|---|---|
| Sensitivity (%) | 35.9 | 72.7 | 40.0 | 28.8 |
| Specificity (%) | 87.2 | 76.6 | 84.9 | 87.1 |
| Positive predictive value (%) | 82.0 | 20.5 | 70.2 | 87.2 |
| Negative predictive value (%) | 45.7 | 97.1 | 61.3 | 20.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clotet-Vidal, S.; Saez Prieto, M.E.; Duch Llorach, P.; Gutiérrez, Á.S.; Casademont Pou, J.; Torres Bonafonte, O.H. Malnutrition, Functional Decline, and Institutionalization in Older Adults after Hospital Discharge Following Community-Acquired Pneumonia. Nutrients 2024, 16, 11. https://doi.org/10.3390/nu16010011
Clotet-Vidal S, Saez Prieto ME, Duch Llorach P, Gutiérrez ÁS, Casademont Pou J, Torres Bonafonte OH. Malnutrition, Functional Decline, and Institutionalization in Older Adults after Hospital Discharge Following Community-Acquired Pneumonia. Nutrients. 2024; 16(1):11. https://doi.org/10.3390/nu16010011
Chicago/Turabian StyleClotet-Vidal, Sandra, M. Encarna Saez Prieto, Pol Duch Llorach, Álvaro Santos Gutiérrez, Jordi Casademont Pou, and Olga H. Torres Bonafonte. 2024. "Malnutrition, Functional Decline, and Institutionalization in Older Adults after Hospital Discharge Following Community-Acquired Pneumonia" Nutrients 16, no. 1: 11. https://doi.org/10.3390/nu16010011
APA StyleClotet-Vidal, S., Saez Prieto, M. E., Duch Llorach, P., Gutiérrez, Á. S., Casademont Pou, J., & Torres Bonafonte, O. H. (2024). Malnutrition, Functional Decline, and Institutionalization in Older Adults after Hospital Discharge Following Community-Acquired Pneumonia. Nutrients, 16(1), 11. https://doi.org/10.3390/nu16010011






