Effects of Garlic on Glucose Parameters and Lipid Profile: A Systematic Review and Meta-Analysis on Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Data Analysis
3. Result
3.1. Process of Study Selection
3.2. Study Characteristics
3.3. Risk of Bias Assessment
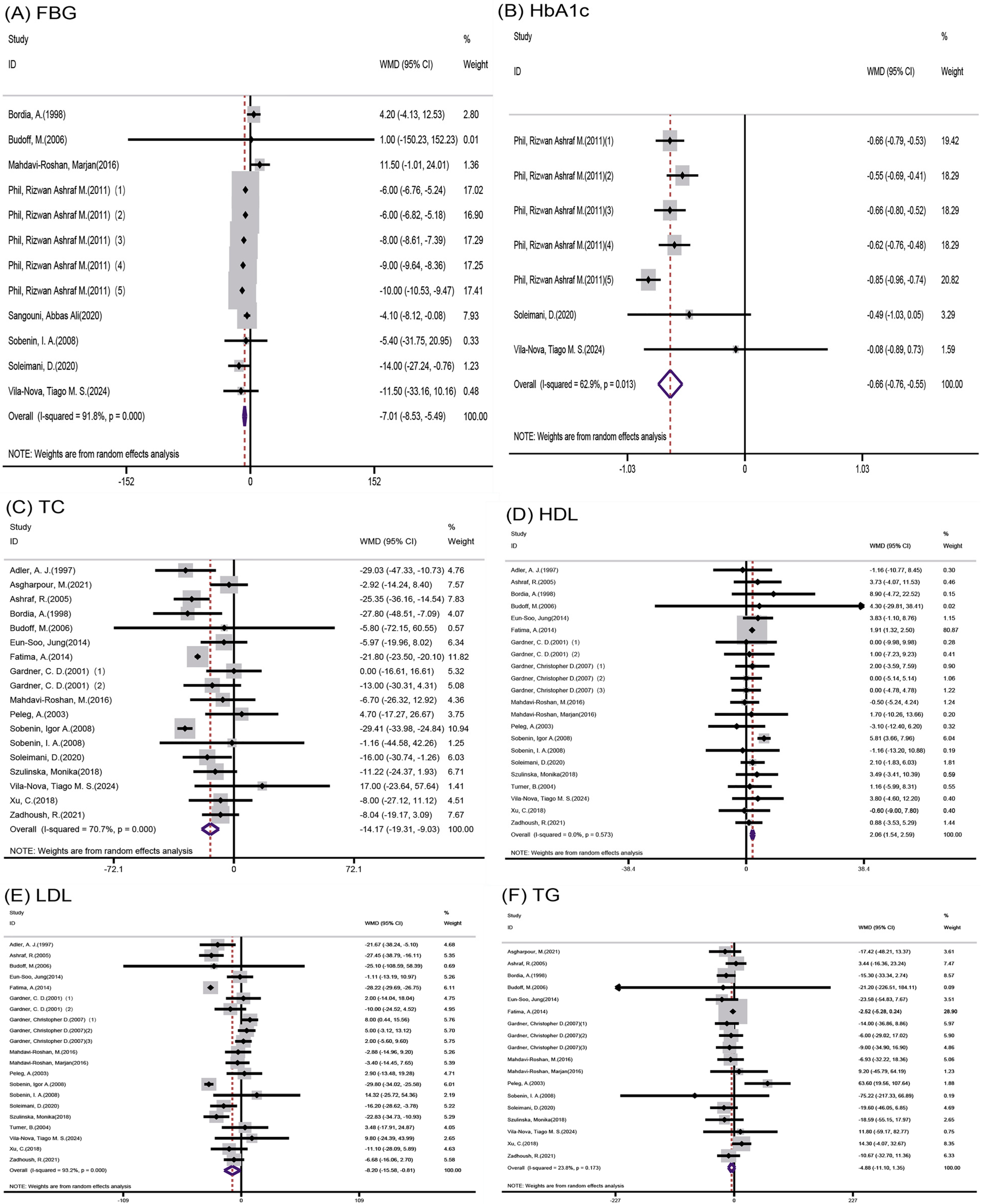
3.4. Results of Meta-Analysis
3.4.1. Effect of Garlic on Indicators Related to Glucose Metabolism
Impact of Garlic on Glucose Parameters
3.4.2. Impact of Garlic o Lipid Profile
3.5. Subgroup Analysis
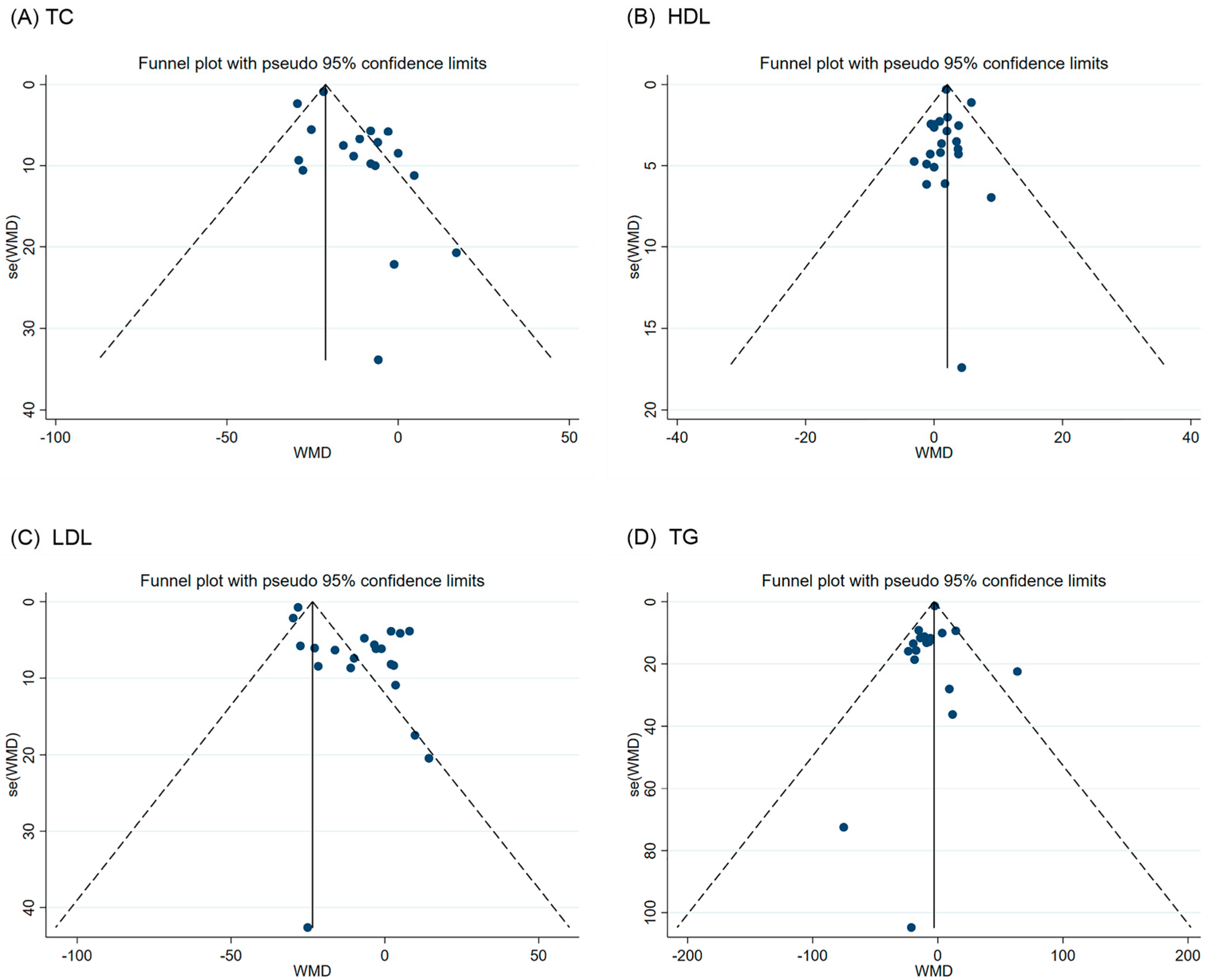
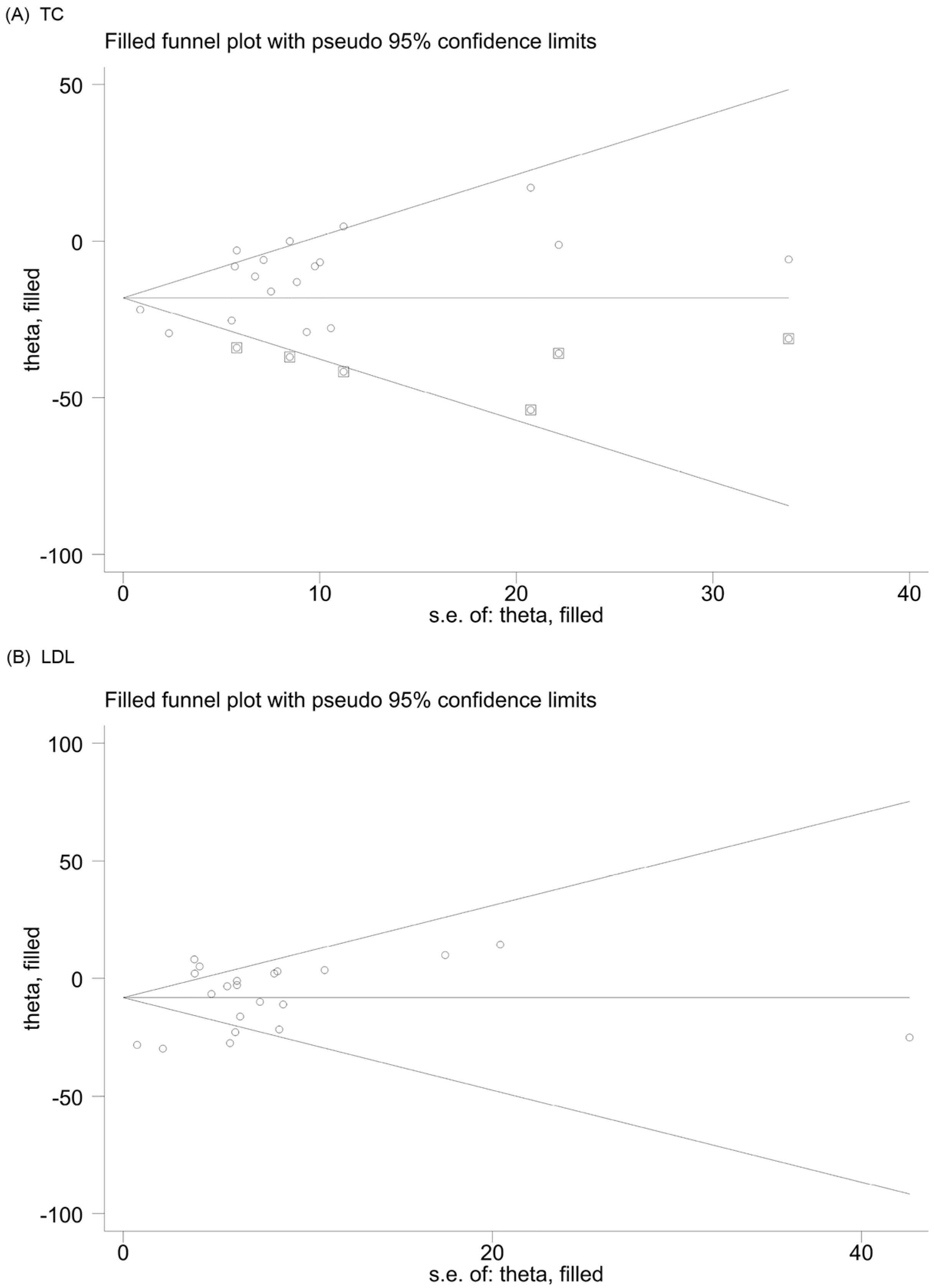
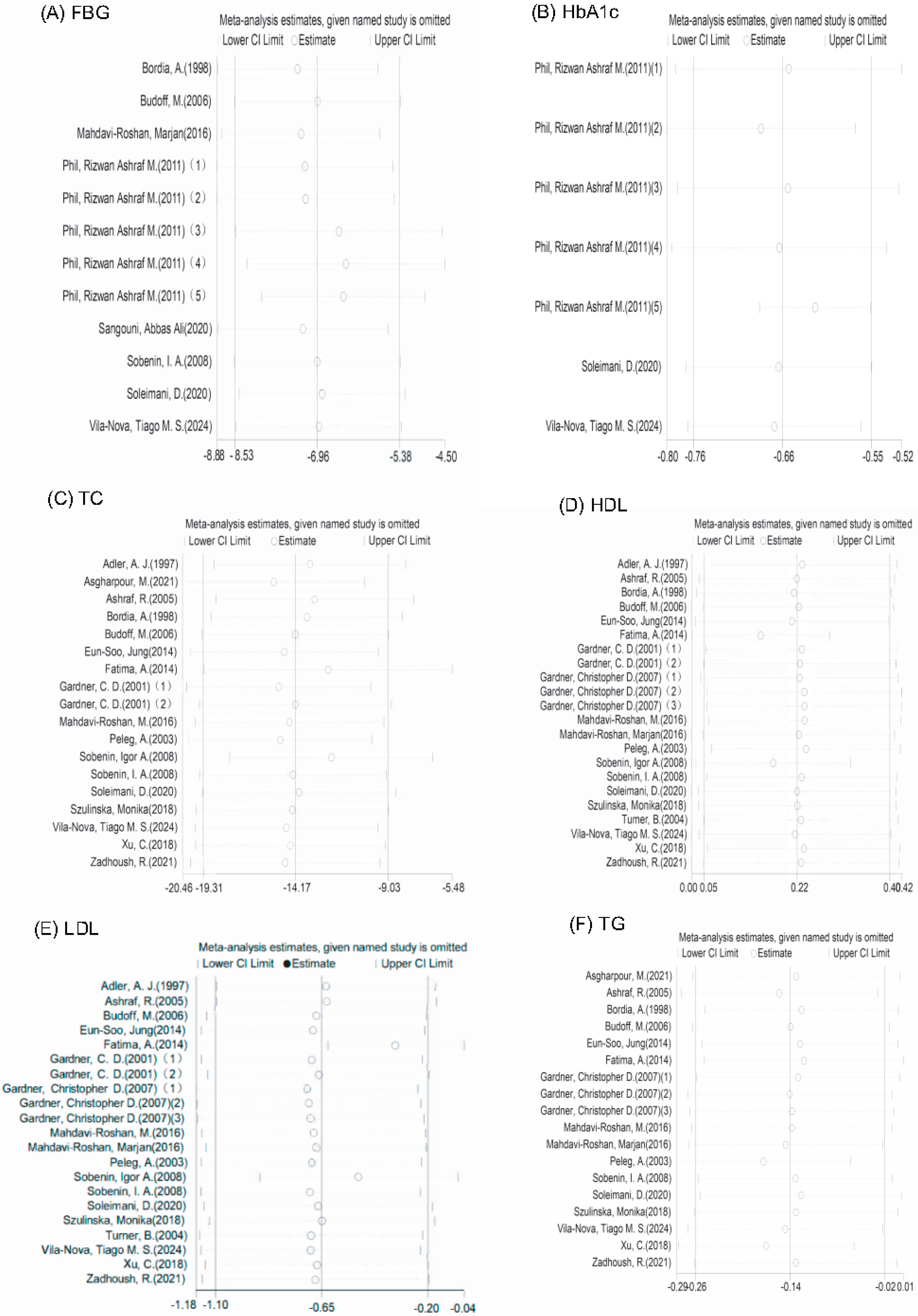
3.6. Publication Bias and Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Williams, J.; Allen, L.; Wickramasinghe, K.; Mikkelsen, B.; Roberts, N.; Townsend, N. A systematic review of associations between non-communicable diseases and socioeconomic status within low- and lower-middle-income countries. J. Glob. Health 2018, 8, 020409. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, X.-W.; Huang, X.; Song, B.-L.; Wang, Y.; Wang, Y. Regulation of glucose and lipid metabolism in health and disease. Sci. China Life Sci. 2019, 62, 1420–1458. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Yan, W.; Gao, S.; Li, X. The effect of PFAS exposure on glucolipid metabolism in children and adolescents: A meta-analysis. Front. Endocrinol. 2024, 15, 1261008. [Google Scholar] [CrossRef] [PubMed]
- Miller, M. Niacin as a component of combination therapy for dyslipidemia. Mayo Clin. Proc. 2003, 78, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.M.; van der Graaff, D.; Kwanten, W.J. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J. Hepatol. 2016, 65, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Wood, D. Established and emerging cardiovascular risk factors. Am. Heart J. 2001, 141 (Suppl. 2), S49–S57. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. Pathogenesis of type 2 diabetes mellitus. Med. Clin. N. Am. 2004, 88, 787–835. [Google Scholar] [CrossRef]
- Alorainy, M.S. Evaluation of antimicrobial activity of garlic (Allium sativum) against E. coli O157: H7. J. Agric. Vet. Sci. 2011, 4, 149–157. [Google Scholar]
- Song, B.; Shu, Y.; Cui, T.; Fu, P. Allicin inhibits human renal clear cell carcinoma progression via suppressing HIF pathway. Int. J. Clin. Exp. Med. 2015, 8, 20573–20580. [Google Scholar]
- Rosner, M.H.; Ronco, C.; Okusa, M.D. The Role of Inflammation in the Cardio-Renal Syndrome: A Focus on Cytokines and Inflammatory Mediators. Semin. Nephrol. 2012, 32, 70–78. [Google Scholar] [CrossRef]
- Jabbes, N.; Arnault, I.; Auger, J.; Al Mohandes Dridi, B.; Hannachi, C. Agro-morphological markers and organo-sulphur compounds to assess diversity in Tunisian garlic landraces. Sci. Hortic. 2012, 148, 47–54. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Derosa, G.; Gaddi, A. What do herbalists suggest to diabetic patients in order to improve glycemic control? Evaluation of scientific evidence and potential risks. Acta Diabetol. 2004, 41, 91–98. [Google Scholar] [CrossRef]
- Jung, Y.-M.; Lee, S.-H.; Lee, D.-S.; You, M.-J.; Chung, I.K.; Cheon, W.H.; Kwon, Y.-S.; Lee, Y.-J.; Ku, S.-K. Fermented garlic protects diabetic, obese mice when fed a high-fat diet by antioxidant effects. Nutr. Res. 2011, 31, 387–396. [Google Scholar] [CrossRef]
- Lin, X.L.; Hu, H.J.; Liu, Y.B.; Hu, X.M.; Fan, X.J.; Zou, W.W.; Pan, Y.Q.; Zhou, W.Q.; Peng, M.W.; Gu, C.H. Allicin induces the upregulation of ABCA1 expression via PPARγ/LXRα signaling in THP-1 macrophage-derived foam cells. Int. J. Mol. Med. 2017, 39, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Orekhov, A.N.; Tertov, V.V. In vitro effect of garlic powder extract on lipid content in normal and atherosclerotic human aortic cells. Lipids 1997, 32, 1055–1060. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Kim, H.G.; Choi, J.H.; Do, M.T.; Chung, Y.C.; Jeong, T.C.; Jeong, H.G. S-Allyl cysteine attenuates free fatty acid-induced lipogenesis in human HepG2 cells through activation of the AMP-activated protein kinase-dependent pathway. J. Nutr. Biochem. 2013, 24, 1469–1478. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Lu, K.; Yu, T.; Cao, X.; Xia, H.; Wang, S.; Sun, G.; Chen, L.; Liao, W. Effect of viscous soluble dietary fiber on glucose and lipid metabolism in patients with type 2 diabetes mellitus: A systematic review and meta-analysis on randomized clinical trials. Front. Nutr. 2023, 10, 1253312. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.J.; Holub, B.J. Effect of garlic and fish-oil supplementation on serum lipid and lipoprotein concentrations in hypercholesterolemic men. Am. J. Clin. Nutr. 1997, 65, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Asgharpour, M.; Khavandegar, A.; Balaei, P.; Enayati, N.; Mardi, P.; Alirezaei, A.; Bakhtiyari, M. Efficacy of Oral Administration of Allium sativum Powder “Garlic Extract” on Lipid Profile, Inflammation, and Cardiovascular Indices among Hemodialysis Patients. Evid.-Based Complement. Altern. Med. Ecam 2021, 2021, 6667453. [Google Scholar] [CrossRef]
- Sangouni, A.A.; Azar, M.R.M.H.; Alizadeh, M. Effects of garlic powder supplementation on insulin resistance, oxidative stress, and body composition in patients with non-alcoholic fatty liver disease: A randomized controlled clinical trial. Complement. Ther. Med. 2020, 51, 102428. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, D.; Paknahad, Z.; Rouhani, M.H. Therapeutic Effects of Garlic on Hepatic Steatosis in Nonalcoholic Fatty Liver Disease Patients: A Randomized Clinical Trial. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2389–2397. [Google Scholar] [CrossRef]
- Zadhoush, R.; Alavi-Naeini, A.; Feizi, A.; Naghshineh, E.; Ghazvini, M.R. The effect of garlic (Allium sativum) supplementation on the lipid parameters and blood pressure levels in women with polycystic ovary syndrome: A randomized controlled trial. Phytother. Res. PTR 2021, 35, 6335–6342. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, R.; Aamir, K.; Shaikh, A.R.; Ahmed, T. Effects of garlic on dyslipidemia in patients with type 2 diabetes mellitus. J. Ayub Med. Coll. Abbottabad JAMC 2005, 17, 60–64. [Google Scholar] [PubMed]
- Fatima, A.; Niaz, K.; Qudoos, A.; Murad, S. Single blind placebo-controlled study on effects of Garlic tablets to reduce serum lipids. Pak. J. Med. Health Sci. 2014, 8, 302–305. [Google Scholar]
- Phil, R.A.M.; Khan, R.A.; Ashraf, I. Effects of garlic on blood glucose levels and HbA1c in patients with type 2 diabetes mellitus. J. Med. Plants Res. 2011, 5, 2922–2928. [Google Scholar]
- Bordia, A.; Verma, S.K.; Srivastava, K.C. Effect of garlic (Allium sativum) on blood lipids, blood sugar, fibrinogen and fibrinolytic activity in patients with coronary artery disease. Prostaglandins Leukot. Essent. Fat. Acids 1998, 58, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi-Roshan, M.; Nasrollahzadeh, J.; Zadeh, A.M.; Zahedmehr, A. Does garlic supplementation control blood pressure in patients with severe coronary artery disease? A clinical trial study. Iran. Red Crescent Med. J. 2016, 18, e23871. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi-Roshan, M.; Rismanchi, M.; Nasrollahzadeh, J. Garlic tablet supplementation reduces lipopolysaccharide-induced TNF-alpha production by peripheral blood mononuclear cells. Eur. J. Inflamm. 2016, 14, 190–195. [Google Scholar] [CrossRef]
- Budoff, M. Aged garlic extract retards progression of coronary artery calcification. J. Nutr. 2006, 136 (Suppl. 3), 741s–744s. [Google Scholar] [CrossRef]
- Gardner, C.D.; Chatterjee, L.M.; Carlson, J.J. The effect of a garlic preparation on plasma lipid levels in moderately hypercholesterolemic adults. Atherosclerosis 2001, 154, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Mathews, A.E.; Rodrigues, C.; Eudy, B.J.; Rowe, C.A.; O’Donoughue, A.; Percival, S.S. Aged garlic extract supplementation modifies inflammation and immunity of adults with obesity: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. ESPEN 2018, 24, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Lawson, L.D.; Chatterjee, L.M.; Kiazand, A.; Balise, R.R.; Kraemer, H.C. Effect of raw garlic vs commercial garlic supplements on plasma lipid concentrations in adults with moderate hypercholesterolemia—A randomized clinical trial. Arch. Intern. Med. 2007, 167, 346–353. [Google Scholar] [CrossRef]
- Jung, E.-S.; Park, S.-H.; Choi, E.-K.; Ryu, B.-H.; Park, B.-H.; Kim, D.-S.; Kim, Y.-G.; Chae, S.-W. Reduction of blood lipid parameters by a 12-wk supplementation of aged black garlic: A randomized controlled trial. Nutrition 2014, 30, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.; Hershcovici, T.; Lipa, R.; Anbar, R.; Redler, M.; Beigel, Y. Effect of garlic on lipid profile and psychopathologic parameters in people with mild to moderate hypercholesterolemia. Isr. Med. Assoc. J. IMAJ 2003, 5, 637–640. [Google Scholar] [PubMed]
- Sobenin, I.A.; Andrianova, I.V.; Demidlova, O.N.; Gorchakova, T.V.; Orekhov, A.N. Lipid-Lowering Effects of Time-Released Garlic Powder Tablets in Double-Blinded Placebo-Controlled Randomized Study. J. Atheroscler. Thromb. 2008, 15, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Sobenin, I.A.; Nedosugova, L.V.; Filatova, L.V.; Balabolkin, M.I.; Gorchakova, T.V.; Orekhov, A.N. Metabolic effects of time-released garlic powder tablets in type 2 diabetes mellitus: The results of double-blinded placebo-controlled study. Acta Diabetol. 2008, 45, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Szulinska, M.; Kregielska-Narozna, M.; Swiatek, J.; Stys, P.; Kuznar-Kaminska, B.; Jakubowski, H.; Walkowiak, J.; Bogdanski, P. Garlic extract favorably modifies markers of endothelial function in obese patients—Randomized double blind placebo-controlled nutritional intervention. Biomed. Pharmacother. 2018, 102, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Vila-Nova, T.M.S.; Barbosa, K.B.F.; Freire, A.R.S.; Cintra, D.E.C.; Silva, D.G.; Rodrigues, T.M.d.A.; Costa, B.M.; Aragao, L.G.S. Effect of aged garlic extract on blood pressure and other cardiovascular markers in hypertensive patients and its relationship with dietary intake. J. Funct. Foods 2024, 112, 105931. [Google Scholar] [CrossRef]
- Turner, B.; Molgaard, C.; Marckmann, P. Effect of garlic (Allium sativum) powder tablets on serum lipids, blood pressure and arterial stiffness in normo-lipidaemic volunteers: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2004, 92, 701–706. [Google Scholar] [CrossRef]
- Sunanta, P.; Kontogiorgos, V.; Pankasemsuk, T.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Sommano, S.R. The nutritional value, bioactive availability and functional properties of garlic and its related products during processing. Front. Nutr. 2023, 10, 1142784. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, L.; Yang, L.; Lǚ, H.; Wang, S.; Sun, G. Anti-obesity and Hypolipidemic effects of garlic oil and onion oil in rats fed a high-fat diet. Nutr. Metab. 2018, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.S.; Kim, J.Y.; Paek, J.E.; Lee, Y.J.; Kim, H.R.; Park, D.S.; Kwon, O. Garlic powder intake and cardiovascular risk factors: A meta-analysis of randomized controlled clinical trials. Nutr. Res. Pract. 2014, 8, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Shabani, E.; Sayemiri, K.; Mohammadpour, M. The effect of garlic on lipid profile and glucose parameters in diabetic patients: A systematic review and meta-analysis. Prim. Care Diabetes 2019, 13, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Liu, Y.; Zhang, Y. Garlic intake lowers fasting blood glucose: Meta-analysis of randomized controlled trials. Asia Pac. J. Clin. Nutr. 2015, 24, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.; Al-Qattan, K.K.; Bordia, T.; Ali, M. Including Garlic in the Diet May Help Lower Blood Glucose, Cholesterol, and Triglycerides. J. Nutr. 2006, 136, 800S–802S. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, F.M.; Yousef, M.I.; El-Naga, N.I.A. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem. Toxicol. 2005, 43, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Padiya, R.; Khatua, T.N.; Bagul, P.K.; Kuncha, M.; Banerjee, S.K. Garlic improves insulin sensitivity and associated metabolic syndromes in fructose fed rats. Nutr. Metab. 2011, 8, 53. [Google Scholar] [CrossRef]
- Augusti, K.T.; Sheela, C.G.J.E. Antiperoxide effect of S-allyl cysteine sulfoxide, an insulin secretagogue, in diabetic rats. Cell. Mol. Life Sci. 1996, 52, 115–119. [Google Scholar] [CrossRef]
- Chen, K.; Xie, K.; Liu, Z.; Nakasone, Y.; Sakao, K.; Hossain, A.; Hou, D.X. Preventive Effects and Mechanisms of Garlic on Dyslipidemia and Gut Microbiome Dysbiosis. Nutrients 2019, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Varade, S.; Nadella, M.; Hirake, A.; Mungase, S.B.; Ali, A.; Adela, R. Effect of garlic on the components of metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. J. Ethnopharmacol. 2024, 318, 116960. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Colagiuri, S. Glycated haemoglobin (HbA1c) for the diagnosis of diabetes mellitus—Practical implications. Diabetes Res. Clin. Pract. 2011, 93, 312–313. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rubio, K.G.; Méndez-Del Villar, M.; Cortez-Navarrete, M. The Role of Garlic in Metabolic Diseases: A Review. J. Med. Food 2022, 25, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, V.M.; Laurindo, L.F.; Manzan, B.; Guiguer, E.L.; Oshiiwa, M.; Otoboni, A.M.M.B.; Araujo, A.C.; Tofano, R.J.; Barbalho, S.M. Garlic: A systematic review of the effects on cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2023, 63, 6797–6819. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.; Lowe, G.M. Garlic and cardiovascular disease: A critical review. J. Nutr. 2006, 136, 736S–740S. [Google Scholar] [CrossRef] [PubMed]
- Stevinson, C.; Pittler, M.H.; Ernst, E. Garlic for Treating Hypercholesterolemia. Ann. Intern. Med. 2000, 133, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Miki, S.; Morihara, N.; Kagawa, Y. Aged garlic extract ameliorates fatty liver and insulin resistance and improves the gut microbiota profile in a mouse model of insulin resistance. Exp. Ther. Med. 2019, 18, 857–866. [Google Scholar] [CrossRef]
- Elmahdi, B.; Maha, M.K.; Afaf, I.A. The effect of fresh crushed garlic bulbs (Allium sativum) on plasma lipids in hypercholesterolemic rats. Res. J. Anim. Vet. Sci. 2008, 3, 15–19. [Google Scholar]
- Warshafsky, S.; Kamer, R.S.; Sivak, S.L. Effect of garlic on total serum cholesterol. A meta-analysis. Ann. Intern. Med. 1993, 119, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Khaleghnejad Tabari, A.; Khsosi Niaki, M.R.; Allahaverdian, S.; Sheikholeslami, M. Effect of dried garlic supplementation on blood lipids in mild and moderate hypercholesterolemic patients. Arch. Iran. Med. 1999, 2, 19–23. [Google Scholar]
- Powolny, A.A.; Singh, S.V. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008, 269, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mukherjee, P.K.; Maulik, S.K. Garlic as an antioxidant: The good, the bad and the ugly. Phytotherapy Res. 2003, 17, 97–106. [Google Scholar] [CrossRef]
- Hughes, B.G.; Lawson, L.D. Antimicrobial effects of Allium sativum L. (garlic), Allium ampeloprasum L. (elephant garlic), and Allium cepa L. (onion), garlic compounds and commercial garlic supplement products. Phytother. Res. 1991, 5, 154–158. [Google Scholar] [CrossRef]

| Study | Year | Study Region | Sample Size | Comorbidities | Dose of Product (mg/Day) | Dose of Active Ingredient per Day | Treatment Duration | Age Range | Type of Intervention(I/C) | Drug Use during the Study Period |
|---|---|---|---|---|---|---|---|---|---|---|
| Adam J Adler [19] | 1997 | Canada | 23 | hyperlipidemia | 900 | NA | 12 weeks | NA | garlic pill/placebo | other drugs that do not affect blood lipids |
| Asgharpour, M. [20] | 2021 | Iran | 140 | hemodialysis | 600 | 2.6 mg garlic extract | 8 weeks | 18–70 | garlic powder/placebo | conventional drugs |
| Ashraf, R. [24] | 2015 | Pakistan | 70 | T2DM | 600 | 7.8 mg alliin | 12 weeks | 25–70 | garlic powder tablets/placebo | NA |
| A. Bordia [27] | 1998 | India | 60 | fold healed myocardial infarction | 4000 | NA | 3 months | NA | garlic oil preparations/placebo | nitrates and aspirin |
| Budoff Matthew [30] | 2006 | US | 19 | CAD | 4 mL/d | NA | 1 year | NA | AGE/placebo | statin (10–40 mg/day) |
| Eun-Soo, Jung [34] | 2014 | Korea | 55 | hyperlipidemia | 6000/d | 3 mg/g SAC | 12 weeks | NA | AGE/placebo | NA |
| AJAZ FATIMA [25] | 2014 | Pakistan | 106 | hyperlipidemia | 900 mg | NA | 3 months | 20–70 | garlic tablets/placebo | NA |
| Gardner, C. D. [31] | 2001 | US | 51 | hyperlipidemia | 1000/500 | 1.5 mg allicin/0.75 mgallicin | 12 weeks | 35–65 | garlic tablets/placebo | NA |
| Gardner, Christopher D. [33] | 2007 | US | 192 | hyperlipidemia | 4000/4000/4000 | NA/3.2 mg allicin/1.5 mg SAC | 6 months | NA | raw garlic/garlic tablets/placebo | NA |
| Mahdavi-Roshan, M. [28] | 2016 | Iran | 56 | CAD | 800 | 2.4 mg allicin | 3 months | 56 | garlic powder tablets/placebo | prescribed medications |
| Mahdavi-Roshan, Marjan [29] | 2016 | Iran | 24 | Healthy | 2400 | 2400 mg allicin | 3 weeks | 25–55 | garlic powder tablets/placebo | NA |
| Peleg, A. [35] | 2003 | Israeli | 33 | hyperlipidemia | 22400 | 22,400 mg alliin | 16 weeks | 18–80 | garlic powder/placebo | NA |
| Rizwan Ashraf M. Phil [26] | 2011 | Pakistan | 180 | T2DM | 300/600/900/1200/1500 | NR | 24 weeks | NA | garlic tablets/placebo | NA |
| Abbas Ali Sangouni [21] | 2020 | Iran | 88 | NAFLD | 1600 | 6 mg allicin | 3 months | >18 | garlic powder tablets/placebo | NA |
| Igor A. Sobenin [36] | 2008 | Russia | 42 | T2DM | 600 | NA | 12 weeks | 35–70 | garlic powder tablets/placebo | NA |
| Igor A. Sobenin [37] | 2008 | Russia | 20 | T2DM | 600 | NA | 28 days | 34–62 | garlic powder Tablets/placebo | NA |
| Soleimani, D. [22] | 2020 | Iran | 98 | NAFLD | 800 | 1.5 mg allicin | 15 weeks | 20–70 | enteric garlic powder/placebo | conventional treatment medications |
| Szulinska, Monika [38] | 2018 | Poland | 92 | Obesity | 400 | 8 mg alliin | 3 months | 25–60 | garlic extract capsules/placebo | NA |
| Turner, B. [40] | 2004 | Denmark | 62 | Healthy | 920 | 9 mg alliin | 12 weeks | 40–60 | garlic powder tablets/placebo | NA |
| Vila-Nova, Tiago M. S. [39] | 2024 | Brazil | 28 | hypertensive | 1200 | 1.2 mg SAC | 12 weeks | 19–59 | AGE/placebo | NA |
| Xu, C. [32] | 2018 | US | 48 | Obesity | 3600 | NA | 6 weeks | 25–65 | AGE/placebo | NA |
| Zadhoush, R. [23] | 2021 | Iran | 80 | PCOS | 800 | NA | 8 weeks | 18–45 | garlic pills/placebo | NA |
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias |
|---|---|---|---|---|---|---|---|
| Adam J Adler [19] | unclear | unclear | low | unclear | low | low | unclear |
| Asgharpour, M. [20] | low | low | low | unclear | low | low | unclear |
| Ashraf, R. [24] | unclear | unclear | unclear | unclear | low | low | unclear |
| A. Bordia [27] | unclear | unclear | low | unclear | low | low | unclear |
| Budoff Matthew [30] | unclear | unclear | low | low | low | low | unclear |
| Eun-Soo, Jung [34] | unclear | unclear | low | unclear | low | low | unclear |
| AJAZ FATIMA [25] | unclear | unclear | low | unclear | low | low | unclear |
| Gardner, C. D. [31] | unclear | low | low | unclear | low | low | unclear |
| Gardner, Christopher D. [33] | unclear | low | low | unclear | low | low | unclear |
| Mahdavi-Roshan, M. [28] | unclear | low | unclear | unclear | low | low | unclear |
| Mahdavi-Roshan, Marjan [29] | unclear | unclear | low | unclear | low | low | unclear |
| Peleg, A. [35] | unclear | unclear | low | unclear | low | low | unclear |
| Rizwan Ashraf M. Phil [26] | unclear | unclear | low | unclear | low | low | unclear |
| Abbas Ali Sangouni [21] | low | unclear | low | unclear | low | low | unclear |
| Igor A. Sobenin [36] | unclear | unclear | low | unclear | low | low | unclear |
| Igor A. Sobenin [37] | unclear | unclear | low | unclear | low | low | unclear |
| Soleimani, D. [22] | unclear | unclear | low | low | low | low | unclear |
| Szulinska, Monika [38] | unclear | unclear | low | unclear | low | low | unclear |
| Turner, B. [40] | unclear | unclear | low | unclear | low | low | unclear |
| Vila-Nova, Tiago M. S. [39] | low | unclear | unclear | unclear | low | low | unclear |
| Xu, C. [32] | unclear | unclear | low | unclear | low | low | unclear |
| Zadhoush, R. [23] | low | low | low | unclear | low | low | unclear |
| Index | Subgroup | Mean Difference | p | I2 (%) | p Value of Heterogeneity | |
|---|---|---|---|---|---|---|
| Mean | 95% CI | |||||
| Type of intervention | ||||||
| FBG | AGE | −11.25 | −32.69, 10.19 | 0.304 | 0.0 | 0.873 |
| Other * | −6.98 | −8.51, −5.44 | 0.000 | 93.3 | 0.000 | |
| TC | AGE | −7.49 | −15.81, 0.83 | 0.078 | 0.0 | 0.781 |
| Other | −14.93 | −20.40, −9.45 | 0.000 | 72.5 | 0.000 | |
| LDL | AGE | −9.91 | −22.00, 2.19 | 0.108 | 49.4 | 0.095 |
| Other | −7.95 | −16.29, 0.38 | 0.061 | 94.9 | 0.000 | |
| Population condition | ||||||
| FBG | Hyperlipidemia | / | / | / | / | / |
| T2DM | −7.01 | −8.53, −5.49 | 0.000 | 95.5 | 0.000 | |
| Healthy | / | / | / | / | / | |
| Other ** | −3.83 | −10.04, 2.38 | 0.227 | 36.6 | 0.177 | |
| TC | Hyperlipidemia | −12.23 | −22.43, −2.04 | 0.019 | 72.5 | 0.003 |
| T2DM | −28.54 | −32.73, −24.34 | 0.000 | 0.0 | 0.368 | |
| Healthy | / | / | / | / | / | |
| Other | −9.24 | −14.61, −3.88 | 0.001 | 0.0 | 0.546 | |
| LDL | Hyperlipidemia | −4.58 | −18.79, 9.63 | 0.527 | 96.6 | 0.000 |
| T2DM | −26.11 | −36.81, −15.42 | 0.000 | 57.5 | 0.095 | |
| Healthy | −1.95 | −11.77, 7.87 | 0.697 | 0.0 | 0.575 | |
| Other | −10.97 | −17.73, −4.21 | 0.001 | 29.8 | 0.200 | |
| Duration | ||||||
| FBG | ≤8 week | 7.19 | −7.25, 21.63 | 0.329 | 22.5 | 0.256 |
| >8 week | −7.29 | −8.78, −5.80 | 0.000 | 92.7 | 0.000 | |
| TC | ≤8 week | −5.76 | −12.99, 1.47 | 0.119 | 0.0 | 0.919 |
| >8 week | −16.86 | −22.20, −11.52 | 0.000 | 66.8 | 0.000 | |
| LDL | ≤8 week | −5.64 | −12.14, 0.86 | 0.089 | 0.0 | 0.669 |
| >8 week | −9.04 | −17.25, −0.83 | 0.031 | 93.9 | 0.000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Cheng, T.; Xia, H.; Yang, Y.; Wang, S. Effects of Garlic on Glucose Parameters and Lipid Profile: A Systematic Review and Meta-Analysis on Randomized Controlled Trials. Nutrients 2024, 16, 1692. https://doi.org/10.3390/nu16111692
Zhao X, Cheng T, Xia H, Yang Y, Wang S. Effects of Garlic on Glucose Parameters and Lipid Profile: A Systematic Review and Meta-Analysis on Randomized Controlled Trials. Nutrients. 2024; 16(11):1692. https://doi.org/10.3390/nu16111692
Chicago/Turabian StyleZhao, Xinyu, Tao Cheng, Hui Xia, Yanhong Yang, and Shaokang Wang. 2024. "Effects of Garlic on Glucose Parameters and Lipid Profile: A Systematic Review and Meta-Analysis on Randomized Controlled Trials" Nutrients 16, no. 11: 1692. https://doi.org/10.3390/nu16111692
APA StyleZhao, X., Cheng, T., Xia, H., Yang, Y., & Wang, S. (2024). Effects of Garlic on Glucose Parameters and Lipid Profile: A Systematic Review and Meta-Analysis on Randomized Controlled Trials. Nutrients, 16(11), 1692. https://doi.org/10.3390/nu16111692






