In Vitro Activation of Human Adrenergic Receptors and Trace Amine-Associated Receptor 1 by Phenethylamine Analogues Present in Food Supplements
Abstract
:1. Introduction
2. Materials and Methods
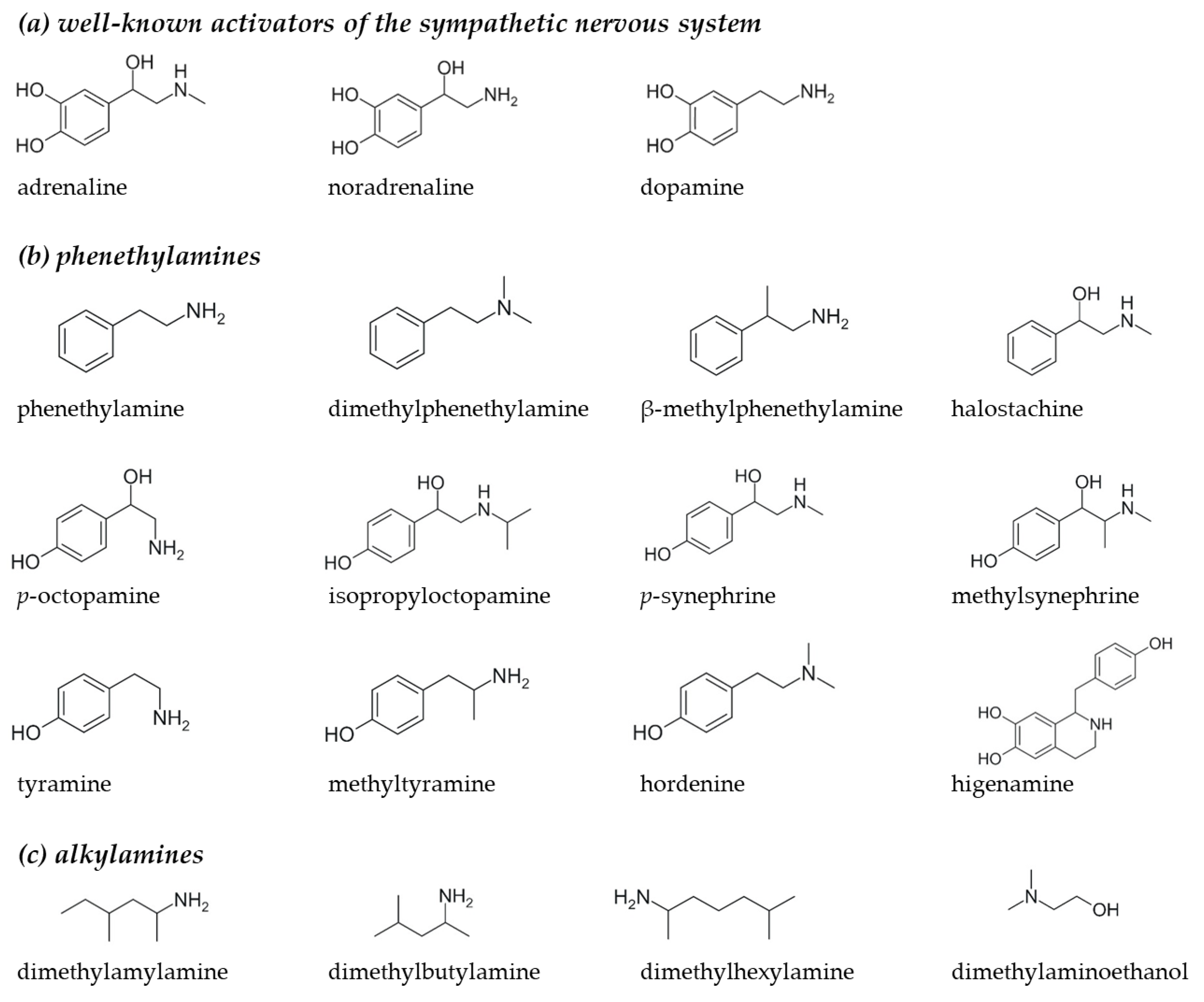
2.1. Selection of Phenethylamine and Alkylamine Analogues for Receptor Activation Assays
2.2. Chemicals
2.3. Adrenergic Receptor (ADR) Activation
2.4. Trace Amine-Associated Receptor 1 (TAAR1) Activation
2.5. Data Analysis
3. Results
3.1. Adrenergic Receptor α1A/B/D Activation
3.2. Adrenergic Receptor α2A/B Activation
3.3. Adrenergic Receptor β1/2 Activation
3.4. Trace Amine-Associated Receptor 1 (TAAR1) Activation
| ADRα1A | ADRα1B | ADRα1D | ADRα2A | ADRβ1 | ADRβ2 | TAAR1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC50 (M) | Emax (%) | EC50 (M) | Emax (%) | EC50 (M) | Emax (%) | EC50 (M) | Emax (%) | EC50 (M) | Emax (%) | EC50 (M) | Emax (%) | EC50 (M) | Emax (%) | |
| Adrenaline | 6.5 × 10−9 (6.0–7.0 × 10−9) | 98 (98–98) | 6.1 × 10−10 (5.6–6.7 × 10−10) | 99 (98–99) | 1.7 × 10−9 (1.5–1.9 × 10−9) | 99 (97–100) | 8.4 × 10−9 (7.4–9.4 × 10−9) | 105 (104–106) | 9.9 × 10−9 (3.6–5.9 × 10−9) | 99 (97–100) | 6.7 × 10−8 (5.4–7.9 × 10−8) | 104 (103–105) | ||
| Phenethylamines | ||||||||||||||
| Phenethylamine | - | - | - | - | - | - | - | - | - | - | - | - | 8.8 × 10−6 (6.8–11 × 10−6) | 97 (96–99) |
| β-Methylphenethyl-amine | - | - | - | - | - | - | - | - | - | - | - | - | 2.1 × 10−6 (1.9–2.3 × 10−6) | 77 (74–80) |
| Tyramine | - | - | - | - | - | - | - | - | - | - | - | - | 9.5 × 10−6 (5.3–14 × 10−6) | 77 (75–80) |
| Methyltyramine | - | - | - | - | - | - | - | - | - | - | - | 2.3 × 10−5 (1.8–2.9 × 10−5) | 83 (78–87) | |
| p-Synephrine | 2.4 × 10−6 (2.1–2.7 × 10−6) | 82 (79–84) | 6.6 × 10−7 (5.8–7.4 × 10−7) | 91 (89–92) | 1.7 × 10−6 (1.4–1.9 × 10−6) | 80 (78–81) | 1.0 × 10−4 (0.87–1.2 × 10−4) | 89 (83–94) | 2.8 × 10−5 (2.6–3.0 × 10−6) | 64 (59–69) | - | - | 9.2 × 10−5 (7.8–11 × 10−5) | 85 (82–87) |
| Methylsynephrine | 4.4 × 10−5 (3.5–5.3 × 10−5) | 16 (14–18) | - | - | - | - | - | - | 2.5 × 10−5 (2.3–2.8 × 10−5) | 75 (70–80) | - | - | - | - |
| Higenamine | - | - | - | - | - | - | - | - | 3.4 × 10−8 (2.8–4.0 × 10−8) | 105 (102–108) | 4.7 × 10−7 (4.2–5.3 × 10−7) | 31 (29–33) | 9.8 × 10−7 (8.4–11 × 10−7) | 93 (80–106) |
| Halostachine | 8.7 × 10−6 (7.1–10 × 10−6) | 59 (57–60) | 1.1 × 10−6 (0.98–1.2 × 10−6) | 77 (75–80) | 2.1 × 10−6 (1.9–2.3 × 10−6) | 82 (79–85) | - | - | - | - | - | - | 7.4 × 10−5 (6.4–8.4 × 10−5) | 100 (94–114) |
| Hordenine | - | - | 5.7 × 10−6 (5.3–6.0 × 10−6) | 37 (34–39) | 3.4 × 10−5 (2.2–4.5 × 10−5) | 23 (20–25) | 6.9 × 10−4 (3.7–10 × 10−4) | 28 (22–33) | - | - | - | - | 4.7 × 10−5 (3.5–5.8 × 10−5) | 82 (78–86) |
| p-Octopamine | 1.1 × 10−5 (0.93–1.2 × 10−5) | 87 (86–89) | 3.9 × 10−6 (3.5–4.3 × 10−6) | 94 (92–96) | 1.2 × 10−6 (1.0–1.3 × 10−6) | 100 (99–102) | - | - | 5.5 × 10−6 (5.0–6.0 × 10−6) | 88 (87–90) | - | - | 4.6 × 10−5 (3.8–5.3 × 10−5) | 85 (77–92) |
| Isopropyloctopamine | - | - | - | - | - | - | - | - | 1.2 × 10−7 (0.99–1.4 × 10−7) | 105 (100–109) | - | - | 1.8 × 10−6 (0.95–2.6 × 10−6) | 40 (35–45) |
| Dimethylphenethylamine | - | - | 6.1 × 10−6 (5.4–6.9 × 10−6) | 19 (17–21) | 8.4 × 10−6 (7.3–9.6 × 10−6) | 7.9 (7.3–8.5) | - | - | - | - | - | - | 2.1 × 10−5 (1.2–2.9 × 10−5) | 64 (59–70) |
| Alkylamines | ||||||||||||||
| 1,3-Dimethylamylamine | - | - | - | - | - | - | - | - | - | - | - | - | * | 55 (50–61) |
| Dimethylbutylamine | - | - | - | - | - | - | - | - | - | - | - | - | * | 20 (17–23) |
| Dimethylhexylamine | - | - | - | - | - | - | - | - | - | - | - | - | * | 5.3 (3.4–7.2) |
| Dimethylaminoethanol | - | - | - | - | - | - | - | - | - | - | - | - | * | 36 (34–38) |
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
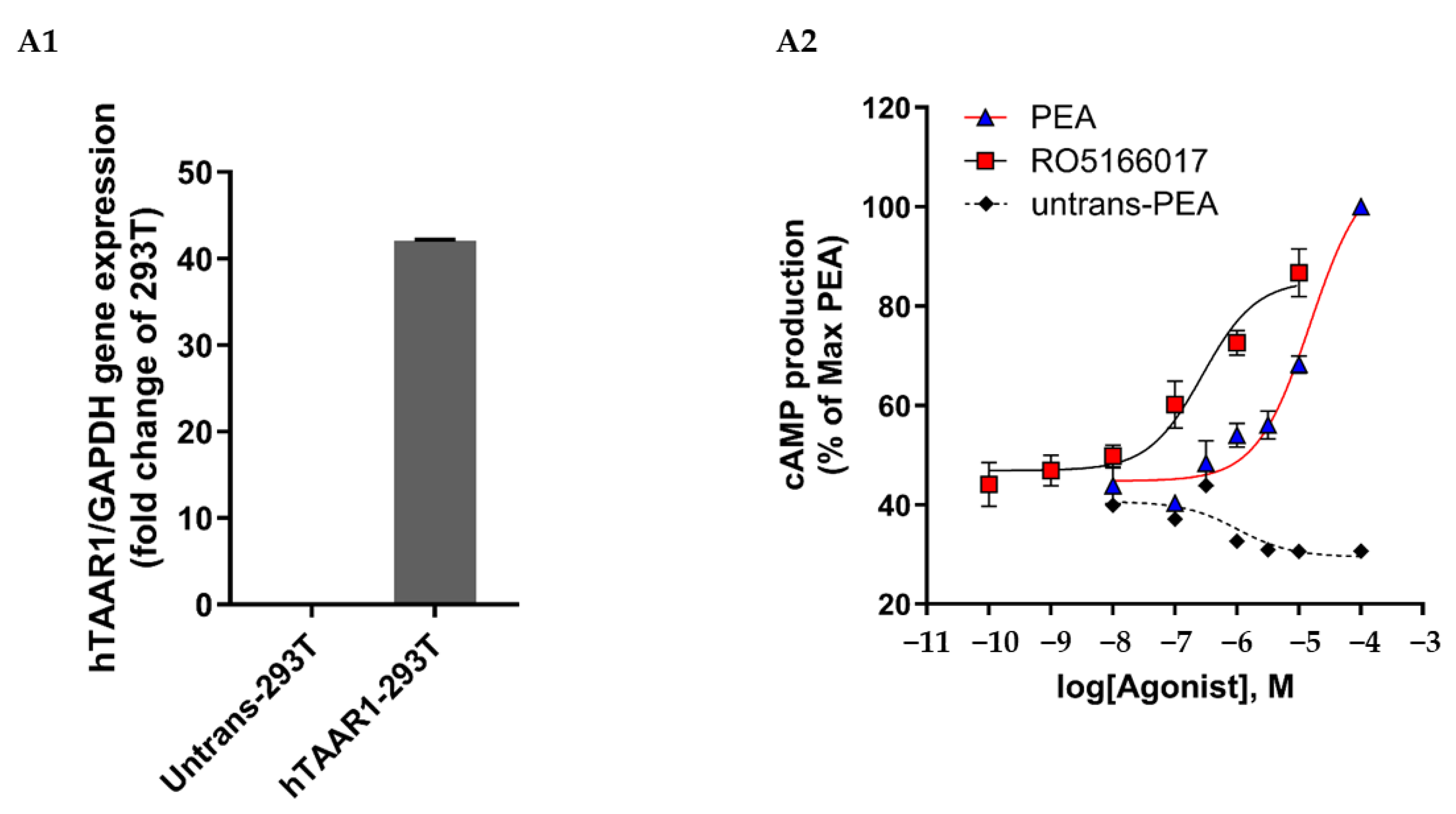
Appendix B

Appendix C

References
- Alonso, M.R.; Fernández-García, B. Evolution of the use of sports supplements. PharmaNutrition 2020, 14, 100239. [Google Scholar] [CrossRef]
- Wardenaar, F.C.; Ceelen, I.J.M.; Van den Dool, R.; Witkamp, R.; Mensink, M. Het gebruik van voedingssupplementen en sportvoeding door meer en minder actieve Nederlanders—Een inventarisatie op basis van het Nationaal Sport Onderzoek 2012 onder de Nederlandse bevolking. Ned. Tijdschr. Voor Voeding Dietiek 2014, 69, 9. [Google Scholar]
- Razenberg, L.; Notenboom, S.; De Wit-Bos, L. Use of Workout Supplements by Sport Participants in The Netherlands; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2021; Volume 0116. [CrossRef]
- Cohen, P.A.; Zeijlon, R.; Nardin, R.; Keizers, P.H.J.; Venhuis, B. Hemorrhagic Stroke Probably Caused by Exercise Combined With a Sports Supplement Containing β-Methylphenyl-ethylamine (BMPEA): A Case Report. Ann. Intern. Med. 2015, 162, 879–880. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.F.; Winn, C.; Ableman, T.B. Hemorrhagic Stroke in a Young Healthy Male Following Use of Pre-Workout Supplement Animal Rage XL. Mil. Med. 2017, 182, e2030–e2033. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.B.; Staub, B.A.; Natarajan, G.M.; Lasorda, D.M.; Poornima, I.G. Acute myocardial infarction associated with dietary supplements containing 1,3-dimethylamylamine and Citrus aurantium. Tex. Heart Inst. J. 2014, 41, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Zeijlon, R.; Hantelius, V.; Wallerstedt, S.M.; Holmqvist, L. Sports nutrition supplements and adverse events—A meta-epidemiological study of case reports specifically addressing causality assessment. Eur. J. Clin. Pharmacol. 2022, 78, 1–9. [Google Scholar] [CrossRef]
- Bovee, T.F.; Mol, H.G.; Bienenmann-Ploum, M.E.; Heskamp, H.H.; Van Bruchem, G.D.; Van Ginkel, L.A.; Kooijman, M.; Lasaroms, J.J.; Van Dam, R.; Hoogenboom, R.L. Dietary supplement for energy and reduced appetite containing the β-agonist isopropyloctopamine leads to heart problems and hospitalisations. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 749–759. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, M.L.L.; Kieviet, L.C.; Sierts, M.; Egberink, L.B.; Van der Heyden, M.A.G. Review of Case Reports on Adverse Events Related to Pre-workout Supplements Containing Synephrine. Cardiovasc. Toxicol. 2023, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Adami, P.E.; Koutlianos, N.; Baggish, A.; Bermon, S.; Cavarretta, E.; Deligiannis, A.; Furlanello, F.; Kouidi, E.; Marques-Vidal, P.; Niebauer, J.; et al. Cardiovascular effects of doping substances, commonly prescribed medications and ergogenic aids in relation to sports: A position statement of the sport cardiology and exercise nucleus of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2022, 29, 559–575. [Google Scholar] [CrossRef]
- Biesterbos, J.W.H.; Sijm, D.; Van Dam, R.; Mol, H.G.J. A health risk for consumers: The presence of adulterated food supplements in the Netherlands. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 1273–1288. [Google Scholar] [CrossRef]
- Cohen, P.A.; Travis, J.C.; Venhuis, B.J. A synthetic stimulant never tested in humans, 1,3-dimethylbutylamine (DMBA), is identified in multiple dietary supplements. Drug Test. Anal. 2015, 7, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Bloszies, C.; Yee, C.; Gerona, R. An amphetamine isomer whose efficacy and safety in humans has never been studied, β-methylphenylethylamine (BMPEA), is found in multiple dietary supplements. Drug Test. Anal. 2016, 8, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Travis, J.C.; Keizers, P.H.J.; Deuster, P.; Venhuis, B.J. Four experimental stimulants found in sports and weight loss supplements: 2-amino-6-methylheptane (octodrine), 1,4-dimethylamylamine (1,4-DMAA), 1,3-dimethylamylamine (1,3-DMAA) and 1,3-dimethylbutylamine (1,3-DMBA). Clin. Toxicol. 2018, 56, 421–426. [Google Scholar] [CrossRef]
- Cohen, P.; Travis, J.C.; Keizers, P.H.J.; Boyer, F.E.; Venhuis, B.J. The stimulant higenamine in weight loss and sports supplements. Clin. Toxicol. 2019, 57, 125–130. [Google Scholar] [CrossRef]
- Cohen, P.A.; Travis, J.C.; Vanhee, C.; Ohana, D.; Venhuis, B.J. Nine prohibited stimulants found in sports and weight loss supplements: Deterenol, phenpromethamine (Vonedrine), oxilofrine, octodrine, beta-methylphenylethylamine (BMPEA), 1,3-dimethylamylamine (1,3-DMAA), 1,4-dimethylamylamine (1,4-DMAA), 1,3-dimethylbutylamine (1,3-DMBA) and higenamine. Clin. Toxicol. 2021, 59, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Venhuis, B.; Keizers, P.; van Riel, A.; De Kaste, D. A cocktail of synthetic stimulants found in a dietary supplement associated with serious adverse events. Drug Test. Anal. 2014, 6, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Bae, J.Y.; Chittiboyina, A.G.; Wang, Y.H.; Wang, M.; Khan, I.A. Liquid chromatography-quadrupole time of flight mass spectrometric method for targeted analysis of 111 nitrogen-based compounds in weight loss and ergogenic supplements. J. Pharm. Biomed. Anal. 2019, 174, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Duiven, E.; Van Loon, L.J.C.; Spruijt, L.; Koert, W.; De Hon, O.M. Undeclared Doping Substances are Highly Prevalent in Commercial Sports Nutrition Supplements. J. Sports Sci. Med. 2021, 20, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, M.; Avula, B.; Khan, I.A. Detection and quantification of phenethylamines in sports dietary supplements by NMR approach. J. Pharm. Biomed. Anal. 2018, 151, 347–355. [Google Scholar] [CrossRef]
- Yun, J.; Kwon, K.; Choi, J.; Jo, C.H. Monitoring of the amphetamine-like substances in dietary supplements by LC-PDA and LC-MS/MS. Food Sci. Biotechnol. 2017, 26, 1185–1190. [Google Scholar] [CrossRef]
- Pawar, R.S.; Sagi, S.; Leontyev, D. Analysis of bitter orange dietary supplements for natural and synthetic phenethylamines by LC-MS/MS. Drug Test. Anal. 2020, 12, 1241–1251. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Gul, W. LC-MS-MS analysis of dietary supplements for N-ethyl-α-ethyl-phenethylamine (ETH), N, N-diethylphenethylamine and phenethylamine. J. Anal. Toxicol. 2014, 38, 63–72. [Google Scholar] [CrossRef]
- Hondebrink, L.; Zwartsen, A.; Westerink, R.H.S. Effect fingerprinting of new psychoactive substances (NPS): What can we learn from in vitro data? Pharmacol. Ther. 2018, 182, 193–224. [Google Scholar] [CrossRef]
- Rickli, A.; Hoener, M.C.; Liechti, M.E. Pharmacological profiles of compounds in preworkout supplements (“boosters”). Eur. J. Pharmacol. 2019, 859, 172515. [Google Scholar] [CrossRef]
- Rebec, G.V. Cocaine and Amphetamines. In Encyclopedia of Life Sciences; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Rutigliano, G.; Accorroni, A.; Zucchi, R. The Case for TAAR1 as a Modulator of Central Nervous System Function. Front. Pharmacol. 2017, 8, 987. [Google Scholar] [CrossRef]
- Fehler, M.; Broadley, K.J.; Ford, W.R.; Kidd, E.J. Identification of trace-amine-associated receptors (TAAR) in the rat aorta and their role in vasoconstriction by β-phenylethylamine. Naunyn Schmiedeberg’s Arch. Pharmacol. 2010, 382, 385–398. [Google Scholar] [CrossRef]
- Broadley, K.J. The vascular effects of trace amines and amphetamines. Pharmacol. Ther. 2010, 125, 363–375. [Google Scholar] [CrossRef]
- Graham, R.M. Adrenergic receptors: Structure and function. Clevel. Clin. J. Med. 1990, 57, 481–491. [Google Scholar] [CrossRef]
- Wardenaar, F.; Van den Dool, R.; Ceelen, I.; Witkamp, R.; Mensink, M. Self-Reported Use and Reasons among the General Population for Using Sports Nutrition Products and Dietary Supplements. Sports 2016, 4, 33. [Google Scholar] [CrossRef]
- Cohen, P.A.; Avula, B.; Venhuis, B.; Travis, J.C.; Wang, Y.H.; Khan, I.A. Pharmaceutical doses of the banned stimulant oxilofrine found in dietary supplements sold in the USA. Drug Test. Anal. 2017, 9, 135–142. [Google Scholar] [CrossRef]
- Waxenbaum, J.A.; Reddy, V.; Varacallo, M. Anatomy, Autonomic Nervous System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539845/ (accessed on 1 December 2023).
- Bylund, D.B.; Eikenberg, D.C.; Hieble, J.P.; Langer, S.Z.; Lefkowitz, R.J.; Minneman, K.P.; Molinoff, P.B.; Ruffolo, R.R., Jr. Trendelenburg, U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol. Rev. 1994, 46, 121–136. [Google Scholar] [PubMed]
- Docherty, J.R. Subtypes of functional α1-adrenoceptor. Cell. Mol. Life Sci. 2010, 67, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Haller, C.A.; Benowitz, N.L.; Jacob, P. Hemodynamic effects of ephedra-free weight-loss supplements in humans. Am. J. Med. 2005, 118, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Haller, C.A.; Duan, M.; Jacob, P., 3rd; Benowitz, N. Human pharmacology of a performance-enhancing dietary supplement under resting and exercise conditions. Br. J. Clin. Pharmacol. 2008, 65, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Miron, A.; Trifan, A.; Luca, V.S.; Costache, I.I. The Cardiovascular Effects of Cocoa Polyphenols-An Overview. Diseases 2016, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Shara, M.; Ray, S.D. p-Synephrine, ephedrine, p-octopamine and m-synephrine: Comparative mechanistic, physiological and pharmacological properties. Phytother. Res. 2020, 34, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J. Physiological functions and pharmacological and toxicological effects of p-octopamine. Drug Chem. Toxicol. 2015, 38, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Sobiech, M.; Giebułtowicz, J.; Luliński, P. Application of Magnetic Core–Shell Imprinted Nanoconjugates for the Analysis of Hordenine in Human Plasma-Preliminary Data on Pharmacokinetic Study after Oral Administration. J. Agric. Food Chem. 2020, 68, 14502–14512. [Google Scholar] [CrossRef] [PubMed]
- Sommer, T.; Göen, T.; Budnik, N.; Pischetsrieder, M. Absorption, Biokinetics, and Metabolism of the Dopamine D2 Receptor Agonist Hordenine (N,N-Dimethyltyramine) after Beer Consumption in Humans. J. Agric. Food Chem. 2020, 68, 1998–2006. [Google Scholar] [CrossRef]
- Frank, M.; Weckman, T.J.; Wood, T.; Woods, W.E.; Tai, C.L.; Chang, S.L.; Ewing, A.; Blake, J.W.; Tobin, T. Hordenine: Pharmacology, pharmacokinetics and behavioural effects in the horse. Equine Vet. J. 1990, 22, 437–441. [Google Scholar] [CrossRef]
- Kanagy, N.L. α2-Adrenergic receptor signalling in hypertension. Clin. Sci. 2005, 109, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Gavras, I.; Manolis, A.J.; Gavras, H. The alpha2—Adrenergic receptors in hypertension and heart failure: Experimental and clinical studies. J. Hypertens. 2001, 19, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Alhayek, S.; Preuss, C.V. Beta 1 Receptors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532904/ (accessed on 14 August 2023).
- Feng, S.; Jiang, J.; Hu, P.; Zhang, J.Y.; Liu, T.; Zhao, Q.; Li, B.L. A phase I study on pharmacokinetics and pharmacodynamics of higenamine in healthy Chinese subjects. Acta Pharmacol. Sin. 2012, 33, 1353–1358. [Google Scholar] [CrossRef]
- Pilkington, T.R.E.; Lowe, R.D.; Foster, R.; Robinson, B.F.; Antonis, A. Effect of sympathomimetic compounds with Β-adrenergic effects on plasma free fatty acids in man. J. Lipid Res. 1966, 7, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Hudzik, T.J.; Patel, M.; Brown, A. β(2)—Adrenoceptor agonist activity of higenamine. Drug Test. Anal. 2021, 13, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.; Kaumann, A.J.; Brown, M.J. β-Adrenoceptor Subtypes in Human Coronary Artery: Desensitization of β2-Adrenergic Vasorelaxation by Chronic β1-Adrenergic Stimulation In Vitro. J. Cardiovasc. Pharmacol. 1995, 25, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R.; Ginsburg, R.; Umans, V.; Fowler, M.; Minobe, W.; Rasmussen, R.; Zera, P.; Menlove, R.; Shah, P.; Jamieson, S. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: Coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ. Res. 1986, 59, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.A.; Galloway, D.A.; Hoener, M.C.; Berry, M.D.; Moore, C.S. TAAR1 Expression in Human Macrophages and Brain Tissue: A Potential Novel Facet of MS Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 11576. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, L.; Ebeling, M.; Kratochwil, N.A.; Bunzow, J.R.; Grandy, D.K.; Hoener, M.C. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics 2005, 85, 372–385. [Google Scholar] [CrossRef]
- Xie, Z.; Miller, G.M. Beta-phenylethylamine alters monoamine transporter function via trace amine-associated receptor 1: Implication for modulatory roles of trace amines in brain. J. Pharmacol. Exp. Ther. 2008, 325, 617–628. [Google Scholar] [CrossRef]
- Koh, A.H.W.; Chess-Williams, R.; Lohning, A.E. Differential mechanisms of action of the trace amines octopamine, synephrine and tyramine on the porcine coronary and mesenteric artery. Sci. Rep. 2019, 9, 10925. [Google Scholar] [CrossRef] [PubMed]
- Broadley, K.J.; Mehta, D. Trace amine-induced vasoconstriction of human mammary artery and saphenous vein. Vasc. Pharmacol. 2023, 151, 107191. [Google Scholar] [CrossRef] [PubMed]
- Broadley, K.J.; Broadley, H.D. Non-adrenergic vasoconstriction and vasodilatation of guinea-pig aorta by β-phenylethylamine and amphetamine—Role of nitric oxide determined with L-NAME and NO scavengers. Eur. J. Pharmacol. 2018, 818, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Herbert, A.A.; Kidd, E.J.; Broadley, K.J. Dietary trace amine-dependent vasoconstriction in porcine coronary artery. Br. J. Pharmacol. 2008, 155, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.D. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J. Neurochem. 2004, 90, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Schindler, C.W.; Thorndike, E.B.; Rice, K.C.; Partilla, J.S.; Baumann, M.H. The Supplement Adulterant β-Methylphenethylamine Increases Blood Pressure by Acting at Peripheral Norepinephrine Transporters. J. Pharmacol. Exp. Ther. 2019, 369, 328–336. [Google Scholar] [CrossRef]
- Underhill, S.M.; Hullihen, P.D.; Chen, J.; Fenollar-Ferrer, C.; Rizzo, M.A.; Ingram, S.L.; Amara, S.G. Amphetamines signal through intracellular TAAR1 receptors coupled to Gα13 and GαS in discrete subcellular domains. Mol. Psychiatry 2021, 26, 1208–1223. [Google Scholar] [CrossRef]
- Lindemann, L.; Hoener, M.C. A renaissance in trace amines inspired by a novel GPCR family. Trends Pharmacol. Sci. 2005, 26, 274–281. [Google Scholar] [CrossRef]
- Berry, M.D.; Shitut, M.R.; Almousa, A.; Alcorn, J.; Tomberli, B. Membrane permeability of trace amines: Evidence for a regulated, activity-dependent, nonexocytotic, synaptic release. Synapse 2013, 67, 656–667. [Google Scholar] [CrossRef]
- Berry, M.D.; Hart, S.; Pryor, A.R.; Hunter, S.; Gardiner, D. Pharmacological characterization of a high-affinity p-tyramine transporter in rat brain synaptosomes. Sci. Rep. 2016, 6, 38006. [Google Scholar] [CrossRef]
- Eliason, M.J.; Eichner, A.; Cancio, A.; Bestervelt, L.; Adams, B.D.; Deuster, P.A. Case Reports: Death of Active Duty Soldiers Following Ingestion of Dietary Supplements Containing 1,3-Dimethylamylamine (DMAA). Mil. Med. 2012, 177, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Gee, P.; Tallon, C.; Long, N.; Moore, G.; Boet, R.; Jackson, S. Use of recreational drug 1,3 Dimethylamylamine (DMAA) [corrected] associated with cerebral hemorrhage. Ann. Emerg. Med. 2012, 60, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Austin, K.G.; Farina, E.K. Surveillance of the armed forces as a sentinel system for detecting adverse effects of dietary supplements in the general population. Public Health Nutr. 2018, 21, 882–887. [Google Scholar] [CrossRef]
- Small, C.; Cheng, M.H.; Belay, S.S.; Bulloch, S.L.; Zimmerman, B.; Sorkin, A.; Block, E.R. The Alkylamine Stimulant 1,3-Dimethylamylamine Exhibits Substrate-Like Regulation of Dopamine Transporter Function and Localization. J. Pharmacol. Exp. Ther. 2023, 386, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Post-marketing withdrawal of anti-obesity medicinal products because of adverse drug reactions: A systematic review. BMC Med. 2016, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Lundsgaard, C. What do pharmacological approaches to obesity management offer? Linking pharmacological mechanisms of obesity management agents to clinical practice. Exp. Clin. Endocrinol. Diabetes 1998, 106 (Suppl. 2), 29–34. [Google Scholar] [CrossRef] [PubMed]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. EClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef]
- White, C.M. Continued Risk of Dietary Supplements Adulterated with Approved and Unapproved Drugs: Assessment of the US Food and Drug Administration’s Tainted Supplements Database 2007 through 2021. J. Clin. Pharmacol. 2022, 62, 928–934. [Google Scholar] [CrossRef]
- Braga, D.; McLaughlin, J. Cactus Alkaloids, V. Isolation of Hordenine and N-Methyltyramine from Ariocarpus retusus. Planta Medica 1969, 17, 87–94. [Google Scholar] [CrossRef]
- Clement, B.A.; Goff, C.M.; Forbes, T.D.A. Toxic amines and alkaloids from Acacia rigidula. Phytochemistry 1998, 49, 1377–1380. [Google Scholar] [CrossRef]
- Sander, L.C.; Putzbach, K.; Nelson, B.C.; Rimmer, C.A.; Bedner, M.; Thomas, J.B.; Porter, B.J.; Wood, L.J.; Schantz, M.M.; Murphy, K.E.; et al. Certification of standard reference materials containing bitter orange. Anal. Bioanal. Chem. 2008, 391, 2023–2034. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.S.; Grundel, E.; Fardin-Kia, A.R.; Rader, J.I. Determination of selected biogenic amines in Acacia rigidula plant materials and dietary supplements using LC–MS/MS methods. J. Pharm. Biomed. Anal. 2014, 88, 457–466. [Google Scholar] [CrossRef]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinckaers, N.E.T.; Blankesteijn, W.M.; Mircheva, A.; Shi, X.; Opperhuizen, A.; Schooten, F.-J.v.; Vrolijk, M.F. In Vitro Activation of Human Adrenergic Receptors and Trace Amine-Associated Receptor 1 by Phenethylamine Analogues Present in Food Supplements. Nutrients 2024, 16, 1567. https://doi.org/10.3390/nu16111567
Pinckaers NET, Blankesteijn WM, Mircheva A, Shi X, Opperhuizen A, Schooten F-Jv, Vrolijk MF. In Vitro Activation of Human Adrenergic Receptors and Trace Amine-Associated Receptor 1 by Phenethylamine Analogues Present in Food Supplements. Nutrients. 2024; 16(11):1567. https://doi.org/10.3390/nu16111567
Chicago/Turabian StylePinckaers, Nicole E. T., W. Matthijs Blankesteijn, Anastasiya Mircheva, Xiao Shi, Antoon Opperhuizen, Frederik-Jan van Schooten, and Misha F. Vrolijk. 2024. "In Vitro Activation of Human Adrenergic Receptors and Trace Amine-Associated Receptor 1 by Phenethylamine Analogues Present in Food Supplements" Nutrients 16, no. 11: 1567. https://doi.org/10.3390/nu16111567
APA StylePinckaers, N. E. T., Blankesteijn, W. M., Mircheva, A., Shi, X., Opperhuizen, A., Schooten, F.-J. v., & Vrolijk, M. F. (2024). In Vitro Activation of Human Adrenergic Receptors and Trace Amine-Associated Receptor 1 by Phenethylamine Analogues Present in Food Supplements. Nutrients, 16(11), 1567. https://doi.org/10.3390/nu16111567






