Physiology of Vitamin D—Focusing on Disease Prevention
Abstract
- Definitions of vitamin D that are used in this review:
- (a).
- Hypovitaminosis D = Insufficient 25(OH)D levels
- (b).
- Severe vitamin D deficiency
- (c).
- Vitamin D sufficiency:
1. Introduction
1.1. The Rationale for This Study
1.2. Importance of Vitamin D for Human Health
1.3. Clinical Signs and Symptoms of Vitamin D Deficiency
1.4. Current Recommendations and Vitamin D Status
1.5. Vitamin D Dose Recommendations
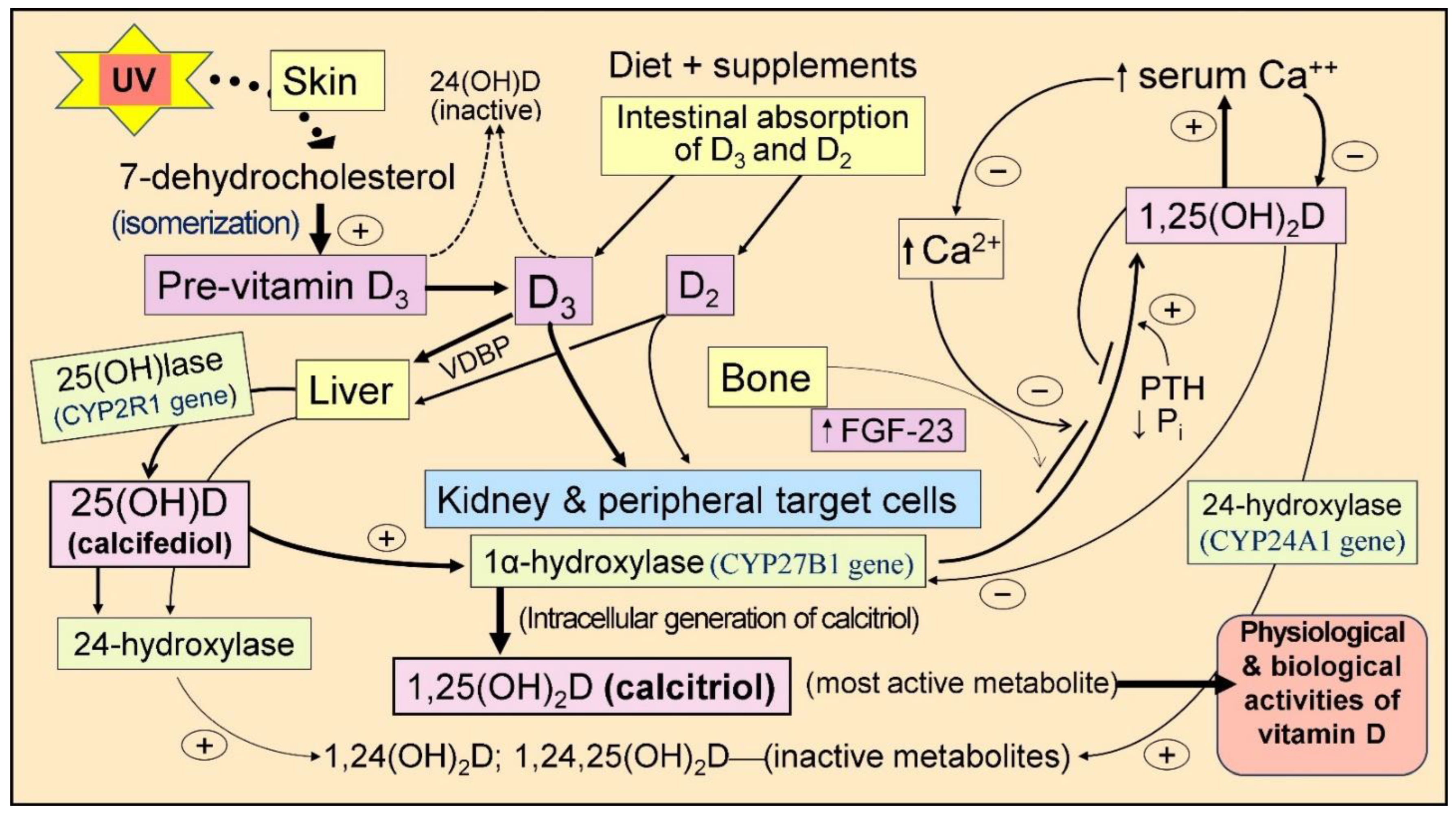
2. Generation of Vitamin D
2.1. Synthesis of Vitamin D
2.2. Vitamin D Supplementation and Its Benefits
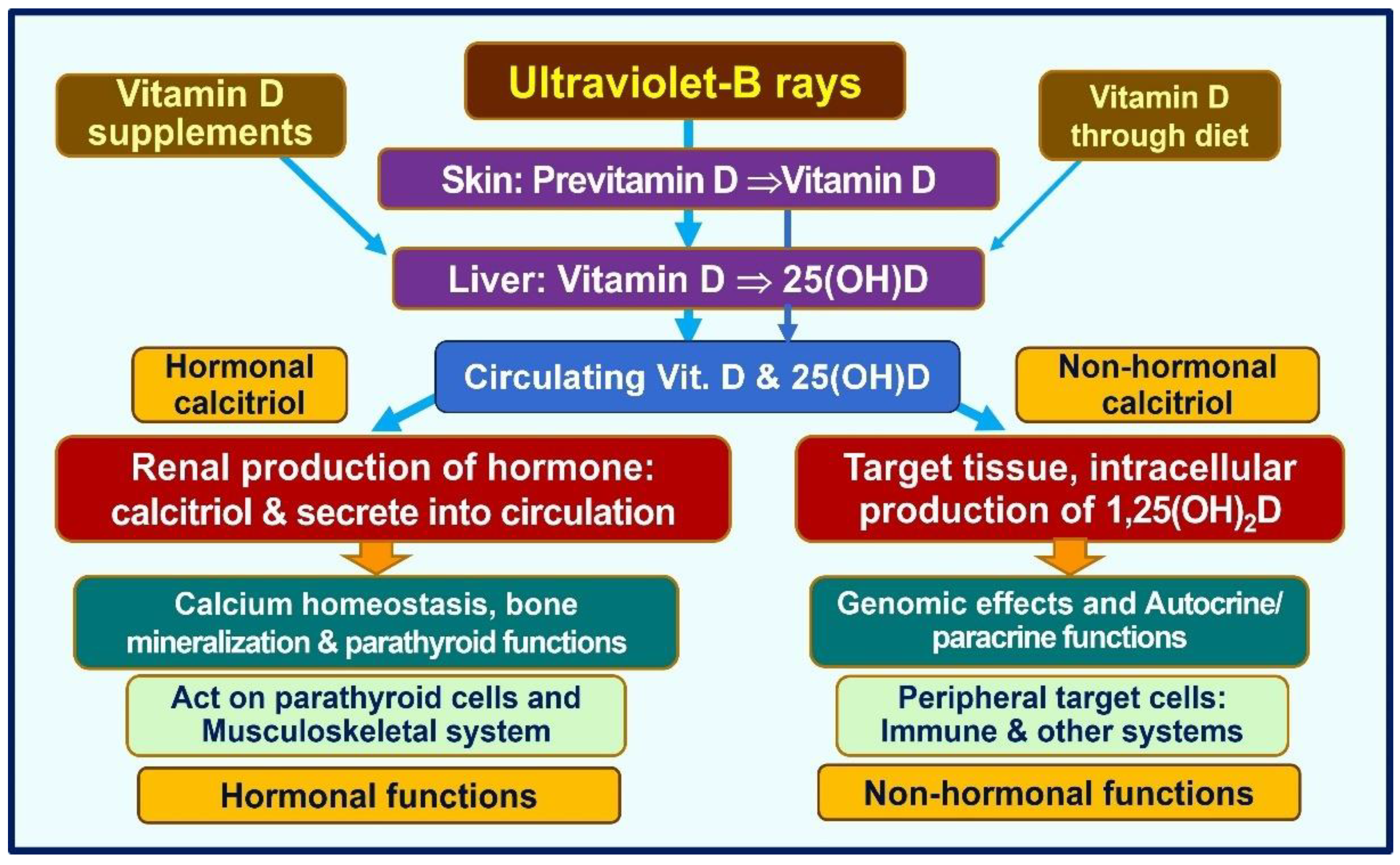
3. Physiology of Vitamin D
3.1. Non-Classical Actions of Calcitriol
3.2. Activation of Vitamin D/CTR
3.3. Genetic Influences on Vitamin D/CTR
3.4. Vitamin D, 25(OH)D, and 1,25(OH)2D
3.5. Intracellular Synthesis of Vitamin D and Binding to CTR
3.6. Binding of Calcitriol to Its Receptors (CTR)
3.7. Vitamin D Is a Crucial Regulator of Calcium Homeostasis
3.8. Maintenace of Calcium Homeostasis
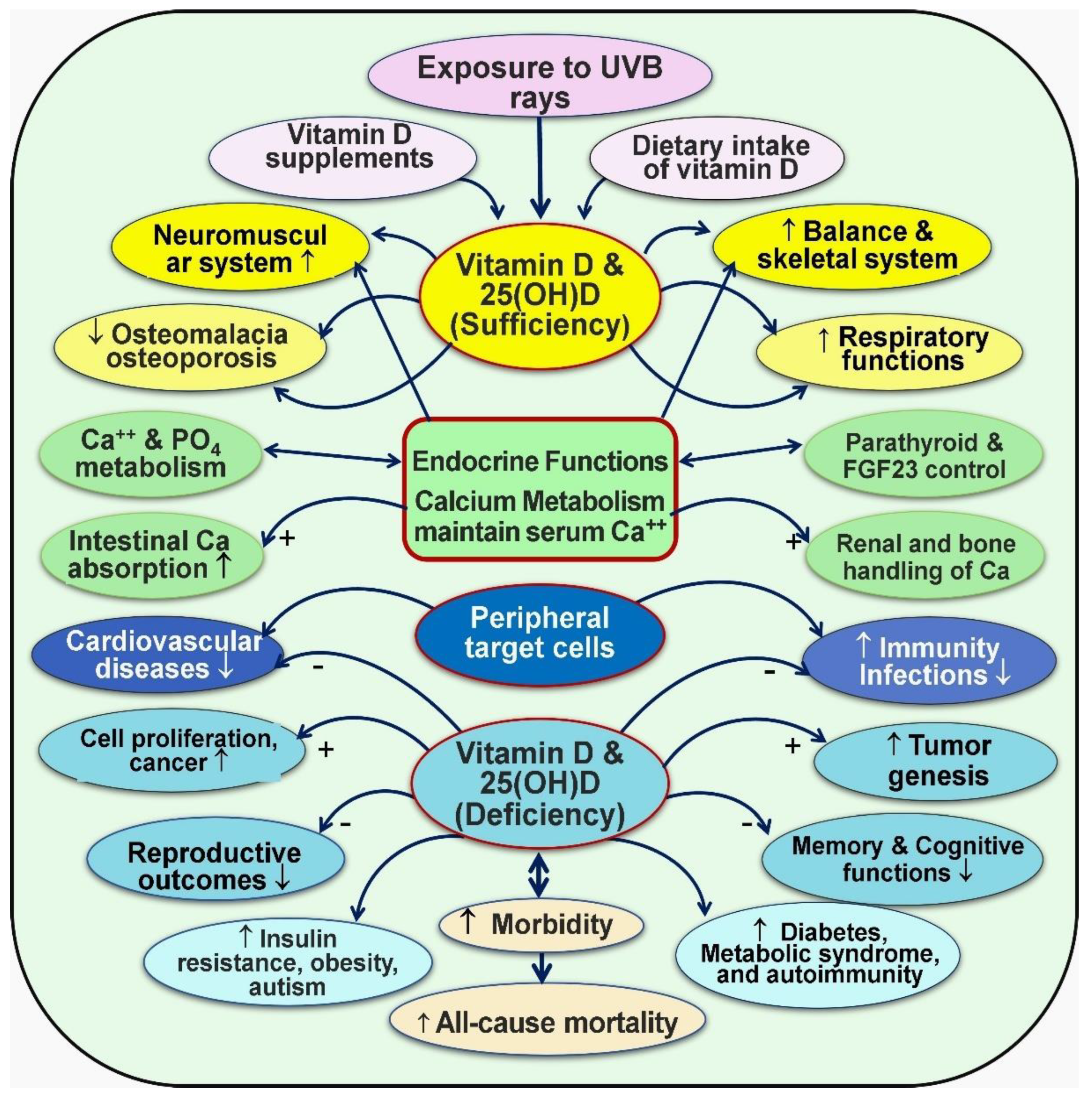
4. Key Physiological Functions of Vitamin D
4.1. Tissue-Specific Regulation of CYP27B1
4.2. Tissue-Specific Thresholds of Vitamin D
4.3. Other Beneficial Effects of Vitamin D
4.4. Clinical Consequences of CTR, CYP27B1, and CYP2R1 Mutations
4.5. Health Economics of Vitamin D—Costs to Maintain Physiological Serum 25(OH)D Concentrations
4.6. Repeated Supra-Pharmacologic Doses Are Unphysiological and Can Be Harmful
4.7. Common Adverse Effects Following an Overdose of Vitamin D
4.8. Sustained Serum 25(OH)D Concentrations Are Necessary for Optimum Outcomes
4.9. Example Conditions Requiring Higher Serum 25(OH)D Concentrations
4.10. How Much Vitamin D Intake Is Necessary?
4.11. Doses of Vitamin D Needed to Boost Serum 25(OH)D Concentration
4.11.1. Vitamin D Requirements for Chronic Conditions
4.11.2. Rapidly Increasing Serum 25(OH)D to Boost the Immune System in a Day
5. Discussion
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1,25(OH)2D | 1,25-dihydroxyvitamin D |
| 25(OH)D | 25-hydroxy vitamin D |
| 7-DHC | 7-dehydrocholesterol |
| CTR | Calcitriol receptor (synonymous with vitamin D receptor; VDR) |
| MR | Mendelian randomization |
| PTH | Parathyroid hormone |
| RAS | Renin–angiotensin system |
| RCT | Randomized controlled clinical trial |
| RDA | Recommended dietary allowance |
| RXR | Retinoid X receptor |
| UVB | Ultraviolet B |
References
- Carlberg, C.; Velleuer, E. Vitamin D and aging: Central role of immunocompetence. Nutrients 2024, 16, 398. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. Why “Vitamin D” is not a hormone, and not a synonym for 1,25-dihydroxy-vitamin D, its analogs or deltanoids. J. Steroid Biochem. Mol. Biol. 2004, 89-90, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.R.; Thacher, T.D. Vitamin D: Immune function, inflammation, infections and auto-immunity. Paediatr. Int. Child. Health 2023, 43, 29–39. [Google Scholar] [CrossRef]
- Middelkoop, K.; Stewart, J.; Walker, N.; Delport, C.; Jolliffe, D.A.; Coussens, A.K.; Nuttall, J.; Tang, J.C.Y.; Fraser, W.D.; Griffiths, C.J.; et al. Vitamin D supplementation to prevent tuberculosis infection in South African schoolchildren: Multicenter phase 3 double-blind randomized placebo-controlled trial (ViDiKids). Int. J. Infect. Dis. 2023, 134, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ganmaa, D.; Enkhmaa, D.; Nasantogtokh, E.; Sukhbaatar, S.; Tumur-Ochir, K.E.; Manson, J.E. Vitamin D, respiratory infections, and chronic disease: Review of meta-analyses and randomized clinical trials. J. Intern. Med. 2022, 291, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Physiological basis for using vitamin D to improve health. Biomedicines 2023, 11, 1542. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Controlling chronic diseases and acute infections with vitamin D sufficiency. Nutrients 2023, 15, 3623. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Tomaschitz, A.; Marz, W.; Drechsler, C.; Ritz, E.; Zittermann, A.; Cavalier, E.; Pieber, T.R.; Lappe, J.M.; Grant, W.B.; et al. Vitamin D, cardiovascular disease and mortality. Clin. Endocrinol. 2011, 75, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Duan, J.W.; Lu, D.H.; Zhang, F.; Liu, H.L. Association between vitamin D status and cardiometabolic risk factors in adults with type 2 diabetes in Shenzhen, China. Front. Endocrinol. 2024, 15, 1346605. [Google Scholar] [CrossRef]
- Cheema, H.A.; Fatima, M.; Shahid, A.; Bouaddi, O.; Elgenidy, A.; Rehman, A.U.; Oussama Kacimi, S.E.; Hasan, M.M.; Lee, K.Y. Vitamin D supplementation for the prevention of total cancer incidence and mortality: An updated systematic review and meta-analysis. Heliyon 2022, 8, e11290. [Google Scholar] [CrossRef]
- Akdere, G.; Efe, B.; Sisman, P.; Yorulmaz, G. The relationship between vitamin D level and organ-specific autoimmune disorders in newly diagnosed type I diabetes mellitus. Bratisl. Lek. Listy 2018, 119, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Sirbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D and cardiovascular diseases: Causality. J. Steroid Biochem. Mol. Biol. 2018, 175, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Wimalawansa, S.J.; Pludowski, P.; Anouti, F.A. Clinical practice guidelines for vitamin D in the United Arab Emirates. J. Steroid Biochem. Mol. Biol. 2018, 175, 4–11. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Infections and autoimmunity-The immune system and vitamin D: A Systematic Review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Makin, G.; Lohnes, D.; Byford, V.; Ray, R.; Jones, G. Target cell metabolism of 1,25-dihydroxyvitamin D3 to calcitroic acid. Evidence for a pathway in kidney and bone involving 24-oxidation. Biochem. J. 1989, 262, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Wimalawansa, S.J.; Carlberg, C. Highlights from the 5th International Conference on Vitamin D Deficiency, Nutrition and Human Health, Abu Dhabi, United Arab Emirates, March 24-25, 2016. J. Steroid Biochem. Mol. Biol. 2018, 175, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Non-musculoskeletal benefits of vitamin D. J. Steroid Biochem. Mol. Biol. 2018, 175, 60–81. [Google Scholar] [CrossRef]
- van Schoor, N.M.; Lips, P. Worldwide vitamin D status. Best. Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Eggemoen, A.R.; Knutsen, K.V.; Dalen, I.; Jenum, A.K. Vitamin D status in recently arrived immigrants from Africa and Asia: A cross-sectional study from Norway of children, adolescents and adults. BMJ Open 2013, 3, e003293. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Gorham, E.D.; Mohr, S.B.; Garland, F.C. Vitamin D for cancer prevention: Global perspective. Ann. Epidemiol. 2009, 19, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014, 111, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Investig. 2006, 116, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.R.; Spector, S.A. Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J. Biol. Chem. 2011, 286, 18890–18902. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, D.; Quinkler, M.; Eardley, K.S.; Bland, R.; Lepenies, J.; Hughes, S.V.; Raymond, N.T.; Howie, A.J.; Cockwell, P.; Stewart, P.M.; et al. Reduction of the vitamin D hormonal system in kidney disease is associated with increased renal inflammation. Kidney Int. 2008, 74, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Krane, S.; Avioli, L.V. Disorders of calcification: Osteomalacia and rickets. In Endocrinology, 3rd ed.; DeGroot, L.J., Besser, M., Burger, H.G., Jameson, J.L., Loriaux, D.L., Marshall, J.C., Eds.; WB Saunders: Philadelphia, PA, USA, 1995; pp. 1204–1227. [Google Scholar]
- Banajeh, S.M. Nutritional rickets and vitamin D deficiency--association with the outcomes of childhood very severe pneumonia: A prospective cohort study. Pediatr. Pulmonol. 2009, 44, 1207–1215. [Google Scholar] [CrossRef]
- Weisman, Y. Vitamin D deficiency rickets and osteomalacia in Israel. Isr. Med. Assoc. J. 2003, 5, 289–290. [Google Scholar]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Willett, W.C.; Staehelin, H.B.; Bazemore, M.G.; Zee, R.Y.; Wong, J.B. Effect of Vitamin D on falls: A meta-analysis. JAMA 2004, 291, 1999–2006. [Google Scholar] [CrossRef]
- Hahn, T.J. Drug-induced disorders of vitamin D and mineral metabolism. Clin. Endocrinol. Metab. 1980, 9, 107–127. [Google Scholar] [CrossRef] [PubMed]
- Gloth, F.M., 3rd; Greenough, W.B., 3rd. Vitamin D deficiency as a contributor to multiple forms of chronic pain. Mayo Clin. Proc. 2004, 79, 696, 699; author reply 699. [Google Scholar] [PubMed]
- Gloth, F.M., 3rd; Lindsay, J.M.; Zelesnick, L.B.; Greenough, W.B., 3rd. Can vitamin D deficiency produce an unusual pain syndrome? Arch. Intern. Med. 1991, 151, 1662–1664. [Google Scholar] [CrossRef]
- Chapuy, M.C.; Arlot, M.E.; Duboeuf, F.; Brun, J.; Crouzet, B.; Arnaud, S.; Delmas, P.D.; Meunier, P.J. Vitamin D3 and calcium to prevent hip fractures in elderly women. N. Engl. J. Med. 1992, 327, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Sahay, M.; Sahay, R. Rickets-vitamin D deficiency and dependency. Indian J. Endocrinol. Metab. 2012, 16, 164–176. [Google Scholar] [CrossRef]
- Holick, M.F. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Tu, C.L.; Menendez, A.; Nguyen, T.; Bikle, D.D. Vitamin D and calcium regulation of epidermal wound healing. J. Steroid Biochem. Mol. Biol. 2016, 164, 379–385. [Google Scholar] [CrossRef]
- Lee, S.; Kim, B.J.; Lee, C.H.; Lee, W.S. Increased prevalence of vitamin D deficiency in patients with alopecia areata: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, R.; Anwar, M.I. Vitamin D deficiency in alopecia areata. J. Coll. Physicians Surg. Pak. JCPSP 2017, 27, 200–202. [Google Scholar]
- Ceglia, L.; Harris, S.S. Vitamin D and its role in skeletal muscle. Calcif. Tissue Int. 2013, 92, 151–162. [Google Scholar] [CrossRef]
- Bollen, S.E.; Bass, J.J.; Wilkinson, D.J.; Hewison, M.; Atherton, P.J. The impact of genetic variation within the vitamin D pathway upon skeletal muscle function: A systematic review. J. Steroid Biochem. Mol. Biol. 2023, 229, 106266. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.D.; Hogler, W.; Craig, M.E.; Verge, C.F.; Walker, J.L.; Piper, A.C.; Woodhead, H.J.; Cowell, C.T.; Ambler, G.R. The re-emerging burden of rickets: A decade of experience from Sydney. Arch. Dis. Child. 2006, 91, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Gogulothu, R.; Nagar, D.; Gopalakrishnan, S.; Garlapati, V.R.; Kallamadi, P.R.; Ismail, A. Disrupted expression of genes essential for skeletal muscle fibre integrity and energy metabolism in vitamin D deficient rats. J. Steroid Biochem. Mol. Biol. 2020, 197, 105525. [Google Scholar] [CrossRef]
- Najada, A.S.; Habashneh, M.S.; Khader, M. The frequency of nutritional rickets among hospitalized infants and its relation to respiratory diseases. J. Trop. Pediatr. 2004, 50, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Sahay, T.; Ananthakrishnan, A.N. Vitamin D deficiency is associated with community-acquired clostridium difficile infection: A case-control study. BMC Infect. Dis. 2014, 14, 661. [Google Scholar] [CrossRef]
- Zasloff, M. Fighting infections with vitamin D. Nat. Med. 2006, 12, 388–390. [Google Scholar] [CrossRef]
- Zanetti, M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 2004, 75, 39–48. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Rapidly increasing serum 25(OH)D boosts the immune system, against infections-sepsis and COVID-19. Nutrients 2022, 14, 2997. [Google Scholar] [CrossRef]
- Polonowita, A.; Wimalawansa, S.J. Impact of Withholding Cost-Effective Early Treatments, such as vitamin D, on COVID-19: An analysis using an innovative logical paradigm. World J. Adanced Pharma. Life Sci. 2023, 5, 13–34. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Biology of vitamin D. J. Steroids Horm. Sci. 2019, 10, 1–8. [Google Scholar]
- Sunil, J.W. Commonsense approaches to minimizing risks from COVID-19. Open J. Pulmonol. Respir. Med. 2020, 2, 28–37. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Catov, J.M.; Simhan, H.N.; Holick, M.F.; Powers, R.W.; Roberts, J.M. Maternal vitamin D deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab. 2007, 92, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Heaney, R.P.; Holick, M.F.; Lips, P.; Meunier, P.J.; Vieth, R. Estimates of optimal vitamin D status. Osteoporos. Int. 2005, 16, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S. Overcoming infections including COVID-19, by maintaining circulating 25(OH)D concentrations above 50 ng/mL. Pathol. Lab. Med. Int. 2022, 14, 37–60. [Google Scholar] [CrossRef]
- Grant, W.B. An estimate of the global reduction in mortality rates through doubling vitamin D levels. Eur. J. Clin. Nutr. 2011, 65, 1016–1026. [Google Scholar] [CrossRef]
- Shen, Y. Role of nutritional vitamin D in chronic kidney disease-mineral and bone disorder: A narrative review. Medicine 2023, 102, e33477. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Andreoli, L.; Mele, C.; Sainaghi, P.P.; Rigamonti, C.; Piantoni, S.; De Benedittis, C.; Aimaretti, G.; Pirisi, M.; Marzullo, P. Pathophysiological role and therapeutic implications of vitamin D in autoimmunity: Focus on chronic autoimmune diseases. Nutrients 2020, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Alonso, P.; Boughanem, H.; Canudas, S.; Becerra-Tomas, N.; Fernandez de la Puente, M.; Babio, N.; Macias-Gonzalez, M.; Salas-Salvado, J. Circulating vitamin D levels and colorectal cancer risk: A meta-analysis and systematic review of case-control and prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Chen, Y.H.; Liang, F.W.; Wu, Y.C.; Wang, J.J.; Lim, S.W.; Ho, C.H. Determinants of cancer incidence and mortality among people with vitamin D deficiency: An epidemiology study using a real-world population database. Front. Nutr. 2023, 10, 1294066. [Google Scholar] [CrossRef]
- Lawler, T.; Warren Andersen, S. Serum 25-hydroxyvitamin D and cancer risk: A systematic review of mendelian randomization studies. Nutrients 2023, 15, 422. [Google Scholar] [CrossRef]
- Gallo, D.; Baci, D.; Kustrimovic, N.; Lanzo, N.; Patera, B.; Tanda, M.L.; Piantanida, E.; Mortara, L. How does vitamin D affect immune cells crosstalk in autoimmune diseases? Int. J. Mol. Sci. 2023, 24, 4689. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Prophylactic use of vitamin D to maintain a robust immune system against infections like SARS-CoV-2. Glob. J. Endocrinol. Metab. GJEM 2023, 3, 1–9. [Google Scholar]
- Aji, A.S.; Yerizel, E.; Desmawati, D.; Lipoeto, N.I. Low maternal vitamin D and calcium food intake during pregnancy associated with place of residence: A coss-sectional study in West Sumatran women, Indonesia. Open Access Maced. J. Med. Sci. 2019, 7, 2879–2885. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; O’Dea, R. Exploration of strategic food vehicles for vitamin D fortification in low/lower-middle income countries. J. Steroid Biochem. Mol. Biol. 2019, 195, 105479. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.; Lewiecki, E.M. Vitamin D and common sense. J. Clin. Densitom. 2011, 14, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J.; Razzaque, M.S.; Al-Daghri, N.M. Calcium and vitamin D in human health: Hype or real? J. Steroid Biochem. Mol. Biol. 2018, 180, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Giovannucci, E.; Willett, W.C.; Dietrich, T.; Dawson-Hughes, B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am. J. Clin. Nutr. 2006, 84, 18–28. [Google Scholar] [CrossRef]
- Wimalawansa, S. Rational food fortification programs to alleviate micronutrient deficiencies. J. Food Process. Technol. 2013, 4, 257–267. [Google Scholar] [CrossRef]
- Grant, W.B.; Holick, M.F.; Wimalawansa, S.J. Vitamin D supplements and reasonable solar UVB should be recommended to prevent escalating incidence of chronic diseases. BMJ 2015, 350, h321. [Google Scholar]
- Ekwaru, J.P.; Zwicker, J.D.; Holick, M.F.; Giovannucci, E.; Veugelers, P.J. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS ONE 2014, 9, e111265. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Lappe, J.M.; Heaney, R.P. Serum 25-Hydroxyvitamin D Concentrations >/=40 ng/ml Are Associated with >65% Lower Cancer Risk: Pooled Analysis of Randomized Trial and Prospective Cohort Study. PLoS ONE 2016, 11, e0152441. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.L.; Baggerly, K.A.; Baggerly, C.A.; Aliano, J.L.; French, C.B.; Baggerly, L.L.; Ebeling, M.D.; Rittenberg, C.S.; Goodier, C.G.; Mateus Nino, J.F.; et al. Maternal 25(OH)D concentrations >/=40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS ONE 2017, 12, e0180483. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Baggerly, C.A.; Cuomo, R.E.; French, C.B.; Garland, C.F.; Gorham, E.D.; Grant, W.B.; Heaney, R.P.; Holick, M.F.; Hollis, B.W.; McDonnell, S.L.; et al. Sunlight and vitamin D: Necessary for public health. J. Am. Coll. Nutr. 2015, 34, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Song, A.; Jin, Y.; Xia, Q.; Song, G.; Xing, X. A dose-response meta-analysis between serum concentration of 25-hydroxy vitamin D and risk of type 1 diabetes mellitus. Eur. J. Clin. Nutr. 2021, 75, 1010–1023. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J. Clin. Endocrinol. Metab. 2012, 97, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Al Anouti, F.; Boucher, B.J.; Fakhoury, H.M.A.; Moukayed, M.; Pilz, S.; Al-Daghri, N.M. Evidence That Increasing Serum 25(OH)D Concentrations to 30 ng/mL in the Kingdom of Saudi Arabia and the United Arab Emirates Could Greatly Improve Health Outcomes. Biomedicines 2023, 11, 994. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, Z.; Sahraian, M.A.; Ebrahimi, M.; Pazoki, M.; Kafan, S.; Tabriz, H.M.; Hadadi, A.; Montazeri, M.; Nasiri, M.; Shirvani, A.; et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS ONE 2020, 15, e0239799. [Google Scholar] [CrossRef]
- Rosen, C.J.; Gallagher, J.C. The 2011 IOM report on vitamin D and calcium requirements for north America: Clinical implications for providers treating patients with low bone mineral density. J Clin Densitom 2011, 14, 79–84. [Google Scholar] [CrossRef]
- Malczewska-Lenczowska, J.; Surala, O.; Granda, D.; Szczepanska, B.; Czaplicki, A.; Kubacki, R. The relationship between bone health parameters, vitamin D and iron status, and dietary calcium intake in young males. Nutrients 2024, 16, 215. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D: Everything You Need to Know (Book); Karunaratne & Sons: Homagama, Sri Lanka, 2012; Volume 1, ISBN 978-955-9098-94-2. [Google Scholar]
- Vieth, R. Vitamin D supplementation: Cholecalciferol, calcifediol, and calcitriol. Eur. J. Clin. Nutr. 2020, 74, 1493–1497. [Google Scholar] [CrossRef]
- Holick, M.F. The cutaneous photosynthesis of previtamin D3: A unique photoendocrine system. J. Investig. Dermatol. 1981, 77, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Vieth, R.; Hollis, B.W. Vitamin D efficacy and safety. Arch. Intern. Med. 2011, 171, 266; author reply 267. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight, UV-radiation, vitamin D and skin cancer: How much sunlight do we need? Adv. Exp. Med. Biol. 2008, 624, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Derm. Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D in the new millennium. Curr. Osteoporo Res. 2012, 10, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Achieving population vitamin D sufficiency will markedly reduce healthcare costs. Eur. J. Biomed. Pharm. Sci. 2020, 7, 136–141. [Google Scholar]
- Wimalawansa, S.J. Maintaining optimum health requires longer-term stable vitamin D concentrations. Int. J. Regen. Med. 2020, 3, 1–5. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Polonowita, A. Boosting immunity with vitamin D for preventing complications and deaths from COVID-19. In Proceedings of the COVID 19: Impact, Mitigation, Opportunities and Building Resilience “From Adversity to Serendipity”, Perspectives of Global Relevance Based on Research, Experience and Successes in Combating COVID-19, Colombo, Sri Lanka, 27–28 January 2021; pp. 171–198. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- McCartney, D.M.; Byrne, D.G. Optimisation of vitamin D status for enhanced immuno-protection against COVID-19. Ir. Med. J. 2020, 113, 58. [Google Scholar] [PubMed]
- Zhou, Y.F.; Luo, B.A.; Qin, L.L. The association between vitamin D deficiency and community-acquired pneumonia: A meta-analysis of observational studies. Medicine 2019, 98, e17252. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, I.; Ushikoshi-Nakayama, R.; Yamazaki, T.; Matsumoto, N.; Saito, I. Pulmonary activation of vitamin D(3) and preventive effect against interstitial pneumonia. J. Clin. Biochem. Nutr. 2019, 65, 245–251. [Google Scholar] [CrossRef] [PubMed]
- FLCCC. Critical Treatment Protocols. Available online: https://covid19criticalcare.com/understanding-vitamin-d/ (accessed on 13 May 2023).
- c19early. Vitamin D for COVID-19. Available online: https://c19vitamind.com/ (accessed on 25 January 2023).
- Lapillonne, A. Vitamin D deficiency during pregnancy may impair maternal and fetal outcomes. Med. Hypotheses 2010, 74, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S. Part 3: Understanding the Forms of Vitamin D: Vitamin D3, 25(OH)D, and 1,25(OH)2D. Available online: https://grassrootshealth.org/blog/understanding-forms-vitamin-d-vitamin-d3-25ohd-125oh2d/ (accessed on 25 January 2023).
- Quraishi, S.A.; Bittner, E.A.; Blum, L.; Hutter, M.M.; Camargo, C.A., Jr. Association between preoperative 25-hydroxyvitamin D level and hospital-acquired infections following Roux-en-Y gastric bypass surgery. JAMA Surg. 2014, 149, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.A.; De Pascale, G.; Needleman, J.S.; Nakazawa, H.; Kaneki, M.; Bajwa, E.K.; Camargo, C.A., Jr.; Bhan, I. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: A randomized, placebo-controlled trial. Crit. Care Med. 2015, 43, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Borsche, L.; Glauner, B.; von Mendel, J. COVID-19 Mortality Risk Correlates Inversely with Vitamin D3 Status, and a Mortality Rate Close to Zero Could Theoretically Be Achieved at 50 ng/mL 25(OH)D3: Results of a Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3596. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Hewison, M.; Burke, F.; Evans, K.N.; Lammas, D.A.; Sansom, D.M.; Liu, P.; Modlin, R.L.; Adams, J.S. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J. Steroid Biochem. Mol. Biol. 2007, 103, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Chen, H.; Chun, R.; Ren, S.; Wu, S.; Gacad, M.; Nguyen, L.; Ride, J.; Liu, P.; Modlin, R.; et al. Substrate and enzyme trafficking as a means of regulating 1,25-dihydroxyvitamin D synthesis and action: The human innate immune response. J. Bone Miner. Res. 2007, 22 (Suppl. 2), V20–V24. [Google Scholar] [CrossRef]
- Kurylowicz, A.; Bednarczuk, T.; Nauman, J. The influence of vitamin D deficiency on cancers and autoimmune diseases development. Endokrynol. Pol. 2007, 58, 140–152. [Google Scholar] [PubMed]
- Hughes, D.A.; Norton, R. Vitamin D and respiratory health. Clin. Exp. Immunol. 2009, 158, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sriramula, S.; Xia, H.; Moreno-Walton, L.; Culicchia, F.; Domenig, O.; Poglitsch, M.; Lazartigues, E. Clinical Relevance and Role of Neuronal AT(1) Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. Circ. Res. 2017, 121, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Hanff, T.C.; Harhay, M.O.; Brown, T.S.; Cohen, J.B.; Mohareb, A.M. Is There an Association Between COVID-19 Mortality and the Renin-Angiotensin System? A Call for Epidemiologic Investigations. Clin. Infect. Dis. 2020, 71, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef] [PubMed]
- Kaparianos, A.; Argyropoulou, E. Local renin-angiotensin II systems, angiotensin-converting enzyme and its homologue ACE2: Their potential role in the pathogenesis of chronic obstructive pulmonary diseases, pulmonary hypertension and acute respiratory distress syndrome. Curr. Med. Chem. 2011, 18, 3506–3515. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Silwal, P.; Kim, I.; Modlin, R.L.; Jo, E.K. Vitamin D-cathelicidin axis: At the crossroads between protective immunity and pathological inflammation during Infection. Immune Netw. 2020, 20, e12. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S. Vitamin D as a defensin. J. Musculoskelet. Neuronal Interact. 2006, 6, 344–346. [Google Scholar]
- Mangin, M.; Sinha, R.; Fincher, K. Inflammation and vitamin D: The infection connection. Inflamm. Res. 2014, 63, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.S.; Dombrowski, Y.; Koglin, S.; Ruzicka, T.; Schauber, J. Impact of vitamin D3 on cutaneous immunity and antimicrobial peptide expression. Derm. Endocrinol. 2011, 3, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lu, R.; Zhang, Y.G.; Sun, J. Vitamin D receptor deletion Leads to the destruction of tight and adheren junctions in lungs. Tissue Barriers 2018, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Booth, D.R.; Ding, N.; Parnell, G.P.; Shahijanian, F.; Coulter, S.; Schibeci, S.D.; Atkins, A.R.; Stewart, G.J.; Evans, R.M.; Downes, M.; et al. Cistromic and genetic evidence that the vitamin D receptor mediates susceptibility to latitude-dependent autoimmune diseases. Genes Immun. 2016, 17, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Racz, A.; Barsony, J. Hormone-dependent translocation of vitamin D receptors is linked to transactivation. J. Biol. Chem. 1999, 274, 19352–19360. [Google Scholar] [CrossRef] [PubMed]
- Seuter, S.; Neme, A.; Carlberg, C. Epigenome-wide effects of vitamin D and their impact on the transcriptome of human monocytes involve CTCF. Nucleic Acids Res. 2016, 44, 4090–4104. [Google Scholar] [CrossRef]
- Karlgren, M.; Miura, S.; Ingelman-Sundberg, M. Novel extrahepatic cytochrome P450s. Toxicol. Appl. Pharmacol. 2005, 207, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J. Clin. Endocrinol. Metab. 2013, 98, 4619–4628. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.N.; Young, M.V.; Persons, K.S.; Wang, L.; Mathieu, J.S.; Whitlatch, L.W.; Holick, M.F.; Chen, T.C. Vitamin D metabolism in human prostate cells: Implications for prostate cancer chemoprevention by vitamin D. Anticancer Res. 2006, 26, 2567–2572. [Google Scholar]
- Zhu, J.; DeLuca, H.F. Vitamin D 25-hydroxylase—Four decades of searching, are we there yet? Arch. Biochem. Biophys. 2012, 523, 30–36. [Google Scholar] [CrossRef]
- Rahman, F.Z.; Hayee, B.; Chee, R.; Segal, A.W.; Smith, A.M. Impaired macrophage function following bacterial stimulation in chronic granulomatous disease. Immunology 2009, 128, 253–259. [Google Scholar] [CrossRef]
- Sunyecz, J.A. The use of calcium and vitamin D in the management of osteoporosis. Ther. Clin. Risk Manag. 2008, 4, 827–836. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Barrile, G.C.; Cavioni, A.; Mansueto, F.; Mazzola, G.; Oberto, L.; Patelli, Z.; Pirola, M.; Tartara, A.; et al. Nutrition, physical activity, and dietary supplementation to prevent bone mineral density loss: A food pyramid. Nutrients 2021, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Wang, M.; Miao, C.; Jin, D.; Wang, H. Mechanism of calcitriol regulating parathyroid cells in secondary hyperparathyroidism. Front. Pharmacol. 2022, 13, 1020858. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Kota, S.; Jammula, S.; Kota, S.; Meher, L.; Modi, K. Correlation of vitamin D, bone mineral density and parathyroid hormone levels in adults with low bone density. Indian J. Orthop. 2013, 47, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Seely, E.W.; Wood, R.J.; Brown, E.M.; Graves, S.W. Lower serum ionized calcium and abnormal calciotropic hormone levels in preeclampsia. J. Clin. Endocrinol. Metab. 1992, 74, 1436–1440. [Google Scholar] [CrossRef]
- Jorde, R.; Grimnes, G. Serum cholecalciferol may be a better marker of vitamin D status than 25-hydroxyvitamin D. Med. Hypotheses 2018, 111, 61–65. [Google Scholar] [CrossRef]
- Fan, H.; Hui, L.; Yan, X.; Hou, W.; Bai, E.; Wang, L.; Yu, X. Serum 25 hydroxyvitamin D levels and affecting factors among preconception fertile women. BMC Womens Health 2020, 20, 146. [Google Scholar] [CrossRef]
- Miller, W.L. Genetic disorders of Vitamin D biosynthesis and degradation. J. Steroid Biochem. Mol. Biol. 2017, 165, 101–108. [Google Scholar] [CrossRef]
- Plum, L.A.; DeLuca, H.F. Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 2010, 9, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and bone. Curr. Osteoporos. Rep. 2012, 10, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and bone health: Potential mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C.; Lu, Z.; Sauter, E. Vitamin D and skin physiology: A D-lightful story. J. Bone Miner. Res. 2007, 22 (Suppl. 2), V28–V33. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.; Genehr, T.; Knuschke, P.; Pietzsch, J.; Meurer, M. UVB-induced conversion of 7-dehydrocholesterol to 1alpha,25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. J. Investig. Dermatol. 2001, 117, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Kagi, L.; Bettoni, C.; Pastor-Arroyo, E.M.; Schnitzbauer, U.; Hernando, N.; Wagner, C.A. Regulation of vitamin D metabolizing enzymes in murine renal and extrarenal tissues by dietary phosphate, FGF23, and 1,25(OH)2D3. PLoS ONE 2018, 13, e0195427. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Sarandeses, L.A.; Allewaert, K.; Zhao, J.; Mascarenas, J.L.; Mourino, A.; Vrielynck, S.; de Clercq, P.; Vandewalle, M. Biologic activity of dihydroxylated 19-nor-(pre)vitamin D3. J. Bone Miner. Res. 1993, 8, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Schuetz, E.G.; Xu, Y.; Thummel, K.E. Interplay between vitamin D and the drug metabolizing enzyme CYP3A4. J. Steroid Biochem. Mol. Biol. 2013, 136, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D: An essential component for skeletal health. Ann. NYAS 2012, 1240, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J. Steroid Biochem. Mol. Biol. 2018, 175, 177–189. [Google Scholar] [CrossRef]
- Adamczak, D.M. The role of Toll-Like receptors and vitamin D in cardiovascular diseases-A review. Int. J. Mol. Sci. 2017, 18, 2252. [Google Scholar] [CrossRef]
- Valdes-Lopez, J.F.; Velilla, P.; Urcuqui-Inchima, S. Vitamin D modulates the expression of Toll-like receptors and pro-inflammatory cytokines without affecting Chikungunya virus replication, in monocytes and macrophages. Acta Trop. 2022, 232, 106497. [Google Scholar] [CrossRef]
- Haussler, M.R.; Whitfield, G.K.; Haussler, C.A.; Hsieh, J.C.; Thompson, P.D.; Selznick, S.H.; Dominguez, C.E.; Jurutka, P.W. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J. Bone Miner. Res. 1998, 13, 325–349. [Google Scholar] [CrossRef]
- Wikvall, K. Cytochrome P450 enzymes in the bioactivation of vitamin D to its hormonal form (review). Int. J. Mol. Med. 2001, 7, 201–209. [Google Scholar] [CrossRef]
- Norman, A.W. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am. J. Clin. Nutr. 2008, 88, 491S–499S. [Google Scholar] [CrossRef]
- Biesalski, H.K.; Aggett, P.J.; Anton, R.; Bernstein, P.S.; Blumberg, J.; Heaney, R.P.; Henry, J.; Nolan, J.M.; Richardson, D.P.; van Ommen, B.; et al. 26th Hohenheim Consensus Conference, September 11, 2010 Scientific substantiation of health claims: Evidence-based nutrition. Nutrition 2011, 27, S1–S20. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.; Coyle, W.J. The microbiome and obesity: Is obesity linked to our gut flora? Curr. Gastroenterol. Rep. 2009, 11, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Zhu, X.; Manson, J.E.; Song, Y.; Li, X.; Franke, A.A.; Costello, R.B.; Rosanoff, A.; Nian, H.; Fan, L.; et al. Magnesium status and supplementation influence vitamin D status and metabolism: Results from a randomized trial. Am. J. Clin. Nutr. 2018, 108, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H. Magnesium and vitamin D deficiency as a potential cause of immune dysfunction, cytokine storm and disseminated intravascular coagulation in covid-19 patients. Mo. Med. 2021, 118, 68–73. [Google Scholar]
- Adams, J.S.; Modlin, R.L.; Diz, M.M.; Barnes, P.F. Potentiation of the macrophage 25-hydroxyvitamin D-1-hydroxylation reaction by human tuberculous pleural effusion fluid. J. Clin. Endocrinol. Metab. 1989, 69, 457–460. [Google Scholar] [CrossRef]
- Dong, D.; Noy, N. Heterodimer formation by retinoid X receptor: Regulation by ligands and by the receptor’s self-association properties. Biochemistry 1998, 37, 10691–10700. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): Genomic and non-genomic mechanisms. Best. Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. ACE inhibitors and angiotensin receptor blockers reduce the complications associated with COVID-19 infection. World J. Pharma Res. 2021, 10, 2579–2600. [Google Scholar] [CrossRef]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of Insulin Synthesis and Secretion and Pancreatic Beta-Cell Dysfunction in Diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch. Biochem. Biophys. 2012, 523, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Taniguchi, M.; Tsuruya, K.; Iida, M. Recurrent sarcoidosis with psoas muscle granuloma and hypercalcaemia in a patient on chronic haemodialysis. Nephrology 2009, 14, 452–453. [Google Scholar] [CrossRef]
- Khan, H.; Kunutsor, S.; Franco, O.H.; Chowdhury, R. Vitamin D, type 2 diabetes and other metabolic outcomes: A systematic review and meta-analysis of prospective studies. Proc. Nutr. Soc. 2013, 72, 89–97. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D and bone health. J. Nutr. 1996, 126, 1159S–1164S. [Google Scholar] [CrossRef]
- Kroll, M.H.; Bi, C.; Garber, C.C.; Kaufman, H.W.; Liu, D.; Caston-Balderrama, A.; Zhang, K.; Clarke, N.; Xie, M.; Reitz, R.E.; et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS ONE 2015, 10, e0118108. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Heaney, R.P.; Dowell, M.S.; Hale, C.A.; Bendich, A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J. Am. Coll. Nutr. 2003, 22, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Mark, K.A.; Dumas, K.J.; Bhaumik, D.; Schilling, B.; Davis, S.; Oron, T.R.; Sorensen, D.J.; Lucanic, M.; Brem, R.B.; Melov, S.; et al. Vitamin D Promotes Protein Homeostasis and Longevity via the Stress Response Pathway Genes skn-1, ire-1, and xbp-1. Cell Rep. 2016, 17, 1227–1237. [Google Scholar] [CrossRef]
- Steingrimsdottir, L.; Gunnarsson, O.; Indridason, O.S.; Franzson, L.; Sigurdsson, G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 2005, 294, 2336–2341. [Google Scholar] [CrossRef]
- Wahl, D.A.; Cooper, C.; Ebeling, P.R.; Eggersdorfer, M.; Hilger, J.; Hoffmann, K.; Josse, R.; Kanis, J.A.; Mithal, A.; Pierroz, D.D.; et al. A global representation of vitamin D status in healthy populations. Arch. Osteoporos. 2012, 7, 155–172. [Google Scholar] [CrossRef]
- Dahlquist, D.T.; Dieter, B.P.; Koehle, M.S. Plausible ergogenic effects of vitamin D on athletic performance and recovery. J. Int. Soc. Sports Nutr. 2015, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Gerster, H. The role of vitamin C in athletic performance. J. Am. Coll. Nutr. 1989, 8, 636–643. [Google Scholar] [CrossRef]
- Lips, P.; Binkley, N.; Pfeifer, M.; Recker, R.; Samanta, S.; Cohn, D.A.; Chandler, J.; Rosenberg, E.; Papanicolaou, D.A. Once-weekly dose of 8400 IU vitamin D(3) compared with placebo: Effects on neuromuscular function and tolerability in older adults with vitamin D insufficiency. Am. J. Clin. Nutr. 2010, 91, 985–991. [Google Scholar] [CrossRef]
- Blain, H.; Jaussent, A.; Thomas, E.; Micallef, J.P.; Dupuy, A.M.; Bernard, P.L.; Mariano-Goulart, D.; Cristol, J.P.; Sultan, C.; Rossi, M.; et al. Appendicular skeletal muscle mass is the strongest independent factor associated with femoral neck bone mineral density in adult and older men. Exp. Gerontol. 2010, 45, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Okuno, J.; Tomura, S.; Yanagi, H.; Kim, M.J.; Okura, T.; Tanaka, K. Evaluation of the association between impaired renal function and physical function among community-dwelling Japanese frail elderly based on the estimated glomerular filtration rate (eGFR). Nihon Ronen Igakkai Zasshi 2009, 46, 63–70. [Google Scholar] [CrossRef]
- Fuchtbauer, L.; Brusgaard, K.; Ledaal, P.; Frost, M.; Frederiksen, A.L. Case report: Vitamin D-dependent rickets type 1 caused by a novel CYP27B1 mutation. Clin. Case Rep. 2015, 3, 1012–1016. [Google Scholar] [CrossRef]
- Hu, W.W.; Ke, Y.H.; He, J.W.; Fu, W.Z.; Wang, C.; Zhang, H.; Yue, H.; Gu, J.M.; Zhang, Z.L. A novel compound mutation of CYP27B1 in a Chinese family with vitamin D-dependent rickets type 1A. J. Pediatr. Endocrinol. Metab. JPEM 2014, 27, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.B.; Wrighton, S.A.; Schuetz, E.G.; Molowa, D.T.; Guzelian, P.S. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J. Clin. Investig. 1987, 80, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Cytochrome P-450 3A4: Regulation and role in drug metabolism. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Dragt, B.S.; Porte, R.J.; Groothuis, G.M. Regulation of VDR expression in rat and human intestine and liver--consequences for CYP3A expression. Toxicol. Vitr. 2010, 24, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Valdivielso, J.M. The physiology of vitamin D receptor activation. Contrib. Nephrol. 2009, 163, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Kim, J.J.; Mohr, S.B.; Gorham, E.D.; Grant, W.B.; Giovannucci, E.L.; Baggerly, L.; Hofflich, H.; Ramsdell, J.W.; Zeng, K.; et al. Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. Am. J. Public. Health 2014, 104, e43–e50. [Google Scholar] [CrossRef] [PubMed]
- Rostand, S.G. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension 1979, 30, 150–156. [Google Scholar]
- Thomas, G.N.; o Hartaigh, B.; Bosch, J.A.; Pilz, S.; Loerbroks, A.; Kleber, M.E.; Fischer, J.E.; Grammer, T.B.; Bohm, B.O.; Marz, W. Vitamin D levels predict all-cause and cardiovascular disease mortality in subjects with the metabolic syndrome: The Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Diabetes Care 2012, 35, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D regulation of immune function during covid-19. Rev. Endocr. Metab. Disord. 2022, 23, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Boucher, B.J.; Bhattoa, H.P.; Lahore, H. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2018, 177, 266–269. [Google Scholar] [CrossRef]
- Ray, J.A.; Meikle, A.W. D-light: Vitamin D and good health. MLO Med. Lab. Obs. 2010, 42, 32, 34–38. [Google Scholar]
- Gruson, D.; Ferracin, B.; Ahn, S.A.; Zierold, C.; Blocki, F.; Hawkins, D.M.; Bonelli, F.; Rousseau, M.F. 1,25-Dihydroxyvitamin D to PTH(1-84) Ratios Strongly Predict Cardiovascular Death in Heart Failure. PLoS ONE 2015, 10, e0135427. [Google Scholar] [CrossRef] [PubMed]
- Bislev, L.S.; Langagergaard Rodbro, L.; Bech, J.N.; Pedersen, E.B.; Kjaergaard, A.D.; Ladefoged, S.A.; Rolighed, L.; Sikjaer, T.; Rejnmark, L. The effect of vitamin D3 supplementation on markers of cardiovascular health in hyperparathyroid, vitamin D insufficient women: A randomized placebo-controlled trial. Endocrine 2018, 62, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Albaaj, F.; Hutchison, A. Hyperphosphataemia in renal failure: Causes, consequences and current management. Drugs 2003, 63, 577–596. [Google Scholar] [CrossRef] [PubMed]
- Umehara, K.; Mukai, N.; Hata, J.; Hirakawa, Y.; Ohara, T.; Yoshida, D.; Kishimoto, H.; Kitazono, T.; Hoka, S.; Kiyohara, Y.; et al. Association between serum vitamin D and all-cause and cause-specific death in a general Japanese population- The Hisayama Study. Circ. J. 2017, 81, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Ernst, J.B.; Pilz, S.; Dreier, J.; Kuhn, J.; Knabbe, C.; Gummert, J.F.; Morshuis, M.; Milting, H. Calciotropic and phosphaturic hormones in end-stage heart failure patients supported by a left-ventricular assist device. PLoS ONE 2016, 11, e0164459. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, S.; Alibrandi, A.; Campenni, A.; Trimarchi, F.; Ruggeri, R.M. Correlation of cardio-metabolic parameters with vitamin D status in healthy premenopausal women. J. Endocrinol. Investig. 2017, 40, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Vigna, L.; Cassinelli, L.; Tirelli, A.S.; Felicetta, I.; Napolitano, F.; Tomaino, L.; Mutti, M.; Barberi, C.E.; Riboldi, L. 25(OH)D Levels in Relation to Gender, Overweight, Insulin Resistance, and Inflammation in a Cross-Sectional Cohort of Northern Italian Workers: Evidence in Support of Preventive Health Care Programs. J. Am. Coll. Nutr. 2017, 36, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.P.; D’Vaz, N.; Meldrum, S.; Palmer, D.J.; Zhang, G.; Prescott, S.L. 25-hydroxyvitamin D3 status is associated with developing adaptive and innate immune responses in the first 6 months of life. Clin. Exp. Allergy 2015, 45, 220–231. [Google Scholar] [CrossRef]
- Israel, A.; Cicurel, A.; Feldhamer, I.; Stern, F.; Dror, Y.; Giveon, S.M.; Gillis, D.; Strich, D.; Lavie, G. Vitamin D deficiency is associated with higher risks for SARS-CoV-2 infection and COVID-19 severity: A retrospective case-control study. Intern. Emerg. Med. 2022, 17, 1053–1063. [Google Scholar] [CrossRef]
- Amital, H.; Szekanecz, Z.; Szucs, G.; Danko, K.; Nagy, E.; Csepany, T.; Kiss, E.; Rovensky, J.; Tuchynova, A.; Kozakova, D.; et al. Serum concentrations of 25-OH vitamin D in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: Is it time to routinely supplement patients with SLE with vitamin D? Ann. Rheum. Dis. 2010, 69, 1155–1157. [Google Scholar] [CrossRef]
- Konstantakis, C.; Tselekouni, P.; Kalafateli, M.; Triantos, C. Vitamin D deficiency in patients with liver cirrhosis. Ann. Gastroenterol. 2016, 29, 297–306. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ichiyama, T.; Ohsaki, A.; Hasegawa, S.; Shiraishi, M.; Furukawa, S. Anti-inflammatory effect of 1alpha,25-dihydroxyvitamin D(3) in human coronary arterial endothelial cells: Implication for the treatment of Kawasaki disease. J. Steroid Biochem. Mol. Biol. 2009, 113, 134–138. [Google Scholar] [CrossRef]
- Ma, R.; Gu, Y.; Zhao, S.; Sun, J.; Groome, L.J.; Wang, Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E928–E935. [Google Scholar] [CrossRef]
- Rondini, E.A.; Pant, A.; Kocarek, T.A. Transcriptional regulation of cytosolic sulfotransferase 1C2 by intermediates of the cholesterol biosynthetic pathway in primary cultured rat hepatocytes. J. Pharmacol. Exp. Ther. 2015, 355, 429–441. [Google Scholar] [CrossRef]
- Lohr, M.; Hummel, F.; Faulmann, G.; Ringel, J.; Saller, R.; Hain, J.; Gunzburg, W.H.; Salmons, B. Microencapsulated, CYP2B1-transfected cells activating ifosfamide at the site of the tumor: The magic bullets of the 21st century. Cancer Chemother. Pharmacol. 2002, 49 (Suppl. 1), S21–S24. [Google Scholar] [CrossRef]
- Morris, H.A.; Anderson, P.H. Autocrine and paracrine actions of vitamin D. Clin. Biochem. Rev. 2010, 31, 129–138. [Google Scholar]
- Powe, C.E.; Karumanchi, S.A.; Thadhani, R. Vitamin D-binding protein and vitamin D in blacks and whites. N. Engl. J. Med. 2014, 370, 880–881. [Google Scholar] [CrossRef]
- Powe, C.E.; Evans, M.K.; Wenger, J.; Zonderman, A.B.; Berg, A.H.; Nalls, M.; Tamez, H.; Zhang, D.; Bhan, I.; Karumanchi, S.A.; et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N. Engl. J. Med. 2013, 369, 1991–2000. [Google Scholar] [CrossRef]
- Feldman, D.; P, J.M. Mutations in the vitamin D receptor and hereditary vitamin D-resistant rickets. BoneKEy Rep. 2014, 3, 510. [Google Scholar] [CrossRef]
- Koren, R. Vitamin D receptor defects: The story of hereditary resistance to vitamin D. Pediatr. Endocrinol. Rev. 2006, 3 (Suppl. 3), 470–475. [Google Scholar]
- Al Mutair, A.N.; Nasrat, G.H.; Russell, D.W. Mutation of the CYP2R1 vitamin D 25-hydroxylase in a Saudi Arabian family with severe vitamin D deficiency. J. Clin. Endocrinol. Metab. 2012, 97, E2022–E2025. [Google Scholar] [CrossRef] [PubMed]
- Molin, A.; Wiedemann, A.; Demers, N.; Kaufmann, M.; Do Cao, J.; Mainard, L.; Dousset, B.; Journeau, P.; Abeguile, G.; Coudray, N.; et al. Vitamin D-dependent rickets Type 1B (25-hydroxylase deficiency): A rare condition or a misdiagnosed condition? J. Bone Miner. Res. 2017, 32, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Thacher, T.D.; Fischer, P.R.; Singh, R.J.; Roizen, J.; Levine, M.A. CYP2R1 mutations impair generation of 25-hydroxyvitamin D and cause an atypical form of vitamin D seficiency. J. Clin. Endocrinol. Metab. 2015, 100, E1005–E1013. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Thacher, T.D.; Levine, M.A. CYP2R1 mutations causing vitamin D-deficiency rickets. J. Steroid Biochem. Mol. Biol. 2017, 173, 333–336. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Decoding the paradox: Understanding elevated hospitalization and reduced mortality in SARS-CoV-2 variants. Int. J. Front. Sci. Technol. Res. 2024, 6, 1–20. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D deficiency: Effects on oxidative stress, epigenetics, gene regulation, and aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Reducing risks from COVID-19: Cost-effective ways of strengthening individual’s and the population immunity with vitamin D. J. Endocrinol. Sci. 2020, 2, 1–13. [Google Scholar] [CrossRef]
- Diamond, T.H.; Ho, K.W.; Rohl, P.G.; Meerkin, M. Annual intramuscular injection of a megadose of cholecalciferol for treatment of vitamin D deficiency: Efficacy and safety data. Med. J. Aust. 2005, 183, 10–12. [Google Scholar] [CrossRef]
- Sanders, K.M.; Stuart, A.L.; Williamson, E.J.; Simpson, J.A.; Kotowicz, M.A.; Young, D.; Nicholson, G.C. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA 2010, 303, 1815–1822. [Google Scholar] [CrossRef]
- Heaney, R.P.; Armas, L.A. Quantifying the vitamin D economy. Nutr. Rev. 2015, 73, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. How to optimize vitamin D supplementation to prevent cancer, based on cellular adaptation and hydroxylase enzymology. Anticancer Res. 2009, 29, 3675–3684. [Google Scholar] [PubMed]
- Ketha, H.; Thacher, T.D.; Oberhelman, S.S.; Fischer, P.R.; Singh, R.J.; Kumar, R. Comparison of the effect of daily versus bolus dose maternal vitamin D3 supplementation on the 24,25-dihydroxyvitamin D3 25-hydroxyvitamin D3 ratio. Bone 2018, 110, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Rooney, M.R.; Harnack, L.; Michos, E.D.; Ogilvie, R.P.; Sempos, C.T.; Lutsey, P.L. Trends in Use of High-Dose Vitamin D Supplements Exceeding 1000 or 4000 International Units Daily, 1999–2014. JAMA 2017, 317, 2448–2450. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, D.M.; Cardona, A.; Urcuqui-Inchima, S. High-dose of vitamin D supplement is associated with reduced susceptibility of monocyte-derived macrophages to dengue virus infection and pro-inflammatory cytokine production: An exploratory study. Clin. Chim. Acta 2017, 478, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Behjat Sasan, S.; Zandvakili, F.; Soufizadeh, N.; Baybordi, E. The Effects of Vitamin D Supplement on Prevention of Recurrence of Preeclampsia in Pregnant Women with a History of Preeclampsia. Obstet. Gynecol. Int. 2017, 2017, 8249264. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Optimum duration and safety of long-term use of potent anti-resorptive medications in osteoporosis. Expert. Rev. Endocrinol. Metab. 2016, 11, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J.; Whittle, R. Vitamin D: A single initial dose is not bogus if followed by an appropriate maintenance intake. JBMR Plus 2022, 6, e10606. [Google Scholar] [CrossRef]
- Cannell, J.J.; Vieth, R.; Willett, W.; Zasloff, M.; Hathcock, J.N.; White, J.H.; Tanumihardjo, S.A.; Larson-Meyer, D.E.; Bischoff-Ferrari, H.A.; Lamberg-Allardt, C.J.; et al. Cod liver oil, vitamin A toxicity, frequent respiratory infections, and the vitamin D deficiency epidemic. Ann. Otol. Rhinol. Laryngol. 2008, 117, 864–870. [Google Scholar] [CrossRef]
- Shamim, N.; Majid, H.; Khemani, S.; Salim, M.; Muneer, S.; Khan, A.H. Inappropriate supplementation of vitamin D can result in toxicity: A cross-sectional study of paediatrics population. JPMA J. Pak. Med. Assoc. 2023, 73, 500–504. [Google Scholar] [CrossRef]
- Aljehani, F.; Qashqari, M.B.; Alghamdi, M.K.; Saadi, A.I.; Alreasini, M.Y.; Alsolami, E.; Alfawaz, M. Prevalence of iatrogenic vitamin D toxicity among the Saudi population of vitamin D users due to overcorrection. Cureus 2023, 15, e37521. [Google Scholar] [CrossRef] [PubMed]
- Asif, A.; Farooq, N. Vitamin D Toxicity. In StatPearls (Internet); Natl Cntr Biotechnol Info: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lim, K.; Thadhani, R. Vitamin D Toxicity. J. Bras. Nefrol. 2020, 42, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. Vitamin D toxicity, policy, and science. J. Bone Miner. Res. 2007, 22 (Suppl. 2), V64–V68. [Google Scholar] [CrossRef] [PubMed]
- Koutkia, P.; Chen, T.C.; Holick, M.F. Vitamin D intoxication associated with an over-the-counter supplement. N. Engl. J. Med. 2001, 345, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Holick, M.F.; Alfonso, B.D.; Charlap, E.; Romero, C.M.; Rizk, D.; Newman, L.G. Vitamin D intoxication with severe hypercalcemia due to manufacturing and labeling errors of two dietary supplements made in the United States. J. Clin. Endocrinol. Metab. 2011, 96, 3603–3608. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [CrossRef] [PubMed]
- Wyon, M.A.; Koutedakis, Y.; Wolman, R.; Nevill, A.M.; Allen, N. The influence of winter vitamin D supplementation on muscle function and injury occurrence in elite ballet dancers: A controlled study. J. Sci. Med. Sport. 2014, 17, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Whiteman, D.C.; Kimlin, M.G.; Janda, M.; Clarke, M.W.; Lucas, R.M.; Neale, R.E. Effect of solar ultraviolet radiation exposure on serum 25(OH)D concentration: A pilot randomised controlled trial. Photochem. Photobiol. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Armas, L.A.; Hollis, B.W.; Heaney, R.P. Vitamin D2 is much less effective than vitamin D3 in humans. J. Clin. Endocrinol. Metab. 2004, 89, 5387–5391. [Google Scholar] [CrossRef]
- Lappe, J.; Watson, P.; Travers-Gustafson, D.; Recker, R.; Garland, C.; Gorham, E.; Baggerly, K.; McDonnell, S.L. Effect of vitamin D and calcium supplementation on cancer incidence in older women: A randomized clinical trial. JAMA 2017, 317, 1234–1243. [Google Scholar] [CrossRef]
- Perez-Lopez, F.R. Vitamin D and its implications for musculoskeletal health in women: An update. Maturitas 2007, 58, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Behradmanesh, S.; Ahmadi, A.; Rafieian-Kopaei, M. Impact of oral vitamin D (cholecalciferol) replacement therapy on blood pressure in type 2 diabetes patients; a randomized, double-blind, placebo controlled clinical trial. J. Nephropathol. 2014, 3, 29–33. [Google Scholar] [CrossRef]
- Gaksch, M.; Jorde, R.; Grimnes, G.; Joakimsen, R.; Schirmer, H.; Wilsgaard, T.; Mathiesen, E.B.; Njolstad, I.; Lochen, M.L.; Marz, W.; et al. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS ONE 2017, 12, e0170791. [Google Scholar] [CrossRef] [PubMed]
- Tretli, S.; Schwartz, G.G.; Torjesen, P.A.; Robsahm, T.E. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: A population-based study. Cancer Causes Control 2012, 23, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Kim, M.K.; Jung, S.; Shin, J.; Choi, B.Y. The cross-sectional relationships of dietary and serum vitamin D with cardiometabolic risk factors: Metabolic components, subclinical atherosclerosis, and arterial stiffness. Nutrition 2016, 32, 1048–1056.e1. [Google Scholar] [CrossRef] [PubMed]
- Souberbielle, J.C.; Body, J.J.; Lappe, J.M.; Plebani, M.; Shoenfeld, Y.; Wang, T.J.; Bischoff-Ferrari, H.A.; Cavalier, E.; Ebeling, P.R.; Fardellone, P.; et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun. Rev. 2010, 9, 709–715. [Google Scholar] [CrossRef]
- Park, S.K.; Garland, C.F.; Gorham, E.D.; BuDoff, L.; Barrett-Connor, E. Plasma 25-hydroxyvitamin D concentration and risk of type 2 diabetes and pre-diabetes: 12-year cohort study. PLoS ONE 2018, 13, e0193070. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Cross, H.S.; Garland, C.F.; Gorham, E.D.; Moan, J.; Peterlik, M.; Porojnicu, A.C.; Reichrath, J.; Zittermann, A. Estimated benefit of increased vitamin D status in reducing the economic burden of disease in western Europe. Prog. Biophys. Mol. Biol. 2009, 99, 104–113. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Hollis, B.W.; Trump, D.L.; Lappe, J.M. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations >/=60 vs <20 ng/mL (150 vs 50 nmol/L): Pooled analysis of two randomized trials and a prospective cohort. PLoS ONE 2018, 13, e0199265. [Google Scholar] [CrossRef]
- Heaney, R.P.; Holick, M.F. Why the IOM recommendations for vitamin D are deficient. J. Bone Miner. Res. 2011, 26, 455–457. [Google Scholar] [CrossRef]
- Pender, M.P. CD8+ T-cell deficiency, Epstein-Barr virus infection, vitamin D deficiency, and steps to autoimmunity: A unifying hypothesis. Autoimmune Dis. 2012, 2012, 189096. [Google Scholar] [CrossRef] [PubMed]
- Gominak, S.C. Vitamin D deficiency changes the intestinal microbiome reducing B vitamin production in the gut. The resulting lack of pantothenic acid adversely affects the immune system, producing a “pro-inflammatory” state associated with atherosclerosis and autoimmunity. Med. Hypotheses 2016, 94, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Dahl, L.; Olson, G.; Thornton, D.; Graff, I.E.; Froyland, L.; Thayer, J.F.; Pallesen, S. Fish consumption, sleep, daily functioning, and heart rate variability. J. Clin. Sleep. Med. 2014, 10, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Shah, S.; Long, Q.; Crankshaw, A.K.; Tangpricha, V. Improvement of pain, sleep, and quality of life in chronic pain patients with vitamin D supplementation. Clin. J. Pain. 2013, 29, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Dudenkov, D.V.; Yawn, B.P.; Oberhelman, S.S.; Fischer, P.R.; Singh, R.J.; Cha, S.S.; Maxson, J.A.; Quigg, S.M.; Thacher, T.D. Changing Incidence of Serum 25-hydroxyvitamin D values above 50 ng/mL: A 10-year population-based study. Mayo Clin. Proc. 2015, 90, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Brashear, M.M.; Johnson, W.D. Prediabetes and prehypertension in healthy adults are associated with low vitamin D levels. Diabetes Care 2011, 34, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Hamed, E.A.; Abu Faddan, N.H.; Adb Elhafeez, H.A.; Sayed, D. Parathormone—25(OH)-vitamin D axis and bone status in children and adolescents with type 1 diabetes mellitus. Pediatr. Diabetes 2011, 12, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, A.; Kamali Sarvestani, H.; Rafat, Z.; Ghaderkhani, S.; Mahmoudi-Aliabadi, M.; Jafarzadeh, B.; Mehrtash, V. The association between the level of serum 25(OH) vitamin D, obesity, and underlying diseases with the risk of developing COVID-19 infection: A case-control study of hospitalized patients in Tehran, Iran. J. Med. Virol. 2021, 93, 2359–2364. [Google Scholar] [CrossRef]
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernan, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef]
- Merlino, L.A.; Curtis, J.; Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Saag, K.G.; Iowa Women’s Health, S. Vitamin D intake is inversely associated with rheumatoid arthritis: Results from the Iowa Women’s Health Study. Arthritis Rheum. 2004, 50, 72–77. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, Y.; Liu, Q.; Zhang, C. Efficacy and safety of vitamin D supplementation on psoriasis: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0294239. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, D.A.; Prindiville, S.; Weiss, D.G.; Willett, W.; Group, V.A.C.S. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 2003, 290, 2959–2967. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Boucher, B.J. Randomized controlled trials of vitamin D and cancer incidence: A modeling study. PLoS ONE 2017, 12, e0176448. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Robertson, A.S.; Rodriguez, C.; Jacobs, E.J.; Chao, A.; Carolyn, J.; Calle, E.E.; Willett, W.C.; Thun, M.J. Calcium, vitamin D, dairy products, and risk of colorectal cancer in the cancer prevention study II nutrition cohort (United States). Cancer Causes Control 2003, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, V.; Vieth, R. Three-year follow-up of serum 25-hydroxyvitamin D, parathyroid hormone, and bone mineral density in nursing home residents who had received 12 months of daily bread fortification with 125 mug of vitamin D(3). Nutr. J. 2013, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J.; Polonowita, A.K. Logical analysis of response of health officials’ worldwide, to cost-effective early remedies for COVID-19. World J. Adv. Pharma Life Sci. 2023, 5, 13–34. [Google Scholar] [CrossRef]
- BishopL, E.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and immune regulation: Antibacterial, antiviral, anti-inflammatory. JBMR Plus 2021, 5, e10405. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Bikle, D.; Hewison, M.; Lazaretti-Castro, M.; Formenti, A.M.; Gupta, A.; Madhavan, M.V.; Nair, N.; Babalyan, V.; Hutchings, N.; et al. Mechanisms in Endocrinology: Vitamin D and COVID-19. Eur. J. Endocrinol. 2020, 183, R133–R147. [Google Scholar] [CrossRef] [PubMed]
- Grenade, N.; Kosar, C.; Steinberg, K.; Avitzur, Y.; Wales, P.W.; Courtney-Martin, G. Use of a Loading Dose of Vitamin D for Treatment of Vitamin D Deficiency in Patients with Intestinal Failure. JPEN J. Parenter. Enter. Nutr. 2017, 41, 512–516. [Google Scholar] [CrossRef]
- Sainaghi, P.P.; Bellan, M.; Nerviani, A.; Sola, D.; Molinari, R.; Cerutti, C.; Pirisi, M. Superiority of a high loading dose of cholecalciferol to correct hypovitaminosis d in patients with inflammatory/autoimmune rheumatic diseases. J. Rheumatol. 2013, 40, 166–172. [Google Scholar] [CrossRef]
- Griffin, G.; Hewison, M.; Hopkin, J.; Kenny, R.A.; Quinton, R.; Rhodes, J.; Subramanian, S.; Thickett, D. Preventing vitamin D deficiency during the COVID-19 pandemic: UK definitions of vitamin D sufficiency and recommended supplement dose are set too low. Clin. Med. 2021, 21, e48–e51. [Google Scholar] [CrossRef]
- van Driel, M.; Koedam, M.; Buurman, C.J.; Hewison, M.; Chiba, H.; Uitterlinden, A.G.; Pols, H.A.; van Leeuwen, J.P. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. FASEB J. 2006, 20, 2417–2419. [Google Scholar] [CrossRef]
- Bader, D.A.; Abed, A.; Mohammad, B.A.; Aljaberi, A.; Sundookah, A.; Habash, M.; Alsayed, A.R.; Abusamak, M.; Al-Shakhshir, S.; Abu-Samak, M. The Effect of Weekly 50,000 IU Vitamin D(3) Supplements on the Serum Levels of Selected Cytokines Involved in Cytokine Storm: A Randomized Clinical Trial in Adults with Vitamin D Deficiency. Nutrients 2023, 15, 1188. [Google Scholar] [CrossRef]
- Binkley, N.; Gemar, D.; Engelke, J.; Gangnon, R.; Ramamurthy, R.; Krueger, D.; Drezner, M.K. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J. Clin. Endocrinol. Metab. 2011, 96, 981–988. [Google Scholar] [CrossRef]
- Binkley, N.; Gemar, D.; Engelke, J.; Gangnon, R.; Ramamurthy, R.; Krueger, D. Dosing with ergocalciferol or cholecalciferol, 1,600 IU daily or 50,000 IU monthly, is safe but does not assure vitamin D adequacy (Abst.). J. Bone Miner. Res. 2009, 24 (Suppl. 1). [Google Scholar]
- Havens, P.L.; Mulligan, K.; Hazra, R.; Flynn, P.; Rutledge, B.; Van Loan, M.D.; Lujan-Zilbermann, J.; Kapogiannis, B.G.; Wilson, C.M.; Stephensen, C.B.; et al. Serum 25-hydroxyvitamin D response to vitamin D3 supplementation 50,000 IU monthly in youth with HIV-1 infection. J. Clin. Endocrinol. Metab. 2012, 97, 4004–4013. [Google Scholar] [CrossRef]
- Mazahery, H.; Stonehouse, W.; von Hurst, P.R. The effect of monthly 50,000 IU or 100,000 IU vitamin D supplements on vitamin D status in premenopausal Middle Eastern women living in Auckland. Eur. J. Clin. Nutr. 2015, 69, 367–372. [Google Scholar] [CrossRef]
- Middleton, L.; Stack, B.C., Jr.; Riggs, A.T.; Bodenner, D.L. Vitamin D deficiency: A simple algorithm employing weekly administration of 50,000 IU of vitamin D. Am. J. Otolaryngol. 2014, 35, 85–88. [Google Scholar] [CrossRef]
- Manaseki-Holland, S.; Maroof, Z.; Bruce, J.; Mughal, M.Z.; Masher, M.I.; Bhutta, Z.A.; Walraven, G.; Chandramohan, D. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: A randomised controlled superiority trial. Lancet 2012, 379, 1419–1427. [Google Scholar] [CrossRef]
- Rothen, J.P.; Rutishauser, J.; Walter, P.N.; Hersberger, K.E.; Arnet, I. Vitamin D oral intermittent treatment (DO IT) study, a randomized clinical trial with individual loading regimen. Sci. Rep. 2021, 11, 18746. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.T.; Cui, Q.Q.; Hong, Y.M.; Yao, W.G. A meta-analysis of high dose, intermittent vitamin D supplementation among older adults. PLoS ONE 2015, 10, e0115850. [Google Scholar] [CrossRef] [PubMed]
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine vitamin D signaling switches off pro-inflammatory programs of T(H)1 cells. Nat. Immunol. 2022, 23, 62–74. [Google Scholar] [CrossRef]
- Chau, A.S.; Weber, A.G.; Maria, N.I.; Narain, S.; Liu, A.; Hajizadeh, N.; Malhotra, P.; Bloom, O.; Marder, G.; Kaplan, B. The Longitudinal Immune Response to Coronavirus Disease 2019: Chasing the Cytokine Storm. Arthritis Rheumatol. 2021, 73, 23–35. [Google Scholar] [CrossRef]
- Suñé Negre, J.; Azpitarte, I.O.; Barrios, P.D.A.; Hernández Herrero, G. Calcifediol Soft Capsules. Available online: https://patents.google.com/patent/WO2016124724A1/en?oq=WO+2016124724 (accessed on 7 April 2024).
- Chiu, K.C.; Chu, A.; Go, V.L.; Saad, M.F. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am. J. Clin. Nutr. 2004, 79, 820–825. [Google Scholar] [CrossRef]
- Feskanich, D.; Willett, W.C.; Colditz, G.A. Calcium, vitamin D, milk consumption, and hip fractures: A prospective study among postmenopausal women. Am. J. Clin. Nutr. 2003, 77, 504–511. [Google Scholar] [CrossRef]
- Meier, C.; Woitge, H.W.; Witte, K.; Lemmer, B.; Seibel, M.J. Supplementation with oral vitamin D3 and calcium during winter prevents seasonal bone loss: A randomized controlled open-label prospective trial. J. Bone Miner. Res. 2004, 19, 1221–1230. [Google Scholar] [CrossRef]
- Theodoratou, E.; Tzoulaki, I.; Zgaga, L.; Ioannidis, J.P. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014, 348, g2035. [Google Scholar] [CrossRef]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef]
- Ryan, J.W.; Anderson, P.H.; Morris, H.A. Pleiotropic Activities of Vitamin D Receptors—Adequate Activation for Multiple Health Outcomes. Clin. Biochem. Rev. 2015, 36, 53–61. [Google Scholar]
- Zipitis, C.S.; Elazabi, A.; Samanta, S. Vitamin D deficiency and guideline awareness. Arch. Dis. Child. Fetal Neonatal Ed. 2011, 96, F310. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Wimalawansa, S.J.; Holick, M.F.; Cannell, J.J.; Pludowski, P.; Lappe, J.M.; Pittaway, M.; May, P. Emphasizing the health benefits of vitamin D for those with neurodevelopmental disorders and intellectual disabilities. Nutrients 2015, 7, 1538–1564. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D adequacy and improvements of comorbidities in persons with intellectual developmental disabilities. J. Child. Dev. Disord. 2016, 2, 22–33. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wimalawansa, S.J. Physiology of Vitamin D—Focusing on Disease Prevention. Nutrients 2024, 16, 1666. https://doi.org/10.3390/nu16111666
Wimalawansa SJ. Physiology of Vitamin D—Focusing on Disease Prevention. Nutrients. 2024; 16(11):1666. https://doi.org/10.3390/nu16111666
Chicago/Turabian StyleWimalawansa, Sunil J. 2024. "Physiology of Vitamin D—Focusing on Disease Prevention" Nutrients 16, no. 11: 1666. https://doi.org/10.3390/nu16111666
APA StyleWimalawansa, S. J. (2024). Physiology of Vitamin D—Focusing on Disease Prevention. Nutrients, 16(11), 1666. https://doi.org/10.3390/nu16111666





