Lycopene in Combination with Insulin Triggers Antioxidant Defenses and Increases the Expression of Components That Detoxify Advanced Glycation Products in Kidneys of Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Induction of DM
2.2. Experimental Design
2.3. Physiological Parameters, Temporal Glycemia Levels and Experiment Conclusion
2.4. Biochemical Parameters
2.5. Biomarkers of Glycoxidative Stress and Antioxidant Defenses
2.6. Semi-Quantitative Analysis of Albuminuria Levels
2.7. Antioxidants’ Defenses in Kidneys
2.8. Western Blotting Analysis in Muscles and Kidneys
2.9. Statistical Analysis
3. Results
3.1. Combined Therapy 1 U/Day Insulin + Lycopene Prevented Weight Loss and Polyphagia in Diabetic Rats
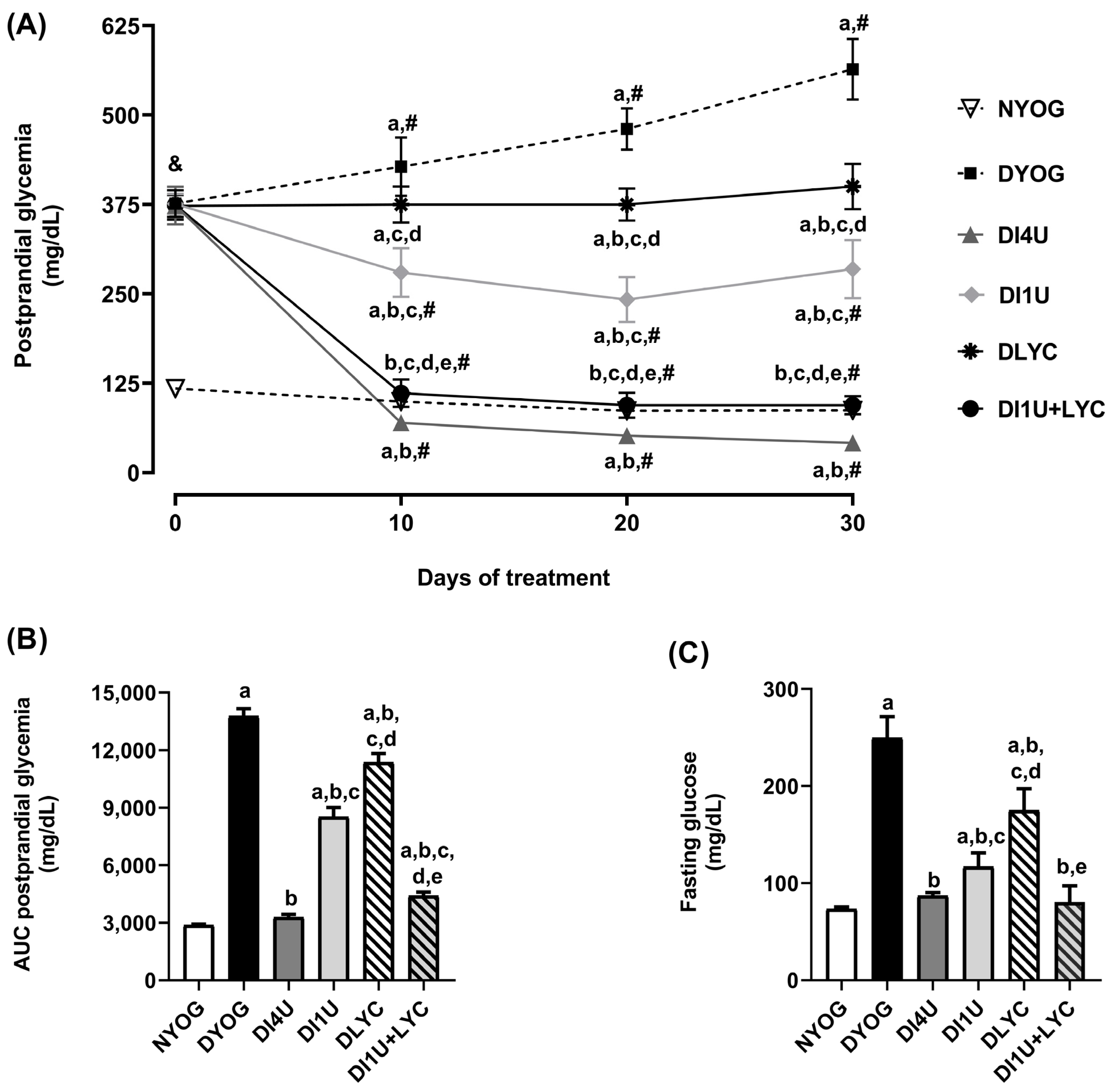
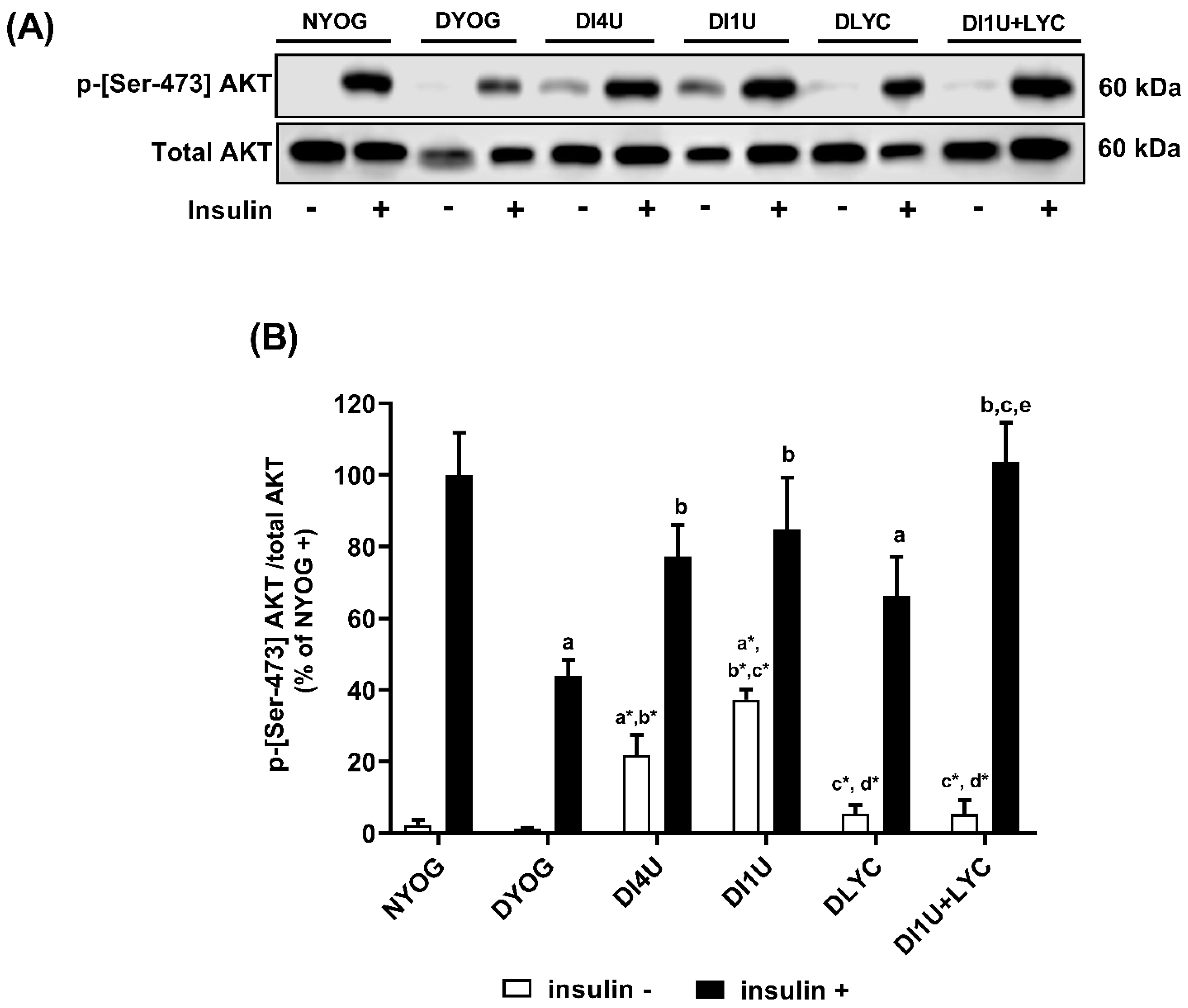
3.2. Combined Therapy 1 U/Day Insulin + Lycopene Decreased Glycemia and Improved Insulin Sensitivity in Diabetic Rats
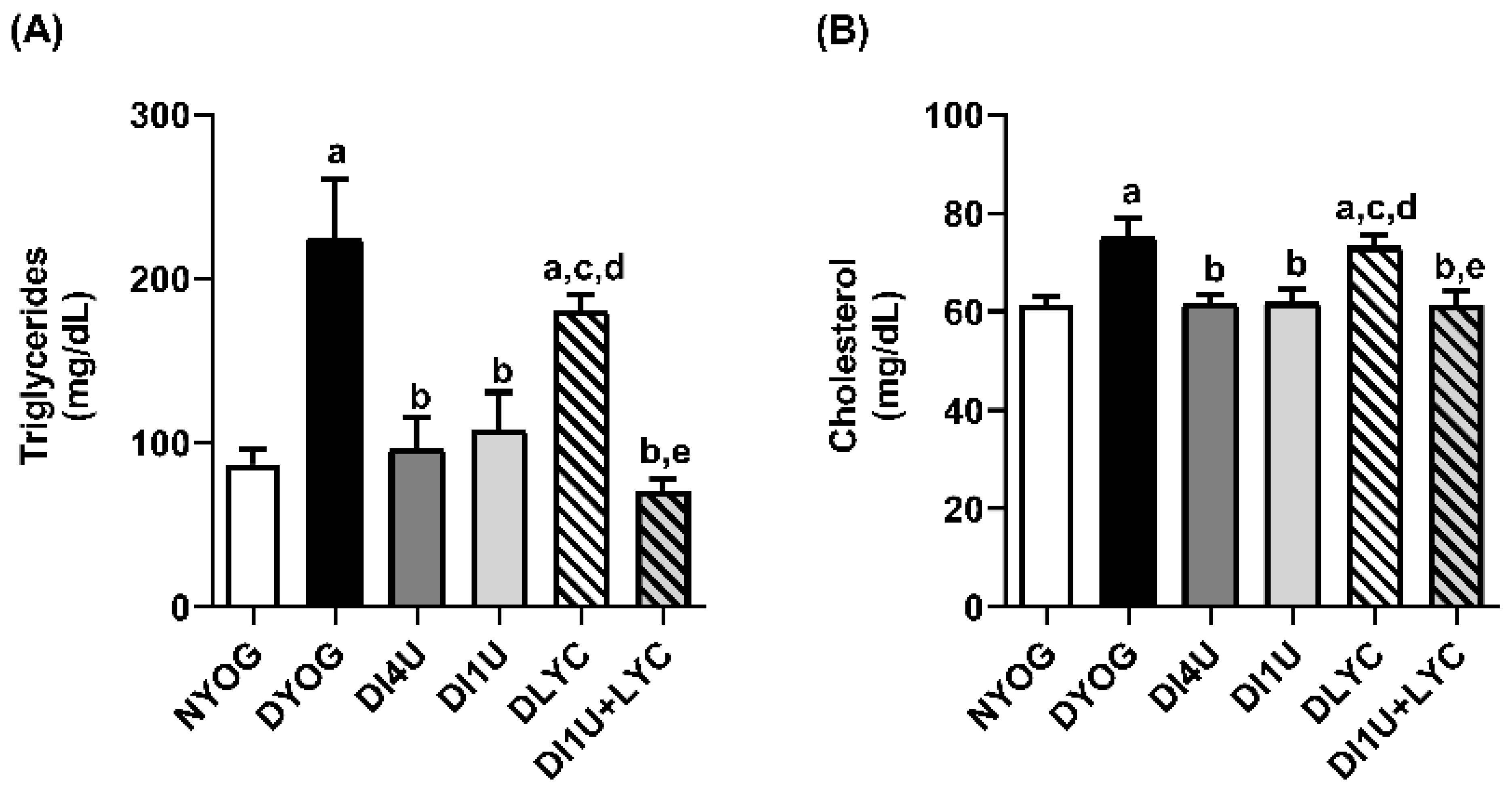
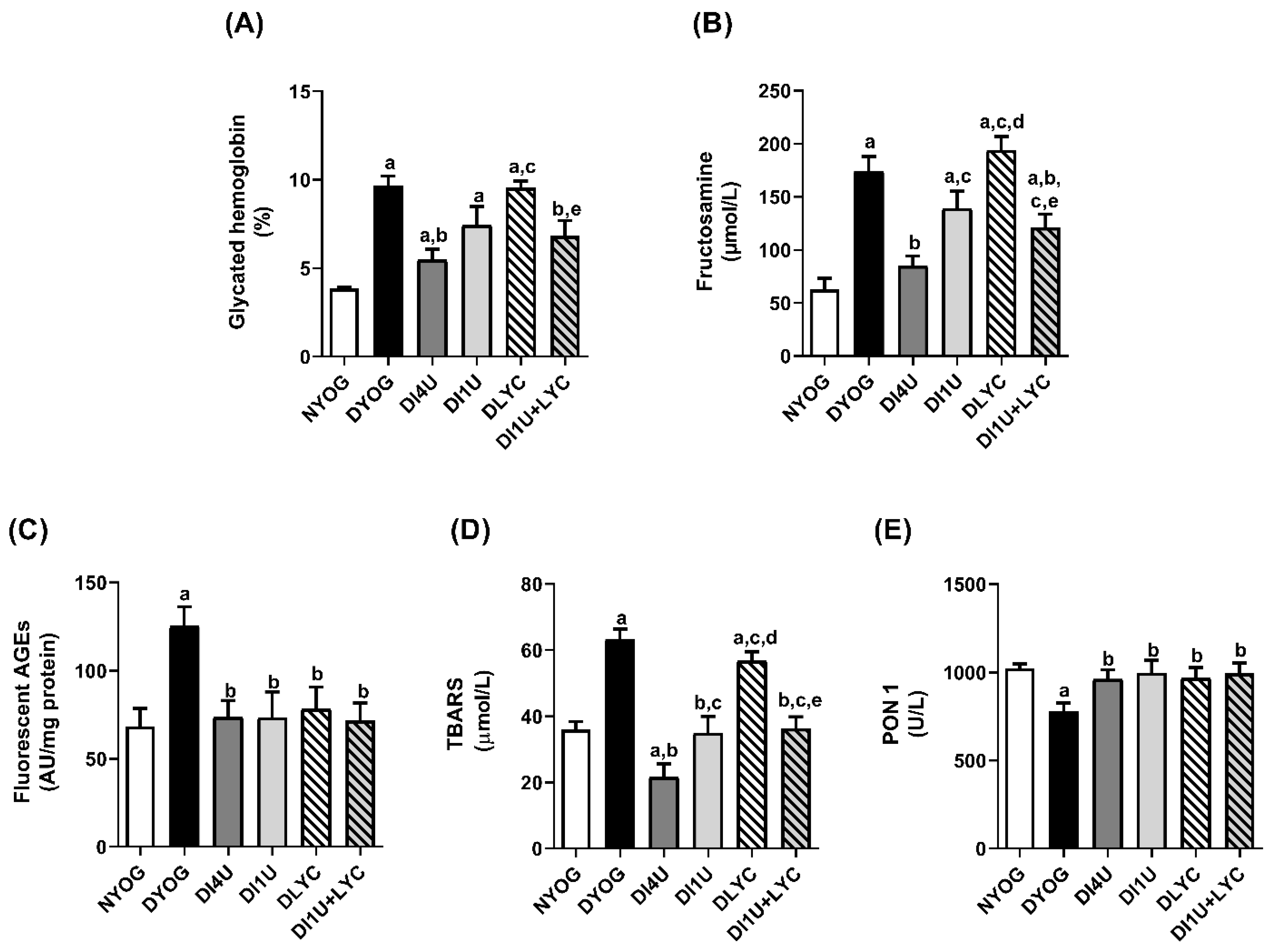
3.3. Combined Therapy 1 U/Day Insulin + Lycopene Decreased Dyslipidemia, HbA1c and Biomarkers of Glycoxidative Stress, and Increased Antioxidant Defenses in Plasma of Diabetic Rats
3.4. Combined Therapy 1 U/Day Insulin + Lycopene Reduced Albuminuria in Diabetic Rats
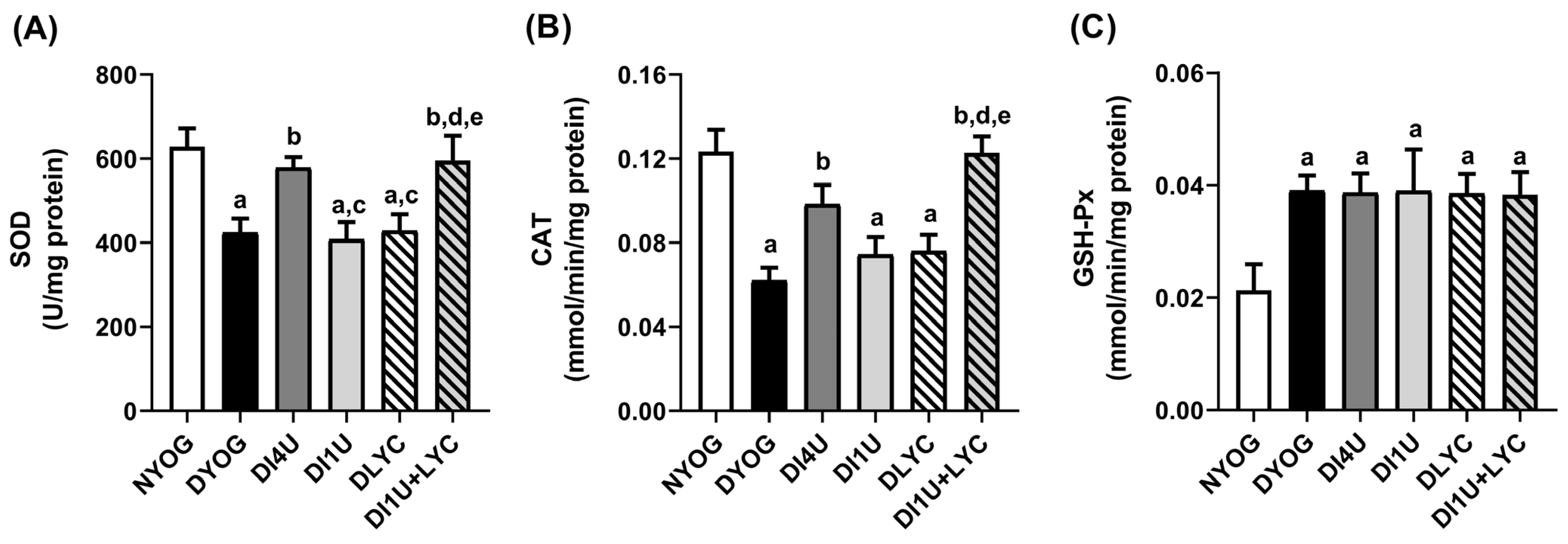
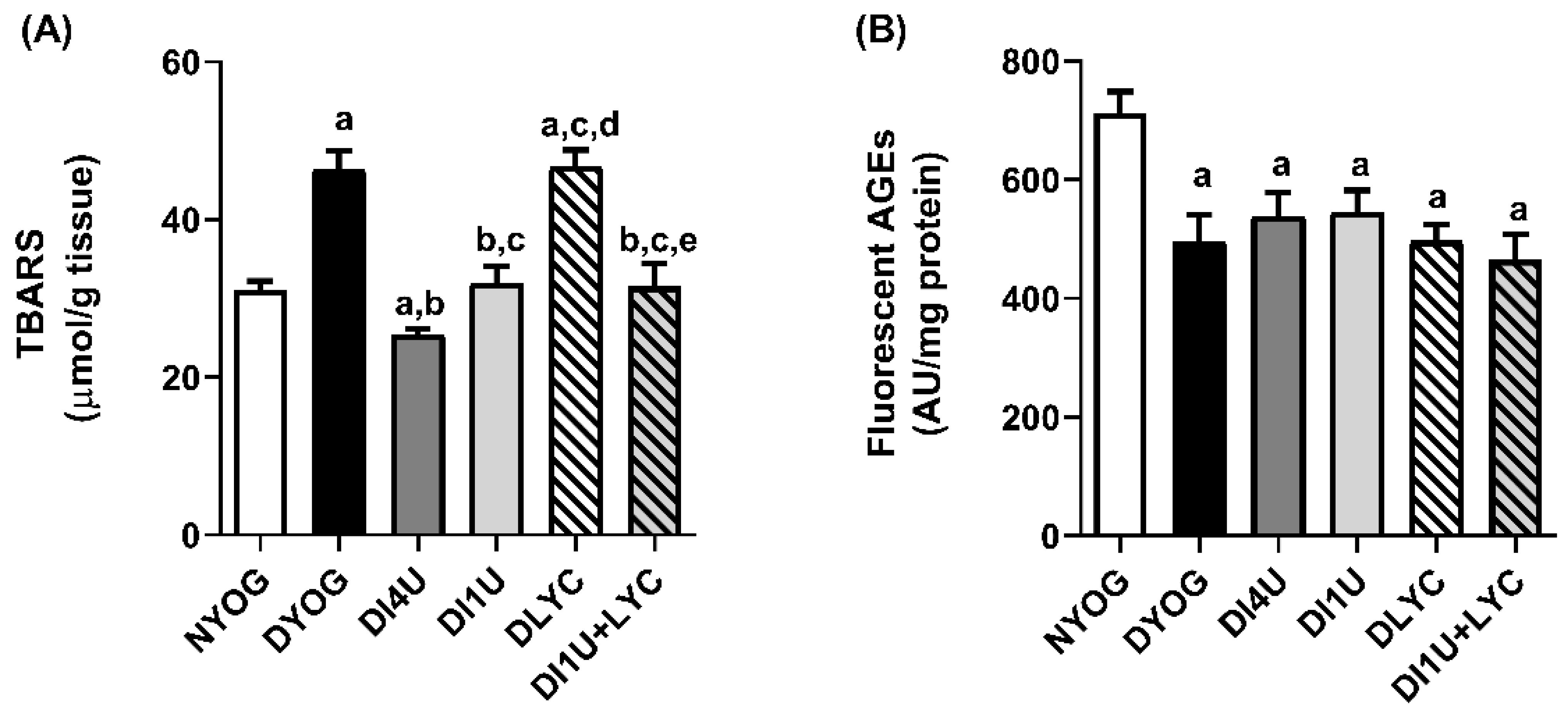
3.5. Combined Therapy 1 U/Day Insulin + Lycopene Stimulated Cytoprotective Mechanisms Related to AGE and ROS Detoxification in Kidneys of Diabetic Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okamoto, M.M.; Anhê, G.F.; Sabino-Silva, R.; dos Snatos Ferreira Marques, M.F.; Freitas, H.S.; Mori, R.C.T.; Melo, K.F.S.; Machado, U.F. Intensive Insulin Treatment Induces Insulin Resistance in Diabetic Rats by Impairing Glucose Metabolism-Related Mechanisms in Muscle and Liver. J. Endocrinol. 2011, 211, 55–64. [Google Scholar] [CrossRef]
- Miller, R.G.; McGurnaghan, S.J.; Onengut-Gumuscu, S.; Chen, W.-M.; Colhoun, H.M.; Rich, S.S.; Orchard, T.J.; Costacou, T. Insulin Resistance-Associated Genetic Variants in Type 1 Diabetes. J. Diabetes Complicat. 2021, 35, 107842. [Google Scholar] [CrossRef]
- Cryer, P.E. Mechanisms of Hypoglycemia-Associated Autonomic Failure and Its Component Syndromes in Diabetes. Diabetes 2013, 54, 3592–3601. [Google Scholar] [CrossRef]
- Heller, S. Weight Gain during Insulin Therapy in Patients with Type 2 Diabetes Mellitus. Diabetes Res. Clin. Pract. 2004, 65, S23–S27. [Google Scholar] [CrossRef]
- Akoumianakis, I.; Badi, I.; Douglas, G.; Chuaiphichai, S.; Herdman, L.; Akawi, N.; Margaritis, M.; Antonopoulos, A.S.; Oikonomou, E.K.; Psarros, C.; et al. Insulin-Induced Vascular Redox Dysregulation in Human Atherosclerosis Is Ameliorated by Dipeptidyl Peptidase 4 Inhibition. Sci. Transl. Med. 2020, 12, eaav8824. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Kumar, A.; Doble, M. Combination Therapy: A New Strategy to Manage Diabetes and Its Complications. Phytomedicine 2014, 21, 123–130. [Google Scholar] [CrossRef]
- Venkatakrishnan, K.; Chiu, H.-F.; Wang, C.-K. Popular Functional Foods and Herbs for the Management of Type-2-Diabetes Mellitus: A Comprehensive Review with Special Reference to Clinical Trials and Its Proposed Mechanism. J. Funct. Foods 2019, 57, 425–438. [Google Scholar] [CrossRef]
- Sharma, S.; Chopra, K.; Kulkarni, S.K. Effect of Insulin and Its Combination with Resveratrol or Curcumin in Attenuation of Diabetic Neuropathic Pain: Participation of Nitric Oxide and TNF-Alpha. Phytother. Res. 2007, 21, 278–283. [Google Scholar] [CrossRef]
- Malekiyan, R.; Abdanipour, A.; Sohrabi, D.; Anarkooli, I.J. Antioxidant and Neuroprotective Effects of Lycopene and Insulin in the Hippocampus of Streptozotocin-Induced Diabetic Rats. Biomed. Rep. 2019, 10, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Ihnat, M.A.; Thorpe, J.E. Clinical Review 2: The “Metabolic Memory”: Is More than Just Tight Glucose Control Necessary to Prevent Diabetic Complications? J. Clin. Endocrinol. Metab. 2009, 94, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Testa, R.; Bonfigli, A.R.; Prattichizzo, F.; La Sala, L.; De Nigris, V.; Ceriello, A. The “Metabolic Memory” Theory and the Early Treatment of Hyperglycemia in Prevention of Diabetic Complications. Nutrients 2017, 9, 437. [Google Scholar] [CrossRef]
- Gutierres, V.O.; Assis, R.P.; Arcaro, C.A.; Oliveira, J.O.; Lima, T.F.O.; Beretta, A.L.R.Z.; Costa, P.I.; Baviera, A.M.; Brunetti, I.L. Curcumin Improves the Effect of a Reduced Insulin Dose on Glycemic Control and Oxidative Stress in Streptozotocin-Diabetic Rats. Phytother. Res. 2019, 33, 976–988. [Google Scholar] [CrossRef]
- Figueiredo, I.D.; Lima, T.F.O.; Inácio, M.D.; Costa, M.C.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Lycopene Improves the Metformin Effects on Glycemic Control and Decreases Biomarkers of Glycoxidative Stress in Diabetic Rats. Diabetes Metab. Syndr. Obes. 2020, 13, 3117–3135. [Google Scholar] [CrossRef]
- Mounien, L.; Tourniaire, F.; Landrier, J.F. Anti-Obesity Effect of Carotenoids: Direct Impact on Adipose Tissue and Adipose Tissue-Driven Indirect Effects. Nutrients 2019, 11, 1562. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Karimi, G.; Iranshahi, M. Carotenoids in the treatment of diabetes mellitus and its complications: A mechanistic review. Biomed. Pharmacother. 2017, 91, 31–42. [Google Scholar] [CrossRef]
- Jin, Y.; Arroo, R. The protective effects of flavonoids and carotenoids against diabetic complications-A review of in vivo evidence. Front. Nutr. 2023, 10, 1020950. [Google Scholar] [CrossRef]
- Grabowska, M.; Wawrzyniak, D.; Rolle, K.; Chomczyński, P.; Oziewicz, S.; Jurga, S.; Barciszewski, J. Let Food Be Your Medicine: Nutraceutical Properties of Lycopene. Food Funct. 2019, 10, 3090–3102. [Google Scholar] [CrossRef]
- Przybylska, S. Lycopene—A Bioactive Carotenoid Offering Multiple Health Benefits: A Review. Int. J. Food Sci. Technol. 2020, 55, 11–32. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, F.; Meng, L.; Chen, D.; Wang, M.; Lu, X.; Chen, D.; Jiang, Y.; Xing, N. Lycopene Attenuates Chronic Prostatitis/Chronic Pelvic Pain Syndrome by Inhibiting Oxidative Stress and Inflammation via the Interaction of NF-ΚB, MAPKs, and Nrf2 Signaling Pathways in Rats. Andrology 2020, 8, 747–755. [Google Scholar] [CrossRef]
- Pierine, D.T.; Navarro, M.E.L.; Minatel, I.O.; Luvizotto, R.A.M.; Nascimento, A.F.; Ferreira, A.L.A.; Yeum, K.J.; Corrêa, C.R. Lycopene Supplementation Reduces TNF-α via RAGE in the Kidney of Obese Rats. Nutr. Diabetes 2014, 4, e142. [Google Scholar] [CrossRef]
- Assis, R.P.; Arcaro, C.A.; Gutierres, V.O.; Oliveira, J.O.; Costa, P.I.; Baviera, A.M.; Brunetti, I.L. Combined Effects of Curcumin and Lycopene or Bixin in Yoghurt on Inhibition of LDL Oxidation and Increases in HDL and Paraoxonase Levels in Streptozotocin-Diabetic Rats. Int. J. Mol. Sci. 2017, 18, 332. [Google Scholar] [CrossRef]
- Motta, B.P.; Pinheiro, C.G.; Figueiredo, I.D.; Cardoso, F.N.; Oliveira, J.O.; Machado, R.T.A.; da Silva, P.B.; Chorilli, M.; Brunetti, I.L.; Baviera, A.M. Combined Effects of Lycopene and Metformin on Decreasing Oxidative Stress by Triggering Endogenous Antioxidant Defenses in Diet-Induced Obese Mice. Molecules 2022, 27, 8503. [Google Scholar] [CrossRef]
- Kohn, H.I.; Liversedge, M. On a New Aerobic Metabolite Whose Production by Brain Is Inhibited by Apomorphine, Emetine, Ergotamine, Epinephrine, and Menadione. J. Pharmacol. Exp. Ther. 1944, 82, 92–300. [Google Scholar]
- Zilin, S.; Naifeng, L.; Bicheng, L.; Jiping, W. The Determination of AGE-Peptides by Flow Injection Assay, a Practical Marker of Diabetic Nephropathy. Clin. Chim. Acta 2001, 313, 69–75. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Kakani, S.; Yardeni, T.; Poling, J.; Ciccone, C.; Niethamer, T.; Klootwijk, E.D.; Manoli, I.; Darvish, D.; Hoogstraten-Miller, S.; Zerfas, P.; et al. The Gne M712T Mouse as a Model for Human Glomerulopathy. Am. J. Pathol. 2012, 180, 1431–1440. [Google Scholar] [CrossRef]
- Inacio, M.D.; Costa, M.C.; Lima, T.F.O.; Figueiredo, I.D.; Motta, B.P.; Spolidorio, L.C.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Pentoxifylline Mitigates Renal Glycoxidative Stress in Obese Mice by Inhibiting AGE/RAGE Signaling and Increasing Glyoxalase Levels. Life Sci. 2020, 258, 118196. [Google Scholar] [CrossRef]
- Watanabe, N.; Kamei, S.; Ohkubo, A.; Yamanaka, M.; Ohsawa, S.; Makino, K.; Tokuda, K. Urinary Protein as Measured with a Pyrogallol Red-Molybdate Complex, Manually and in a Hitachi 726 Automated Analyzer. Clin. Chem. 1986, 32, 1551–1554. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A Spectrophotometric Method for Measuring the Breakdown of Hydrogen Peroxide by Catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Rush, J.W.E.; Sandiford, S.D. Plasma Glutathione Peroxidase in Healthy Young Adults: Influence of Gender and Physical Activity. Clin. Biochem. 2003, 36, 345–351. [Google Scholar] [CrossRef]
- Janež, A.; Guja, C.; Mitrakou, A.; Lalic, N.; Tankova, T.; Czupryniak, L.; Tabák, A.G.; Prazny, M.; Martinka, E.; Smircic-Duvnjak, L. Insulin Therapy in Adults with Type 1 Diabetes Mellitus: A Narrative Review. Diabetes Ther. 2020, 11, 387–409. [Google Scholar] [CrossRef]
- Jafari Anarkooli, I.; Barzegar Ganji, H.; Pourheidar, M. The Protective Effects of Insulin and Natural Honey against Hippocampal Cell Death in Streptozotocin-Induced Diabetic Rats. J. Diabetes Res. 2014, 2014, 491571. [Google Scholar] [CrossRef]
- Bashir, S.O. Concomitant Administration of Resveratrol and Insulin Protects against Diabetes Mellitus Type-1-Induced Renal Damage and Impaired Function via an Antioxidant-Mediated Mechanism and up-Regulation of Na+/K+-ATPase. Arch. Physiol. Biochem. 2019, 125, 104–113. [Google Scholar] [CrossRef]
- Ip, E.J.; Doroudgar, S.; Salehi, A.; Salehi, F.; Najmi, M. Diabulimia: A Risky Trend Among Adults with Type 1 Diabetes Mellitus. Endocr. Pract. 2023, 29, 849–854. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zeng, X.; Cheng, Y.; Tang, L.; Hong, D.; Yang, X. Lycopene Ameliorates Insulin Resistance and Increases Muscle Capillary Density in Aging via Activation of SIRT1. J. Nutr. Biochem. 2022, 99, 108862. [Google Scholar] [CrossRef]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; He, J.; Zheng, P.; Yu, J.; Luo, Y.; Yan, H.; Yu, B. Lycopene Promotes a Fast-to-Slow Fiber Type Transformation through Akt/FoxO1 Signaling Pathway and MiR-22-3p. J. Funct. Foods 2021, 80, 104430. [Google Scholar] [CrossRef]
- Otocka-Kmiecik, A. Effect of Carotenoids on Paraoxonase-1 Activity and Gene Expression. Nutrients 2022, 14, 2842. [Google Scholar] [CrossRef]
- Rosenblat, M.; Aviram, M. Paraoxonases Role in the Prevention of Cardiovascular Diseases. BioFactors 2009, 35, 98–104. [Google Scholar] [CrossRef]
- Hedayati, N.; Oskouei, Z.; Tabeshpour, J.; Naeini, M.B. Berberine and Lycopene as Alternative or Add-on Therapy to Metformin and Statins, a Review. Eur. J. Pharmacol. 2021, 913, 174590. [Google Scholar] [CrossRef]
- Ried, K.; Fakler, P. Protective effect of lycopene on serum cholesterol and blood pressure: Meta-analyses of intervention trials. Maturitas 2011, 68, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Yoshida, K.; Sasaki, E.; Aizawa, K.; Kamioka, H. Effects of lycopene intake on HDL-cholesterol and triglyceride levels: A systematic review with meta-analysis. J. Food Sci. 2021, 86, 3285–3302. [Google Scholar] [CrossRef] [PubMed]
- Leh, H.E.; Lee, L.K. Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus. Molecules 2022, 27, 2335. [Google Scholar] [CrossRef]
- Tabrez, S.; Al-Shali, K.Z.; Ahmad, S. Lycopene powers the inhibition of glycation-induced diabetic nephropathy: A novel approach to halt the AGE-RAGE axis menace. Biofactors 2015, 41, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Szaleczky, E.; Prechl, J.; Fehér, J.; Somogyi, A. Alterations in enzymatic antioxidant defence in diabetes mellitus—A rational approach. Postgrad. Med. J. 1999, 75, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Li, B.; Qiang, Y.; Yuan, T.; Tan, X.; Wang, Z.; Liu, Z.; Liu, X. Lycopene Attenuates Western-Diet-Induced Cognitive Deficits via Improving Glycolipid Metabolism Dysfunction and Inflammatory Responses in Gut–Liver–Brain Axis. Int. J. Obes. 2018, 43, 1735–1746. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Ma, J.; Xv, Q.; Gao, H.; Yin, H.; Yan, G.; Jiang, X.; Yu, W. Lycopene attenuates the inflammation and apoptosis in aristolochic acid nephropathy by targeting the Nrf2 antioxidant system. Redox Biol. 2022, 57, 102494. [Google Scholar] [CrossRef]
- Albrahim, T.; Robert, A.A. Lycopene Effects on Metabolic Syndrome and Kidney Injury in Rats Fed a High-Fat Diet: An Experimental Study. ACS Omega 2022, 7, 30930–30938. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.J.; Stehouwer, C.D.A.; Schalkwijk, C.G. Methylglyoxal Stress, the Glyoxalase System, and Diabetic Chronic Kidney Disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 26–33. [Google Scholar] [CrossRef]
- Zhuang, A.; Forbes, J.M. Diabetic Kidney Disease: A Role for Advanced Glycation End-Product Receptor 1 (AGE-R1)? Glycoconj. J. 2016, 33, 645–652. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Glyoxalase in Diabetes, Obesity and Related Disorders. Semin. Cell Dev. Biol. 2011, 22, 309–317. [Google Scholar] [CrossRef]
- He, C.J.; Zheng, F.; Stitt, A.; Striker, L.; Hattori, M.; Vlassara, H. Differential expression of renal AGE-receptor genes in NOD mice: Possible role in nonobese diabetic renal disease. Kidney Int. 2000, 58, 1931–1940. [Google Scholar] [CrossRef]
- Chaparro, R.J.; Konigshofer, Y.; Beilhack, G.F.; Shizuru, J.A.; McDevitt, H.O.; Chien, Y.H. Nonobese diabetic mice express aspects of both type 1 and type 2 diabetes. Proc. Natl. Acad. Sci. USA 2006, 103, 12475–12480. [Google Scholar] [CrossRef]
- Dehnad, A.; Fan, W.; Jiang, J.X.; Fish, S.R.; Li, Y.; Das, S.; Mozes, G.; Wong, K.A.; Olson, K.A.; Charville, G.W.; et al. AGER1 downregulation associates with fibrosis in nonalcoholic steatohepatitis and type 2 diabetes. J. Clin. Investig. 2020, 130, 4320–4330. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Advanced Glycation End Products in the Pathogenesis of Chronic Kidney Disease. Kidney Int. 2018, 93, 803–813. [Google Scholar] [CrossRef]
- He, Y.; Zhou, C.; Huang, M.; Tang, C.; Liu, X.; Yue, Y.; Diao, Q.; Zheng, Z.; Liu, D. Glyoxalase System: A Systematic Review of Its Biological Activity, Related-Diseases, Screening Methods and Small Molecule Regulators. Biomed. Pharmacother. 2020, 131, 110663. [Google Scholar] [CrossRef]



| Physiological Parameters | NYOG | DYOG | DI4U | DI1U | DLYC | DI1U + LYC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 30 | Day 0 | Day 30 | Day 0 | Day 30 | Day 0 | Day 30 | Day 0 | Day 30 | Day 0 | Day 30 | |
| Body weight (g) | 181 ± 1.6 | 328 ± 7.6 (#) | 180 ± 4.2 | 246 ± 7.8 (a,#) | 173 ± 2.8 | 321 ± 6.8 (b,#) | 166 ± 4.7 | 280 ± 17.8 (a,c,#) | 172 ± 4.2 | 254 ± 10.5 (a,c,#) | 175 ± 3.4 | 319 ± 15.5 (b,d,e,#) |
| Body weight gain (g) | 163 ± 18.5 | 68 ± 8.5 (a) | 148 ± 6.8 (b) | 112 ± 17.3 (a,b,c) | 80 ± 10.1 (a,c) | 142 ± 13.7 (b,e) | ||||||
| Food intake (g/24 h) | 21 ± 0.1 | 23 ± 1.4 | 31 ± 3.1 (a) | 44 ± 1.2 (a,#) | 29 ± 2.3 (a) | 22 ± 1.7 (b,#) | 31 ± 5.2 (a) | 31 ± 2.1 (a,b,c) | 31 ± 1.5 (a) | 31 ± 2.1 (a,b,c) | 30 ± 2.7 (a) | 26 ± 4.2 (b,d,e) |
| Water intake (mL/24 h) | 37 ± 2.1 | 40 ± 3.0 | 136 ± 19.0 (a) | 197 ± 1.1 (a,#) | 139 ± 21.4 (a) | 54 ± 7.0 (b,#) | 131 ± 23.0 (a) | 113 ± 11.5 (a,b,c) | 138 ± 17.1 (a) | 136 ± 11.6 (a,b,c) | 136 ± 13.7 (a) | 111 ± 27.1 (a,b,c) |
| Urinary volume (mL/24 h) | 5 ± 0.1 | 6 ± 1.2 | 80 ± 14.7 (a) | 146 ± 10.2 (a,#) | 74 ± 15.0 (a) | 16 ± 6.6 (b,#) | 71 ± 14.4 (a) | 71 ± 12.1 (a,b,c) | 90 ± 13.4 (a) | 81 ± 15.1 (a,b,c) | 72 ± 15.1 | 74 ± 23.7 (a,b,c) |
| Tissues | NYOG | DYOG | DI4U | DI1U | DLYC | DI1U + LYC |
|---|---|---|---|---|---|---|
| Gastrocnemius muscle (g/100 g b.w) | 1.59 ± 0.05 | 0.98 ± 0.16 (a) | 1.57 ± 0.05 (b) | 1.36 ± 0.19 (b) | 1.10 ± 0.12 (a,c) | 1.46 ± 0.16 (b,e) |
| Epididymal adipose tissue (g/100 g b.w) | 4.67 ± 0.67 | 1.60 ± 0.32 (a) | 4.22 ± 0.38 (b) | 2.97 ± 0.46 (a,b,c) | 1.88 ± 0.17 (a,c,d) | 3.64 ± 0.62 (b,e) |
| Retroperitoneal adipose tissue (g/100 g b.w) | 5.92 ± 0.74 | 1.02 ± 0.26 (a) | 4.79 ± 0.66 (b) | 2.93 ± 0.60 (a,b,c) | 1.22 ± 0.12 (a,c,d) | 3.63 ± 0.87 (a,b,e) |
| Kidneys (g/100 g b.w) | 1.37 ± 0.05 | 1.62 ± 0.14 (a) | 1.42 ± 0.06 (b) | 1.50 ± 0.14 | 1.49 ± 0.11 | 1.44 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, I.D.; Lima, T.F.O.; Carlstrom, P.F.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Lycopene in Combination with Insulin Triggers Antioxidant Defenses and Increases the Expression of Components That Detoxify Advanced Glycation Products in Kidneys of Diabetic Rats. Nutrients 2024, 16, 1580. https://doi.org/10.3390/nu16111580
Figueiredo ID, Lima TFO, Carlstrom PF, Assis RP, Brunetti IL, Baviera AM. Lycopene in Combination with Insulin Triggers Antioxidant Defenses and Increases the Expression of Components That Detoxify Advanced Glycation Products in Kidneys of Diabetic Rats. Nutrients. 2024; 16(11):1580. https://doi.org/10.3390/nu16111580
Chicago/Turabian StyleFigueiredo, Ingrid Delbone, Tayra Ferreira Oliveira Lima, Paulo Fernando Carlstrom, Renata Pires Assis, Iguatemy Lourenço Brunetti, and Amanda Martins Baviera. 2024. "Lycopene in Combination with Insulin Triggers Antioxidant Defenses and Increases the Expression of Components That Detoxify Advanced Glycation Products in Kidneys of Diabetic Rats" Nutrients 16, no. 11: 1580. https://doi.org/10.3390/nu16111580
APA StyleFigueiredo, I. D., Lima, T. F. O., Carlstrom, P. F., Assis, R. P., Brunetti, I. L., & Baviera, A. M. (2024). Lycopene in Combination with Insulin Triggers Antioxidant Defenses and Increases the Expression of Components That Detoxify Advanced Glycation Products in Kidneys of Diabetic Rats. Nutrients, 16(11), 1580. https://doi.org/10.3390/nu16111580






