Serum 25-Hydroxyvitamin D Level Is Positively Associated with Vascular Reactivity Index in Patients with Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Anthropometric and Blood Pressure Measurements
2.3. Biochemical Investigations
2.4. Endothelial Function Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maruhashi, T.; Higashi, Y. Pathophysiological association between diabetes mellitus and endothelial dysfunction. Antioxidants 2021, 10, 1306. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional, and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef]
- Villano, A.; Mencarelli, E.; Melita, V.; Rizzi, A.; Lamendola, P.; De Vita, A.; Manfredonia, L.; Ravenna, S.E.; Pitocco, D.; Lanza, G.A.; et al. Endothelial dysfunction and cardiovascular outcomes in asymptomatic patients with type 2 diabetes: A pilot study. Diabetes Metab. Res. Rev. 2020, 36, e3215. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanisms to pharmacokinetics. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Metzler, M.; Duerr, S.; Granata, R.; Krismer, F.; Robertson, D.; Wenning, G.K. Neurogenic orthostatic hypotension: Pathophysiology, evaluation, and management. J. Neurol. 2013, 260, 2212–2219. [Google Scholar] [CrossRef]
- Uberti, F.; Lattuada, D.; Morsanuto, V.; Nava, U.; Bolis, G.; Vacca, G.; Squarzanti, D.F.; Cisari, C.; Molinari, C. Vitamin D protects human endothelial cells from oxidative stress through autophagic and survival pathways. J. Clin. Endocrinol. Metab. 2014, 99, 1367–1374. [Google Scholar] [CrossRef]
- Oz, F.; Cizgici, A.Y.; Oflaz, H.; Elitok, A.; Karaayvaz, E.B.; Mercanoglu, F.; Bugra, Z.; Omer, B.; Adalet, K.; Oncul, A. Impact of vitamin D insufficiency on epicardial coronary flow velocity and endothelial function. Coron. Artery Dis. 2013, 24, 392–397. [Google Scholar] [CrossRef]
- Dalan, R.; Liew, H.; Tan, W.K.A.; Chew, D.E.; Leow, M.K.-S. Vitamin D and the endothelium: Basic, translational, and clinical research updates. IJC Metab. Endocr. 2014, 4, 4–17. [Google Scholar] [CrossRef]
- Dalan, R.; Liew, H.; Assam, P.N.; Chan, E.S.; Siddiqui, F.J.; Tan, A.W.; Chew, D.E.; Boehm, B.O.; Leow, M.K. A randomized controlled trial evaluating the impact of targeted vitamin D supplementation on endothelial function in patients with type 2 diabetes mellitus: The DIMENSION trial. Diab. Vasc. Dis. Res. 2016, 13, 192–200. [Google Scholar] [CrossRef]
- Hsu, B.G.; Wu, D.A.; Yang, H.Y.; Chen, M.C. Serum sclerostin level is positively associated with endothelial dysfunction measured by digital thermal monitoring in patients with type 2 diabetes: A prospective cross-sectional study. Medicine 2023, 102, e34649. [Google Scholar] [CrossRef]
- Chen, M.C.; Lee, C.J.; Lin, Y.L.; Wang, C.H.; Hsu, B.G. Association between serum adiponectin levels and endothelial function in patients with non-dialysis-dependent chronic kidney disease patients. Biomedicines 2023, 11, 2174. [Google Scholar] [CrossRef]
- Lee, C.J.; Hsieh, Y.J.; Lin, Y.L.; Wang, C.H.; Hsu, B.G.; Tsai, J.P. Correlation between serum 25-hydroxyvitamin d level and peripheral arterial stiffness in chronic kidney disease stage 3-5 patients. Nutrients 2022, 14, 2429. [Google Scholar] [CrossRef]
- Beveridge, L.A.; Khan, F.; Struthers, A.D.; Armitage, J.; Barchetta, I.; Bressendorff, I.; Cavallo, M.G.; Clarke, R.; Dalan, R.; Dreyer, G.; et al. Effects of vitamin D supplementation on vascular function markers: A systematic review and individual participant meta-analysis. J. Am. Heart Assoc. 2018, 7, e008273. [Google Scholar] [CrossRef]
- Cohn, J.N.; Quyyumi, A.A.; Hollenberg, N.K.; Jamerson, K.A. Surrogate markers for cardiovascular disease: Functional markers. Circulation 2004, 109 (25 Suppl. 1), IV31–IV46. [Google Scholar] [CrossRef]
- Suetsugu, M.; Takebayashi, K.; Aso, Y. Association between diabetic microangiopathy and vascular endothelial function evaluated by flow-mediated vasodilation in patients with type 2 diabetes mellitus. Int. J. Clin. Pract. 2007, 61, 920–926. [Google Scholar] [CrossRef]
- Naghavi, M.; Yen, A.A.; Lin, A.W.; Tanaka, H.; Kleis, S. New indices of endothelial function measured by digital thermal monitoring of vascular reactivity: Data from 6084 patients registry. Int. J. Vasc. Med. 2016, 2016, 1348028. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Kiss, T.; Wren, J.D.; Giles, C.B.; Griffin, C.T.; Murfee, W.L.; Pacher, P.; Csiszar, A. Endothelial dysfunction and angiogenesis impairment in the aging vasculature. Nat. Rev. Cardiol. 2018, 15, 555–565. [Google Scholar] [CrossRef]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial dysfunction in obesity-induced inflammation: Molecular mechanisms and clinical implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Larson, M.G.; Keyes, M.J.; Mitchell, G.F.; Vasan, R.S.; Keaney, J.F., Jr.; Lehman, B.T.; Fan, S.; Osypiuk, E.; Vita, J.A. Clinical correlates and heritability of flow-mediated dilation in the community: The Framingham Heart Study. Circulation 2004, 109, 613–619. [Google Scholar] [CrossRef]
- Wang, S.; Randall, D.C.; Knapp, C.F.; Patwardhan, A.R.; Nelson, K.R.; Karounos, D.G.; Evans, J.M. Blood pressure regulation in patients with diabetes with and without peripheral neuropathy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R541–R550. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, Y.; Morioka, T.; Mori, K.; Yamazaki, Y.; Ochi, A.; Kurajoh, M.; Fukumoto, S.; Shioi, A.; Shoji, T.; Inaba, M.; et al. Albuminuria, rather than glomerular filtration rate, is associated with vascular endothelial function in patients with type 2 diabetes. J. Diabetes Complicat. 2020, 34, 107702. [Google Scholar] [CrossRef]
- Rensma, S.P.; van Sloten, T.T.; Houben, A.J.H.M.; Köhler, S.; van Boxtel, M.P.J.; Berendschot, T.T.J.M.; Jansen, J.F.A.; Verhey, F.R.J.; Kroon, A.A.; Koster, A.; et al. Microvascular dysfunction was associated with poor cognitive performance in the Maastricht study. Hypertension 2020, 75, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Ando, A.; Miyamoto, M.; Saito, N.; Kotani, K.; Kamiya, H.; Ishibashi, S.; Tavakoli, M. Small fiber neuropathy is associated with impaired vascular endothelial function in patients with type 2 diabetes. Front. Endocrinol. 2021, 12, 653277. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wei, L.; Xiong, X.; Yang, M.; Sun, L. Association between serum 25-hydroxyvitamin D and diabetic kidney disease in Chinese patients with type 2 diabetes. Front. Endocrinol. 2020, 11, 564738. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Okada, Y.; Hajime, M.; Tanaka, Y. Low vitamin D levels are associated with vascular endothelial dysfunction in patients with poorly controlled type 2 diabetes: A retrospective study. J. Atheroscler. Thromb. 2022, 29, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Molinari, C.; Uberti, F.; Grossini, E.; Vacca, G.; Carda, S.; Invernizzi, M.; Cisari, C. 1α,25-dihydroxycholecalciferol induces nitric oxide production in cultured endothelial cells. Cell Physiol. Biochem. 2011, 27, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Andrukhova, O.; Slavic, S.; Zeitz, U.; Riesen, S.C.; Heppelmann, M.S.; Ambrisko, T.D.; Markovic, M.; Kuebler, W.M.; Erben, R.G. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol. Endocrinol. 2014, 28, 53–64. [Google Scholar] [CrossRef]
- Kalkan, G.Y.; Gür, M.; Koyunsever, N.Y.; Şeker, T.; Gözükara, M.Y.; Uçar, H.; Kaypaklı, O.; Baykan, A.O.; Akyol, S.; Türkoğlu, C.; et al. Serum 25-Hydroxyvitamin D Level and aortic intima-media thickness in patients without clinical manifestation of atherosclerotic cardiovascular disease. J. Clin. Lab. Anal. 2015, 29, 305–311. [Google Scholar] [CrossRef]
- Joergensen, C.; Gall, M.A.; Schmedes, A.; Tarnow, L.; Parving, H.H.; Rossing, P. Vitamin D levels and mortality in type 2 diabetes. Diabetes Care 2010, 33, 2238–2243. [Google Scholar] [CrossRef] [PubMed]
- Yiu, Y.F.; Chan, Y.H.; Yiu, K.H.; Siu, C.W.; Li, S.W.; Wong, L.Y.; Lee, S.W.; Tam, S.; Wong, E.W.; Cheung, B.M.; et al. Vitamin D deficiency is associated with depletion of circulating endothelial progenitor cells and endothelial dysfunction in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2011, 96, E830–E835. [Google Scholar] [CrossRef] [PubMed]
- Juonala, M.; Voipio, A.; Pahkala, K.; Viikari, J.S.; Mikkilä, V.; Kähönen, M.; Hutri-Kähönen, N.; Jula, A.; Burgner, D.; Sabin, M.A.; et al. Childhood 25-OH vitamin D levels and carotid intima-media thickness in adulthood: Cardiovascular risk in young Finns. J. Clin. Endocrinol. Metab. 2015, 100, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Bacha, F.; Bartz, S.K.; Tomsa, A.; Sharma, S. Free vitamin D: Relationship to insulin sensitivity and vascular health in youth. J. Pediatr. 2019, 212, 28–34.e2. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Evans, M.K.; Wenger, J.; Zonderman, A.B.; Berg, A.H.; Nalls, M.; Tamez, H.; Zhang, D.; Bhan, I.; Karumanchi, S.A.; et al. Vitamin D-binding protein and vitamin D status in black and white Americans. N. Engl. J. Med. 2013, 369, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Gee, E.; Halloran, B.; Kowalski, M.A.; Ryzen, E.; Haddad, J.G. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and vitamin D binding protein. J. Clin. Endocrinol. Metab. 1986, 63, 954–959. [Google Scholar] [CrossRef]
- Ciarambino, T.; Crispino, P.; Minervini, G.; Giordano, M. Vitamin D: Can gender medicine have a role? Biomedicines 2023, 11, 1762. [Google Scholar] [CrossRef]
| Characteristics | All Patients (n = 102) | Good Vascular Reactivity (n = 33) | Intermediate Vascular Reactivity (n = 39) | Poor Vascular Reactivity (n = 30) | p Value |
|---|---|---|---|---|---|
| Age (years) | 64.61 ± 9.27 | 63.09 ± 8.94 | 66.49 ± 8.08 | 63.83 ± 10.86 | 0.262 |
| Height (cm) | 161.38 ± 9.21 | 159.43 ± 9.42 | 162.23 ± 9.60 | 162.43 ± 8.36 | 0.336 |
| Body weight (kg) | 71.25 ± 14.15 | 72.63 ± 14.92 | 69.08 ± 10.99 | 72.54 ± 16.83 | 0.482 |
| Body mass index (kg/m2) | 27.21 ± 4.16 | 28.47 ± 4.64 | 26.09 ± 2.70 | 27.29 ± 4.82 | 0.051 |
| Vascular reactivity index | 1.48 ± 0.70 | 2.34 ± 0.22 | 1.37 ± 0.27 | 0.68 ± 0.20 | <0.001 * |
| Systolic BP (mmHg) | 133.83 ± 17.91 | 137.61 ± 15.49 | 136.23 ± 18.37 | 126.57 ± 18.21 | 0.027 * |
| Diastolic BP (mmHg) | 69.84 ± 12.95 | 77.12 ± 10.36 | 67.00 ± 11.95 | 65.53 ± 13.72 | <0.001 * |
| Total cholesterol (mg/dL) | 156.44 ± 34.64 | 153.70 ± 37.35 | 149.67 ± 27.35 | 168.27 ± 37.99 | 0.073 |
| Triglyceride (mg/dL) | 123.00 (81.00–170.25) | 129.00 (86.00–208.50) | 136.00 (81.00–170.00) | 105.50 (71.00–163.50) | 0.346 |
| LDL-C (mg/dL) | 85.47 ± 29.21 | 82.85 ± 28.93 | 81.41 ± 27.47 | 93.63 ± 31.00 | 0.187 |
| Fasting glucose (mg/dL) | 138.50 (112.75–170.25) | 131.00 (110.00–141.50) | 147.00 (118.00–180.00) | 156.50 (120.25–183.25) | 0.019 * |
| Glycated hemoglobin (%) | 7.35 (6.48–9.15) | 7.00 (6.20–7.50) | 7.70 (6.60–9.10) | 7.90 (6.58–10.13) | 0.009 * |
| Blood urea nitrogen (mg/dL) | 17.00 (13.00–23.00) | 15.00 (13.00–21.00) | 19.00 (14.00–24.50) | 17.00 (13.00–23.00) | 0.197 |
| Creatinine (mg/dL) | 1.10 (0.90–1.40) | 1.00 (0.90–1.45) | 1.10 (1.00–1.30) | 1.30 (0.90-1.83) | 0.290 |
| eGFR (mL/min) | 65.44 ± 22.71 | 71.26 ± 26.51 | 63.22 ± 16.92 | 61.94 ± 24.23 | 0.197 |
| UACR (mg/g) | 182.21 (81.873–311.77) | 121.27 (28.61–270.81) | 167.40 (63.30–315.48) | 231.28 (157.65–390.50) | 0.006 * |
| Total 25(OH)D (ng/mL) | 14.76 ± 5.90 | 18.54 ± 6.33 | 13.56 ± 5.00 | 12.17 ± 4.34 | <0.001 * |
| Female, n (%) | 31 (30.4) | 10 (30.3) | 12 (30.8) | 9 (30.0) | 0.998 |
| Hypertension, n (%) | 58 (56.9) | 22 (66.7) | 25 (64.1) | 11 (36.7) | 0.029 * |
| ARB use, n (%) | 53 (52.0) | 19 (57.6) | 22 (56.4) | 12 (40.0) | 0.294 |
| β-blocker use, n (%) | 18 (17.6) | 6 (18.2) | 7 (17.9) | 5 (16.7) | 0.986 |
| CCB use, n (%) | 33 (32.4) | 10 (30.3) | 14 (35.9) | 9 (30.0) | 0.834 |
| Statin use, n (%) | 51 (50.0) | 16 (48.5) | 19 (48.7) | 16 (53.3) | 0.910 |
| Fibrate use, n (%) | 32 (31.4) | 11 (33.3) | 13 (33.3) | 8 (26.7) | 0.804 |
| Metformin use, n (%) | 64 (62.7) | 22 (66.7) | 23 (59.0) | 19 (63.3) | 0.795 |
| Sulfonylureas use, n (%) | 54 (52.9) | 17 (51.5) | 24 (61.5) | 13 (43.3) | 0.317 |
| DDP-4 inhibitors use, n (%) | 59 (57.8) | 21 (63.6) | 22 (56.4) | 16 (53.3) | 0.692 |
| Thiazolidinedione use, n (%) | 10 (9.8) | 4 (12.1) | 4 (10.3) | 2 (6.7) | 0.762 |
| SGLT2 inhibitors use, n (%) | 23 (22.5) | 7 (21.2) | 9 (23.1) | 7 (23.3) | 0.975 |
| Insulin use, n (%) | 31 (30.4) | 9 (27.3) | 11 (28.2) | 11 (36.7) | 0.671 |
| Model | 25(OH)D (per 1 ng/mL of Increase) for Vascular Reactivity Dysfunction | 25(OH)D (per 1 ng/mL of Increase) for Poor Vascular Reactivity | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Crude model | 0.826 (0.751–0.909) | <0.001 * | 0.878 (0.800–0.963) | 0.006 * |
| Adjusted model | 0.845 (0.753–0.948) | 0.004 * | 0.878 (0.790–0.976) | 0.016 * |
| Vascular ReactivityDysfunction | |||||||
| AUC (95% CI) | p Value | Cut-off | Sen (%) | Spe (%) | PPV (%) | NPV (%) | |
| 25(OH)D (ng/mL) | 0.762 (0.659–0.866) | <0.001 * | 14.94 | 69.57 | 75.76 | 85.72 | 54.35 |
| Poor Vascular Reactivity | |||||||
| AUC (95% CI) | p Value | Cut-off | Sen (%) | Spe (%) | PPV (%) | NPV (%) | |
| 25(OH)D (ng/mL) | 0.674 (0.562–0.786) | 0.0023 * | 13.38 | 66.67 | 63.89 | 43.48 | 82.15 |
| Variables | Vascular Reactivity Index | ||||
|---|---|---|---|---|---|
| Simple Regression | Multivariable Regression | ||||
| r | p Value | Beta | Adjusted r2 Change | p Value | |
| Female | 0.022 | 0.823 | – | – | – |
| Hypertension | 0.202 | 0.042 * | – | – | – |
| Age (years) | −0.063 | 0.527 | – | – | – |
| Height (cm) | −0.172 | 0.084 | – | – | – |
| Body weight (kg) | −0.004 | 0.970 | – | – | – |
| Body mass index (kg/m2) | 0.146 | 0.144 | – | – | – |
| Systolic blood pressure (mmHg) | 0.214 | 0.031 * | – | – | – |
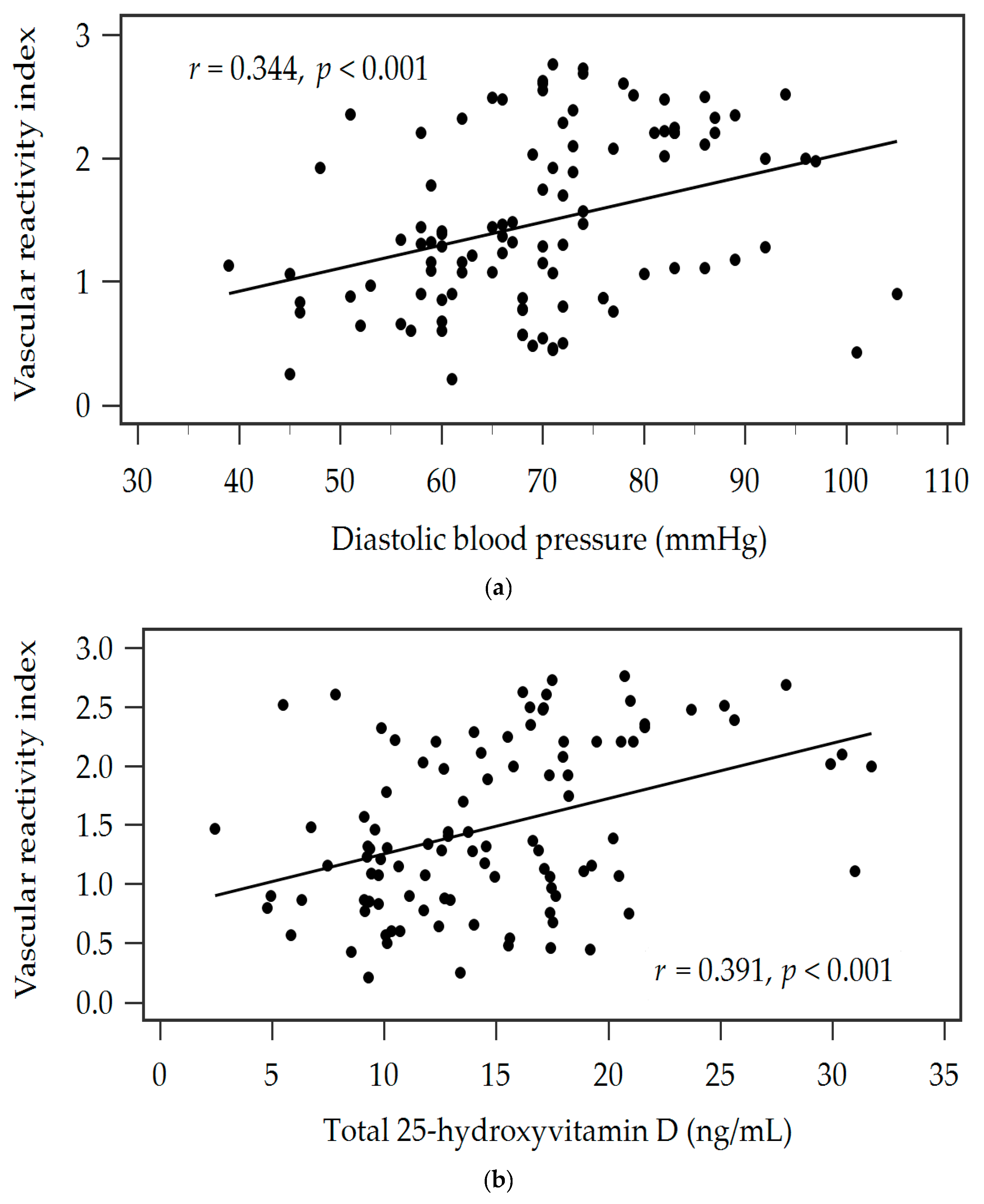
| Diastolic blood pressure (mmHg) | 0.344 | <0.001 * | 0.282 | 0.070 | 0.002 * |
| Total cholesterol (mg/dL) | −0.116 | 0.246 | – | – | – |
| Log-Triglyceride (mg/dL) | 0.086 | 0.392 | – | – | – |
| LDL-C (mg/dL) | −0.171 | 0.085 | – | – | – |
| Log-Glucose (mg/dL) | −0.199 | 0.045 * | – | – | – |
| Log-Glycated hemoglobin (%) | −0.249 | 0.012 * | – | – | – |
| Log-BUN (mg/dL) | −0.018 | 0.857 | – | – | – |
| Log-Creatinine (mg/dL) | −0.142 | 0.156 | – | – | – |
| eGFR (mL/min) | 0.161 | 0.107 | – | – | – |
| Log-UACR (mg/g) | −0.207 | 0.037 * | – | – | – |
| Total 25-hydroxyvitamin D (ng/mL) | 0.392 | <0.001 * | 0.342 | 0.146 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, B.-G.; Wang, Y.-C.; Wu, D.-A.; Chen, M.-C. Serum 25-Hydroxyvitamin D Level Is Positively Associated with Vascular Reactivity Index in Patients with Type 2 Diabetes Mellitus. Nutrients 2024, 16, 1575. https://doi.org/10.3390/nu16111575
Hsu B-G, Wang Y-C, Wu D-A, Chen M-C. Serum 25-Hydroxyvitamin D Level Is Positively Associated with Vascular Reactivity Index in Patients with Type 2 Diabetes Mellitus. Nutrients. 2024; 16(11):1575. https://doi.org/10.3390/nu16111575
Chicago/Turabian StyleHsu, Bang-Gee, Yi-Cheng Wang, Du-An Wu, and Ming-Chun Chen. 2024. "Serum 25-Hydroxyvitamin D Level Is Positively Associated with Vascular Reactivity Index in Patients with Type 2 Diabetes Mellitus" Nutrients 16, no. 11: 1575. https://doi.org/10.3390/nu16111575








