Effects of Different Caffeine Dosages on Maximal Physical Performance and Potential Side Effects in Low-Consumer Female Athletes: Morning vs. Evening Administration
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
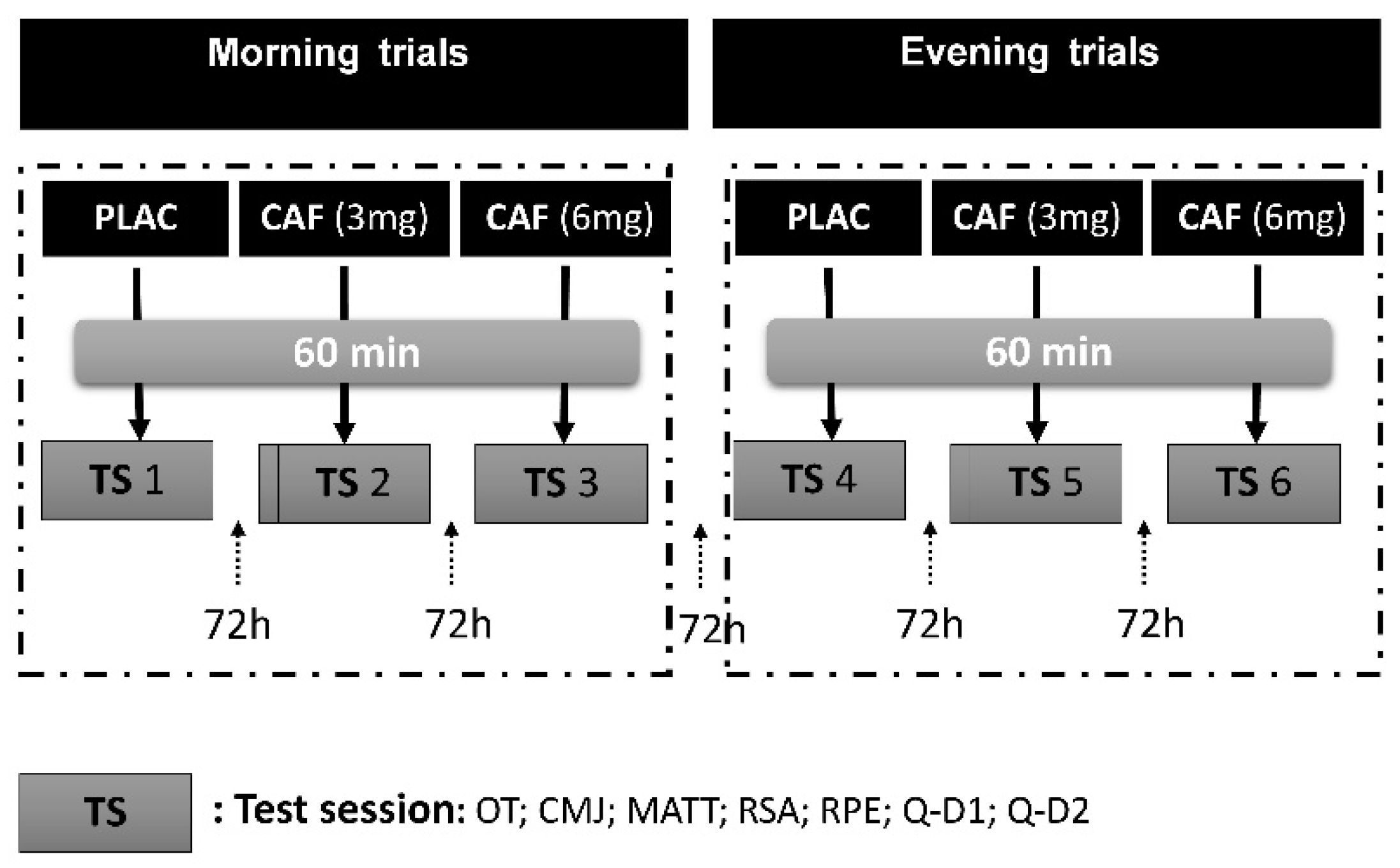
2.2. Experimental Design
2.3. CMJ
2.4. MATT
2.5. RSA
2.6. RPE
2.7. CAF Side Effects Questionnaire
2.8. Habitual CAF Intake Assessment
2.9. Statistical Analysis
3. Results
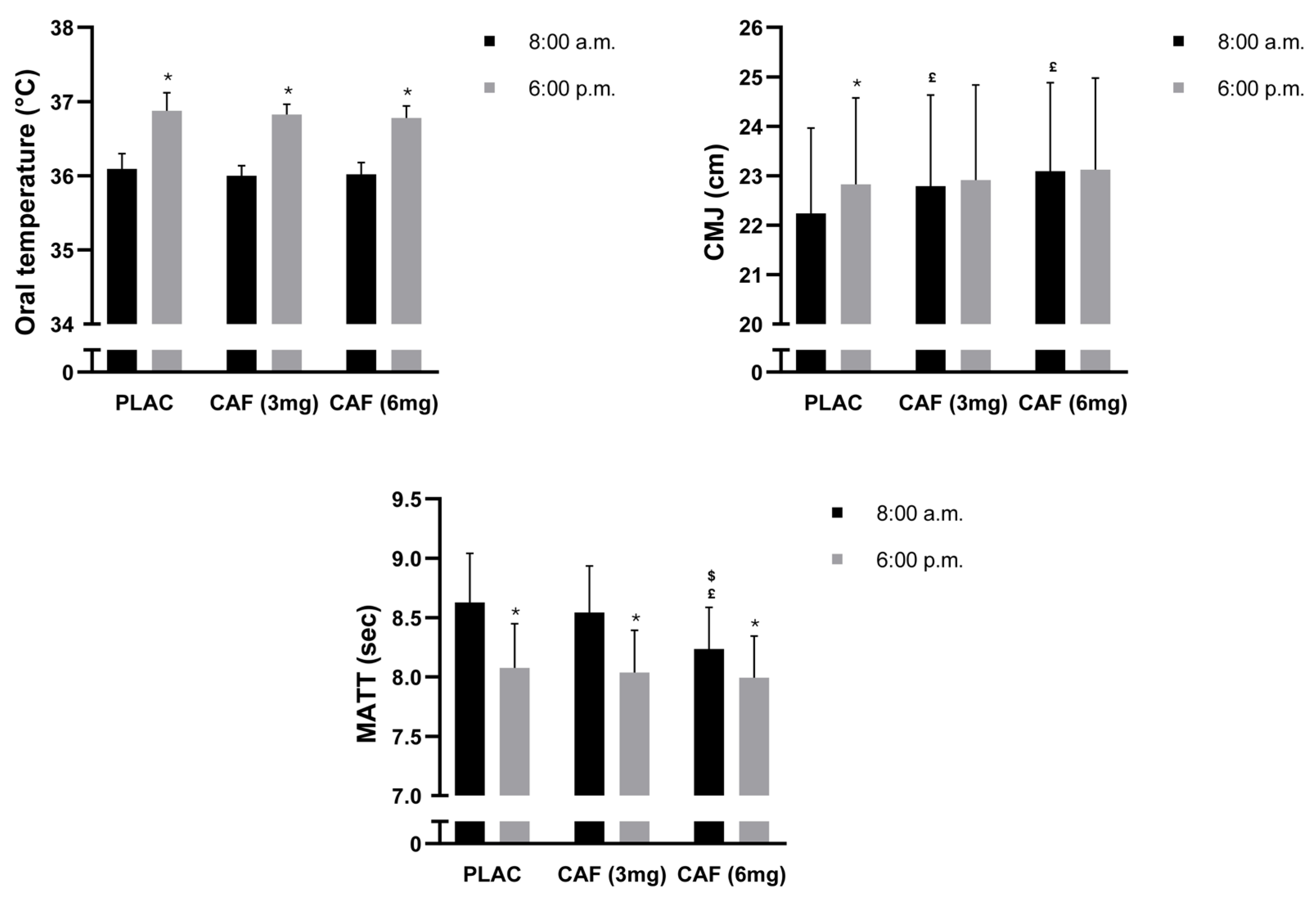
3.1. Oral Temperature (OT)
3.2. CMJ
3.3. MATT
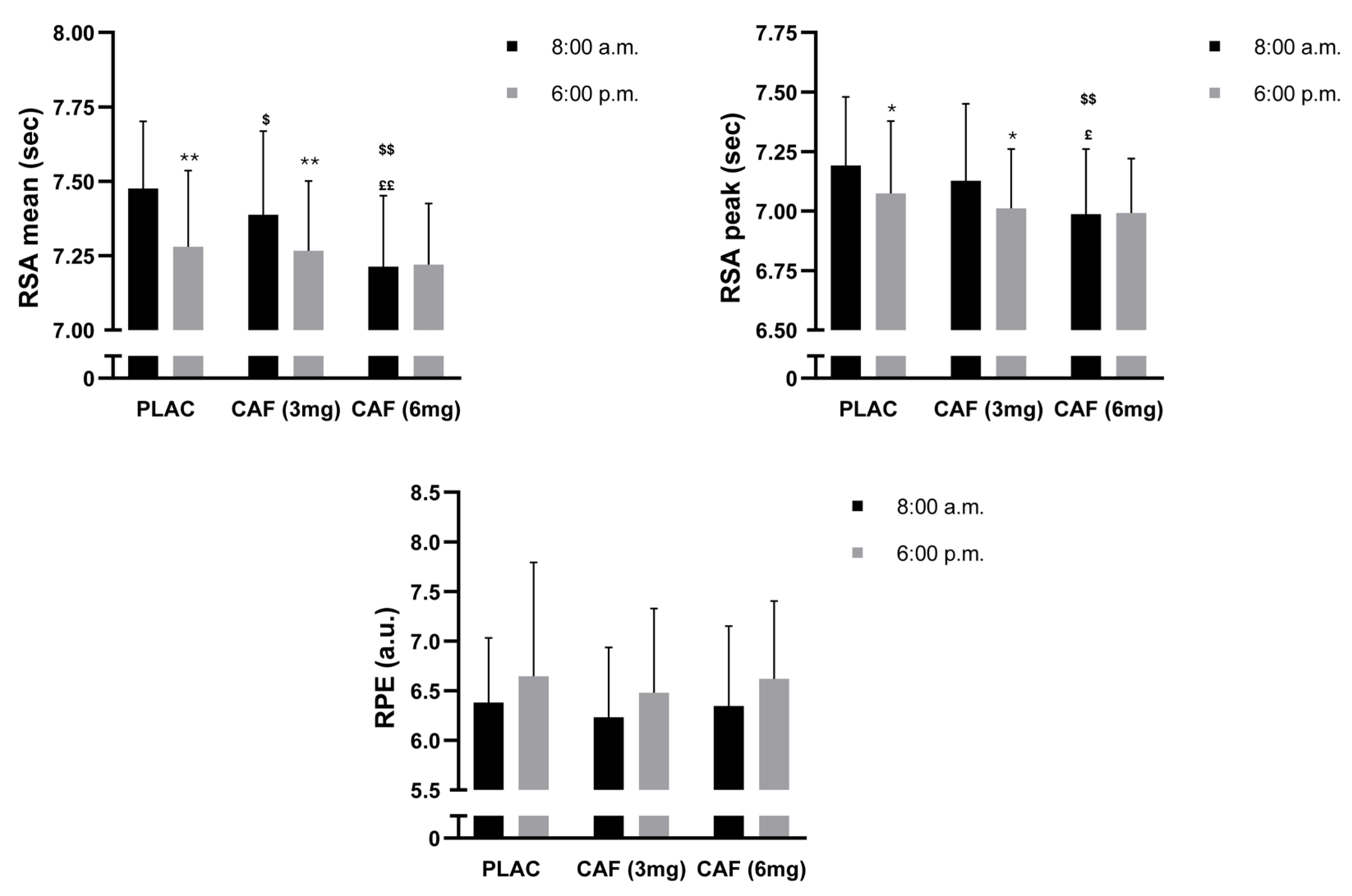
3.4. RSA Mean
3.5. RSA Peak
3.6. RPE
3.7. CAF Side Effects Questionnaire
4. Discussion
4.1. TOD Effects
4.2. CAF Dosage Effects
4.3. CAF and TOD Effects
4.4. CAF Side Effects
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguilar-Navarro, M.; Muñoz, G.; Salinero, J.; Muñoz-Guerra, J.; Fernández-Álvarez, M.; Plata, M.; Del Coso, J. Urine Caffeine Concentration in Doping Control Samples from 2004 to 2015. Nutrients 2019, 11, 286. [Google Scholar] [CrossRef] [PubMed]
- Salinero, J.J.; Lara, B.; Jiménez-Ormeño, E.; Romero-Moraleda, B.; Giráldez-Costas, V.; Baltazar-Martins, G.; Del Coso, J. More Research Is Necessary to Establish the Ergogenic Effect of Caffeine in Female Athletes. Nutrients 2019, 11, 1600. [Google Scholar] [CrossRef] [PubMed]
- Chia, J.S.; Barrett, L.A.; Chow, J.Y.; Burns, S.F. Effects of Caffeine Supplementation on Performance in Ball Games. Sports Med. 2017, 47, 2453–2471. [Google Scholar] [CrossRef]
- Bougrine, H.; Salem, A.; Ammar, A.; Souissi, N. Caffeine and Team Ball Performances: A Mini-Review. Tunis. J. Sports Sci. Med. 2023, 1, 57–63. [Google Scholar] [CrossRef]
- Bougrine, H.; Ammar, A.; Salem, A.; Trabelsi, K.; Jahrami, H.; Chtourou, H.; Souissi, N. Optimizing Short-Term Maximal Exercise Performance: The Superior Efficacy of a 6 Mg/Kg Caffeine Dose over 3 or 9 Mg/Kg in Young Female Team-Sports Athletes. Nutrients 2024, 16, 640. [Google Scholar] [CrossRef]
- Perez-Lopez, A.; Salinero, J.J.; Abian-Vicen, J.; Valades, D.; Lara, B.; Hernandez, C.; Areces, F.; Gonzalez, C.; Del Coso, J. Caffeinated Energy Drinks Improve Volleyball Performance in Elite Female Players. Med. Sci. Sports Exerc. 2015, 47, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Siquier-Coll, J.; Delgado-García, G.; Soto-Méndez, F.; Liñán-González, A.; García, R.; González-Fernández, F.T. The Effect of Caffeine Supplementation on Female Volleyball Players’ Performance and Wellness during a Regular Training Week. Nutrients 2023, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Bougrine, H.; Cherif, M.; Chtourou, H.; Souissi, N. Can Caffeine Supplementation Reverse the Impact of Time of Day on Cognitive and Short-Term High Intensity Performances in Young Female Handball Players? Chronobiol. Int. 2022, 39, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Bougrine, H.; Nasser, N.; Abdessalem, R.; Ammar, A.; Chtourou, H.; Souissi, N. Pre-Exercise Caffeine Intake Attenuates the Negative Effects of Ramadan Fasting on Several Aspects of High-Intensity Short-Term Maximal Performances in Adolescent Female Handball Players. Nutrients 2023, 15, 3432. [Google Scholar] [CrossRef] [PubMed]
- Lara, B.; Gonzalez-Millán, C.; Salinero, J.J.; Abian-Vicen, J.; Areces, F.; Barbero-Alvarez, J.C.; Muñoz, V.; Portillo, L.J.; Gonzalez-Rave, J.M.; Del Coso, J. Caffeine-Containing Energy Drink Improves Physical Performance in Female Soccer Players. Amino Acids 2014, 46, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; O’Donnell, J.; Foskett, A.; Rutherfurd-Markwick, K. The Influence of Caffeine Ingestion on Strength and Power Performance in Female Team-Sport Players. J. Int. Soc. Sports Nutr. 2016, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Campos, C.; Dengo, A.L.; Moncada-Jiménez, J. Acute Consumption of an Energy Drink Does Not Improve Physical Performance of Female Volleyball Players. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 271–277. [Google Scholar] [CrossRef]
- Lee, C.-L.; Cheng, C.-F.; Astorino, T.A.; Lee, C.-J.; Huang, H.-W.; Chang, W.-D. Effects of Carbohydrate Combined with Caffeine on Repeated Sprint Cycling and Agility Performance in Female Athletes. J. Int. Soc. Sports Nutr. 2014, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.; López-Samanes, Á.; Pérez-López, A.; Aguilar-Navarro, M.; Moreno-Heredero, B.; Rivilla-García, J.; González-Frutos, P.; Pino-Ortega, J.; Morencos, E.; Del Coso, J. Effects of Caffeine Ingestion on Physical Performance in Elite Women Handball Players: A Randomized, Controlled Study. Int. J. Sports Physiol. Perform. 2020, 15, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Del Coso, J.; Ramírez, J.A.; Muñoz, G.; Portillo, J.; Gonzalez-Millán, C.; Muñoz, V.; Barbero-Álvarez, J.C.; Muñoz-Guerra, J. Caffeine-Containing Energy Drink Improves Physical Performance of Elite Rugby Players during a Simulated Match. Appl. Physiol. Nutr. Metab. 2013, 38, 368–374. [Google Scholar] [CrossRef]
- Pfeifer, D.R.; Arvin, K.M.; Herschberger, C.N.; Haynes, N.J.; Renfrow, M.S. A Low Dose Caffeine and Carbohydrate Supplement Does Not Improve Athletic Performance during Volleyball Competition. Int. J. Exerc. Sci. 2017, 10, 340–353. [Google Scholar] [PubMed]
- Stojanović, E.; Stojiljković, N.; Scanlan, A.T.; Dalbo, V.J.; Stanković, R.; Antić, V.; Milanović, Z. Acute Caffeine Supplementation Promotes Small to Moderate Improvements in Performance Tests Indicative of In-Game Success in Professional Female Basketball Players. Appl. Physiol. Nutr. Metab. 2019, 44, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.; Guelfi, K.; Dawson, B.; McNaughton, L.; Wallman, K. Effects of Sodium Phosphate and Caffeine Loading on Repeated-Sprint Ability. J. Sports Sci. 2015, 33, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Karayigit, R.; Naderi, A.; Akca, F.; da Cruz, C.J.G.; Sarshin, A.; Yasli, B.C.; Ersoz, G.; Kaviani, M. Effects of Different Doses of Caffeinated Coffee on Muscular Endurance, Cognitive Performance, and Cardiac Autonomic Modulation in Caffeine Naive Female Athletes. Nutrients 2020, 13, 2. [Google Scholar] [CrossRef]
- Karayigit, R.; Forbes, S.C.; Osmanov, Z.; Yilmaz, C.; Yasli, B.C.; Naderi, A.; Buyukcelebi, H.; Benesova, D.; Gabrys, T.; Esen, O. Low and Moderate Doses of Caffeinated Coffee Improve Repeated Sprint Performance in Female Team Sport Athletes. Biology 2022, 11, 1498. [Google Scholar] [CrossRef]
- Arazi, H.; Hoseinihaji, M.; Eghbali, E. The Effects of Different Doses of Caffeine on Performance, Rating of Perceived Exertion and Pain Perception in Teenagers Female Karate Athletes. Braz. J. Pharm. Sci. 2016, 52, 685–692. [Google Scholar] [CrossRef]
- Souissi, Y.; Souissi, M.; Chtourou, H. Effects of Caffeine Ingestion on the Diurnal Variation of Cognitive and Repeated High-Intensity Performances. Pharmacol. Biochem. Behav. 2019, 177, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, W.; Xue, Y.; Hou, D.; Chen, S.; Xu, Z.; Peng, S.; Zhao, H.; Wang, C.; Liu, C. Timing Matters: Time of Day Impacts the Ergogenic Effects of Caffeine—A Narrative Review. Nutrients 2024, 16, 1421. [Google Scholar] [CrossRef] [PubMed]
- Mora-Rodríguez, R.; Pallarés, J.G.; López-Samanes, Á.; Ortega, J.F.; Fernández-Elías, V.E. Caffeine Ingestion Reverses the Circadian Rhythm Effects on Neuromuscular Performance in Highly Resistance-Trained Men. PLoS ONE 2012, 7, e33807. [Google Scholar] [CrossRef]
- Muñoz, A.; Aguilar-Navarro, M.; Ruiz-Moreno, C.; Varillas-Delgado, D.; Amaro-Gahete, F.J.; Gutiérrez-Hellín, J.; Del Coso, J.; López-Samanes, Á. Influence of the Time of Day in the Effect of Caffeine on Maximal Fat Oxidation during Exercise in Women: A Randomized, Crossover, Double-Blind, and Placebo-Controlled Study. Eur. J. Appl. Physiol. 2024, 124, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Souissi, M.; Abedelmalek, S.; Chtourou, H.; Boussita, A.; Hakim, A.; Sahnoun, Z. Effects of Time-of-Day and Caffeine Ingestion on Mood States, Simple Reaction Time, and Short-Term Maximal Performance in Elite Judoists. Biol. Rhythm. Res. 2013, 44, 897–907. [Google Scholar] [CrossRef]
- Boyett, J.; Giersch, G.; Womack, C.; Saunders, M.; Hughey, C.; Daley, H.; Luden, N. Time of Day and Training Status Both Impact the Efficacy of Caffeine for Short Duration Cycling Performance. Nutrients 2016, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- Mora-Rodríguez, R.; Pallarés, J.G.; López-Gullón, J.M.; López-Samanes, Á.; Fernández-Elías, V.E.; Ortega, J.F. Improvements on Neuromuscular Performance with Caffeine Ingestion Depend on the Time-of-Day. J. Sci. Med. Sport 2015, 18, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Silva, J.P.; da Silva Santos, J.F.; Franchini, E. Can Caffeine Supplementation Reverse the Effect of Time of Day on Repeated-Sprint Exercise Performance? Appl. Physiol. Nutr. Metab. 2019, 44, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, D.; Welsh, B.T.; Henderson, R.; Brorby, G.P.; Britt, J.; Myers, E.; Goldberger, J.; Lieberman, H.R.; O’Brien, C.; Peck, J. Systematic Review of the Potential Adverse Effects of Caffeine Consumption in Healthy Adults, Pregnant Women, Adolescents, and Children. Food Chem. Toxicol. 2017, 109, 585–648. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo Calvo, J.; Fei, X.; Domínguez, R.; Pareja-Galeano, H. Caffeine and Cognitive Functions in Sports: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 868. [Google Scholar] [CrossRef] [PubMed]
- Willson, C. The Clinical Toxicology of Caffeine: A Review and Case Study. Toxicol. Rep. 2018, 5, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.W. The Importance of A Priori Sample Size Estimation in Strength and Conditioning Research. J. Strength. Cond. Res. 2013, 27, 2323–2337. [Google Scholar] [CrossRef] [PubMed]
- Filip, A.; Wilk, M.; Krzysztofik, M.; Del Coso, J. Inconsistency in the Ergogenic Effect of Caffeine in Athletes Who Regularly Consume Caffeine: Is It Due to the Disparity in the Criteria That Defines Habitual Caffeine Intake? Nutrients 2020, 12, 1087. [Google Scholar] [CrossRef] [PubMed]
- Bougrine, H.; Ammar, A.; Salem, A.; Trabelsi, K.; Jahrami, H.; Chtourou, H.; Souissi, N. Effects of Various Caffeine Doses on Cognitive Abilities in Female Athletes with Low Caffeine Consumption. Brain Sci. 2024, 14, 280. [Google Scholar] [CrossRef] [PubMed]
- Filip-Stachnik, A. Does Acute Caffeine Intake before Evening Training Sessions Impact Sleep Quality and Recovery-Stress State? Preliminary Results from a Study on Highly Trained Judo Athletes. Appl. Sci. 2022, 12, 9957. [Google Scholar] [CrossRef]
- Ribeiro-Alves, M.A.; Trugo, L.C.; Donangelo, C.M. Use of Oral Contraceptives Blunts the Calciuric Effect of Caffeine in Young Adult Women. J. Nutr. 2003, 133, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.A.; Ostberg, O. A Self-Assessment Questionnaire to Determine Morningness-Eveningness in Human Circadian Rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar] [PubMed]
- Suleiman, K.H.; Yates, B.C.; Berger, A.M.; Pozehl, B.; Meza, J. Translating the Pittsburgh Sleep Quality Index into Arabic. West. J. Nurs. Res. 2010, 32, 250–268. [Google Scholar] [CrossRef] [PubMed]
- Janse de Jonge, X.A.K. Effects of the Menstrual Cycle on Exercise Performance. Sports Med. 2003, 33, 833–851. [Google Scholar] [CrossRef]
- Portaluppi, F.; Smolensky, M.H.; Touitou, Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol. Int. 2010, 27, 1911–1929. [Google Scholar] [CrossRef] [PubMed]
- Chtourou, H.; Souissi, N. The Effect of Training at a Specific Time of Day: A Review. J. Strength. Cond. Res. 2012, 26, 1984–2005. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Shirreffs, S.M. Development of Individual Hydration Strategies for Athletes. Int. J. Sport. Nutr. Exerc. Metab. 2008, 18, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Kavouras, S.A. Caffeine Use in Sports, Pharmacokinetics in Man, and Cellular Mechanisms of Action. Crit. Rev. Food Sci. Nutr. 2005, 45, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Sassi, R.H.; Dardouri, W.; Yahmed, M.H.; Gmada, N.; Mahfoudhi, M.E.; Gharbi, Z. Relative and Absolute Reliability of a Modified Agility T-Test and Its Relationship with Vertical Jump and Straight Sprint. J. Strength. Cond. Res. 2009, 23, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Chaouachi, A.; Castagna, C.; Hue, O.; Wong, D.P.; Tabben, M.; Behm, D.G.; Chamari, K. Validity and Psychometric Evaluation of the French Version of RPE Scale in Young Fit Males When Monitoring Training Loads. Sci. Sports 2013, 28, e29–e35. [Google Scholar] [CrossRef]
- Pallarés, J.G.; Fernández-Elías, V.E.; Ortega, J.F.; Muñoz, G.; Muñoz-Guerra, J.; Mora-Rodríguez, R. Neuromuscular Responses to Incremental Caffeine Doses: Performance and Side Effects. Med. Sci. Sports Exerc. 2013, 45, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Bühler, E.; Lachenmeier, D.W.; Winkler, G. Development of a Tool to Assess Caffeine Intake among Teenagers and Young Adults. Ernahr. Umsch. 2014, 61, 58–63. [Google Scholar] [CrossRef]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Hopkins, W.G. A Scale of Magnitudes for Effect Statistics. New View Stat. 2002, 502, 321. [Google Scholar]
- Ayala, V.; Martínez-Bebia, M.; Latorre, J.A.; Gimenez-Blasi, N.; Jimenez-Casquet, M.J.; Conde-Pipo, J.; Bach-Faig, A.; Mariscal-Arcas, M. Influence of Circadian Rhythms on Sports Performance. Chronobiol. Int. 2021, 38, 1522–1536. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, S.; Kosmidis, I.; Sougioultzis, M.; Kabasakalis, A.; Mougios, V. Diurnal Variation and Reliability of the Urine Lactate Concentration after Maximal Exercise. Chronobiol. Int. 2018, 35, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Unver, S.; Atan, T. Investigation of the Changes in Performance Measurements Based on Circadian Rhythm. Anthropologist 2015, 19, 423–430. [Google Scholar] [CrossRef]
- Bougrine, H.; Cherif, M.; Chtourou, H.; Souissi, N. Does Ramadan Intermittent Fasting Affect the Intraday Variations of Cognitive and High-Intensity Short-Term Maximal Performances in Young Female Handball Players? Biol. Rhythm. Res. 2023, 54, 399–418. [Google Scholar] [CrossRef]
- Mhenni, T.; Michalsik, L.B.; Mejri, M.A.; Yousfi, N.; Chaouachi, A.; Souissi, N.; Chamari, K. Morning–Evening Difference of Team-Handball-Related Short-Term Maximal Physical Performances in Female Team Handball Players. J. Sports Sci. 2017, 35, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Sabzevari Rad, R.; Mahmoodzadeh Hosseini, H.; Shirvani, H. Circadian Rhythm Effect on Military Physical Fitness and Field Training: A Narrative Review. Sport. Sci. Health 2021, 17, 43–56. [Google Scholar] [CrossRef]
- Serin, Y.; Acar Tek, N. Effect of Circadian Rhythm on Metabolic Processes and the Regulation of Energy Balance. Ann. Nutr. Metab. 2019, 74, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, S.; Shibata, S. Time-of-Day-Dependent Physiological Responses to Meal and Exercise. Front. Nutr. 2020, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Bellastella, G.; De Bellis, A.; Maiorino, M.I.; Paglionico, V.A.; Esposito, K.; Bellastella, A. Endocrine Rhythms and Sport: It Is Time to Take Time into Account. J. Endocrinol. Invest. 2019, 42, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Facer-Childs, E.; Brandstaetter, R. The Impact of Circadian Phenotype and Time since Awakening on Diurnal Performance in Athletes. Curr. Biol. 2015, 25, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Bruton, A.; Marin-Puyalto, J.; Muñiz-Pardos, B.; Matute-Llorente, A.; Del Coso, J.; Gomez-Cabello, A.; Vicente-Rodriguez, G.; Casajus, J.A.; Lozano-Berges, G. Does Acute Caffeine Supplementation Improve Physical Performance in Female Team-Sport Athletes? Evidence from a Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3663. [Google Scholar] [CrossRef] [PubMed]
- Astorino, T.A.; Matera, A.J.; Basinger, J.; Evans, M.; Schurman, T.; Marquez, R. Effects of Red Bull Energy Drink on Repeated Sprint Performance in Women Athletes. Amino Acids 2012, 42, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L. Exercise and Sport Performance with Low Doses of Caffeine. Sports Med. 2014, 44, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.G.; Simon, B.J.; Schneider, M.F. Effects of Caffeine on Calcium Release from the Sarcoplasmic Reticulum in Frog Skeletal Muscle Fibres. J. Physiol. 1990, 425, 599–626. [Google Scholar] [CrossRef] [PubMed]
- Souissi, M.; Chikh, N.; Affès, H.; Sahnoun, Z. Caffeine Reversal of Sleep Deprivation Effects on Alertness, Mood and Repeated Sprint Performances in Physical Education Students. Biol. Rhythm. Res. 2018, 49, 746–760. [Google Scholar] [CrossRef]
- Facer-Childs, E.R.; Boiling, S.; Balanos, G.M. The Effects of Time of Day and Chronotype on Cognitive and Physical Performance in Healthy Volunteers. Sports Med. Open 2018, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ágoston, C.; Urbán, R.; Rigó, A.; Griffiths, M.D.; Demetrovics, Z. Morningness-Eveningness and Caffeine Consumption: A Largescale Path-Analysis Study. Chronobiol. Int. 2019, 36, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.M.; Halter, K.A.; Prosser, R.A. Circadian Rhythm and Sleep-Wake Systems Share the Dynamic Extracellular Synaptic Milieu. Neurobiol. Sleep Circadian Rhythm. 2018, 5, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.F.; Deboer, T.; Landolt, H.-P. Adenosine, Caffeine, and Sleep–Wake Regulation: State of the Science and Perspectives. J. Sleep Res. 2022, 31, e13597. [Google Scholar] [CrossRef] [PubMed]
- Hilaire, M.A.S.; Lockley, S.W. Caffeine Does Not Entrain the Circadian Clock but Improves Daytime Alertness in Blind Patients with Non-24-Hour Rhythms. Sleep Med. 2015, 16, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.K.; Bouâouda, H.; Gourmelen, S.; Dumont, S.; Fuchs, F.; Goumon, Y.; Bourgin, P.; Kalsbeek, A.; Challet, E. Sleep Deprivation and Caffeine Treatment Potentiate Photic Resetting of the Master Circadian Clock in a Diurnal Rodent. J. Neurosci. 2017, 37, 4343–4358. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Campo, D.J.; Pérez, A.; Ávila-Gandía, V.; Pérez-Piñero, S.; Rubio-Arias, J.Á. Impact of Caffeine Intake on 800-m Running Performance and Sleep Quality in Trained Runners. Nutrients 2019, 11, 2040. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.G.; Del Coso, J.; de Souza Fonseca, F.; Silva, B.V.C.; de Souza, D.B.; da Silva Gianoni, R.L.; Filip-Stachnik, A.; Serrão, J.C.; Claudino, J.G. Risk or Benefit? Side Effects of Caffeine Supplementation in Sport: A Systematic Review. Eur. J. Nutr. 2022, 61, 3823–3834. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, D.A.; Julia, H.L.; Turin, A.C. Caffeine and Human Behavior: Arousal, Anxiety, and Performance Effects. J. Behav. Med. 1982, 5, 415–439. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.B.; Greenblatt, D.J.; Ehrenberg, B.L.; Goddard, J.E.; Cotreau, M.M.; Harmatz, J.S.; Shader, R.I. Dose-dependent Pharmacokinetics and Psychomotor Effects of Caffeine in Humans. J. Clin. Pharmacol. 1997, 37, 693–703. [Google Scholar] [CrossRef]
- Paz-Graniel, I.; Kose, J.; Babio, N.; Hercberg, S.; Galan, P.; Touvier, M.; Salas-Salvadó, J.; Andreeva, V.A. Caffeine Intake and Its Sex-Specific Association with General Anxiety: A Cross-Sectional Analysis among General Population Adults. Nutrients 2022, 14, 1242. [Google Scholar] [CrossRef] [PubMed]
- Juliano, L.M.; Evatt, D.P.; Richards, B.D.; Griffiths, R.R. Characterization of Individuals Seeking Treatment for Caffeine Dependence. Psychol. Addict. Behav. 2012, 26, 948. [Google Scholar] [CrossRef] [PubMed]
- Mills, L.; Boakes, R.A.; Colagiuri, B. The Effect of Dose Expectancies on Caffeine Withdrawal Symptoms during Tapered Dose Reduction. J. Psychopharmacol. 2019, 33, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- de Mejia, E.G.; Ramirez-Mares, M.V. Impact of Caffeine and Coffee on Our Health. Trends Endocrinol. Metab. 2014, 25, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Li, Y.-F.; Wang, Y.; Tan, L.; Cao, Z.-Q.; Xie, C.; Xie, G.; Gong, H.-B.; Sun, W.-Y.; Ouyang, S.-H. Identification and Characterization of N 9-Methyltransferase Involved in Converting Caffeine into Non-Stimulatory Theacrine in Tea. Nat. Commun. 2020, 11, 1473. [Google Scholar] [CrossRef] [PubMed]
- Shilo, L.; Sabbah, H.; Hadari, R.; Kovatz, S.; Weinberg, U.; Dolev, S.; Dagan, Y.; Shenkman, L. The Effects of Coffee Consumption on Sleep and Melatonin Secretion. Sleep Med. 2002, 3, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Fernández, O.H.; Liu, J.A.; Nelson, R.J. Circadian Rhythms Disrupted by Light at Night and Mistimed Food Intake Alter Hormonal Rhythms and Metabolism. Int. J. Mol. Sci. 2023, 24, 3392. [Google Scholar] [CrossRef] [PubMed]
- Segu, A.; Kannan, N.N. The Duration of Caffeine Treatment Plays an Essential Role in Its Effect on Sleep and Circadian Rhythm. Sleep. Adv. 2023, 4, zpad014. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC Consensus Statement: Dietary Supplements and the High-Performance Athlete. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Halson, S.L.; Sargent, C.; Roach, G.D.; Nédélec, M.; Gupta, L.; Leeder, J.; Fullagar, H.H.; Coutts, A.J.; Edwards, B.J.; et al. Sleep and the Athlete: Narrative Review and 2021 Expert Consensus Recommendations. Br. J. Sports Med. 2021, 55, 356–368. [Google Scholar] [CrossRef] [PubMed]
| PLAC | CAF (3 mg) | CAF (6 mg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8:00 a.m. | 6:00 p.m. | 8:00 a.m. | 6:00 p.m. | 8:00 a.m. | 6:00 p.m. | |||||||
| Questionnaire | D1 | D2 | D1 | D2 | D1 | D2 | D1 | D2 | D1 | D2 | D1 | D2 |
| Muscle soreness | 0 | 6.66 | 6.66 | 6.66 | 0 | 0 | 6.66 | 6.66 | 6.66 | 0 | 6.66 | 13.33 |
| Increased urine output | 0 | 6.66 | 0 | 6.66 | 0 | 13.33 | 13.33 | 20 | 6.66 | 6.66 | 6.66 | 26.66 |
| Tachycardia | 0 | 6.66 | 13.33 | 6.6 | 13.33 | 6.66 | 13.33 | 20 | 13.33 | 6.66 | 33.33 | 20 |
| Anxiety or nervousness | 6.66 | 6.66 | 0 | 6.66 | 0 | 6.66 | 6.66 | 6.66 | 6.66 | 0 | 13.33 | 13.33 |
| Headache | 6.66 | 6.66 | 6.66 | 0 | 13.33 | 6.66 | 13.33 | 26.66 | 20 | 13.33 | 20 | 40 |
| Gastrointestinal problems | 0 | 6.66 | 0 | 13.33 | 6.66 | 0 | 6.66 | 26.66 | 13.33 | 0 | 26.66 | 26.66 |
| Insomnia | - | 6.66 | - | 6.66 | - | 6.66 | - | 40 | - | 20 | - | 46.66 |
| Increased vigor/activeness | 6.66 | 0 | 13.33 | 0 | 6.66 | 6.66 | 13.33 | 6.66 | 13.33 | 6.66 | 13.33 | 13.33 |
| Perception of performance improvement | 13.33 | - | 6.66 | - | 13.33 | - | 13.33 | - | 13.33 | - | 13.33 | - |
| Blinding | 13.33 | 13.33 | 6.66 | 13.33 | 0 | 13.33 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bougrine, H.; Ammar, A.; Salem, A.; Trabelsi, K.; Żmijewski, P.; Jahrami, H.; Chtourou, H.; Souissi, N. Effects of Different Caffeine Dosages on Maximal Physical Performance and Potential Side Effects in Low-Consumer Female Athletes: Morning vs. Evening Administration. Nutrients 2024, 16, 2223. https://doi.org/10.3390/nu16142223
Bougrine H, Ammar A, Salem A, Trabelsi K, Żmijewski P, Jahrami H, Chtourou H, Souissi N. Effects of Different Caffeine Dosages on Maximal Physical Performance and Potential Side Effects in Low-Consumer Female Athletes: Morning vs. Evening Administration. Nutrients. 2024; 16(14):2223. https://doi.org/10.3390/nu16142223
Chicago/Turabian StyleBougrine, Houda, Achraf Ammar, Atef Salem, Khaled Trabelsi, Piotr Żmijewski, Haitham Jahrami, Hamdi Chtourou, and Nizar Souissi. 2024. "Effects of Different Caffeine Dosages on Maximal Physical Performance and Potential Side Effects in Low-Consumer Female Athletes: Morning vs. Evening Administration" Nutrients 16, no. 14: 2223. https://doi.org/10.3390/nu16142223
APA StyleBougrine, H., Ammar, A., Salem, A., Trabelsi, K., Żmijewski, P., Jahrami, H., Chtourou, H., & Souissi, N. (2024). Effects of Different Caffeine Dosages on Maximal Physical Performance and Potential Side Effects in Low-Consumer Female Athletes: Morning vs. Evening Administration. Nutrients, 16(14), 2223. https://doi.org/10.3390/nu16142223









