Chemical Compositions before and after Lactic Acid Fermentation of Isoflavone-Enriched Soybean Leaves and Their Anti-Obesity and Gut Microbiota Distribution Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants, Bacteria, Chemicals, and Instruments
2.2. Fermentation of IESLs
2.3. Physicochemical Properties
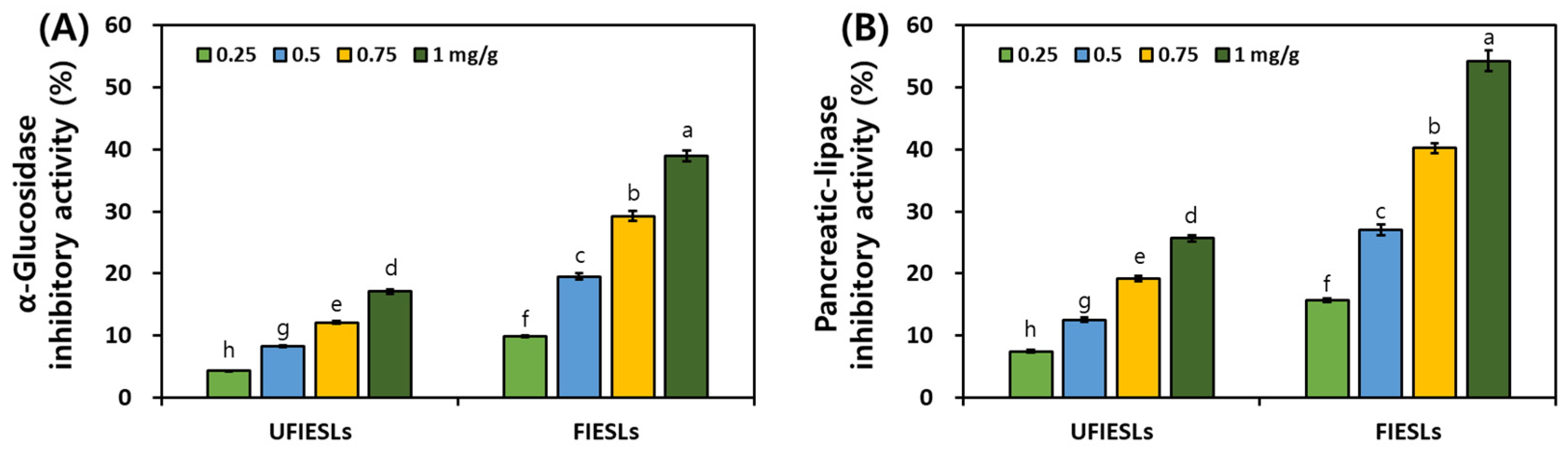
2.4. Analysis of Digestive Enzyme Inhibitory Activities
2.5. Analysis of Fatty Acids
2.6. Analysis of Free Amino Acids
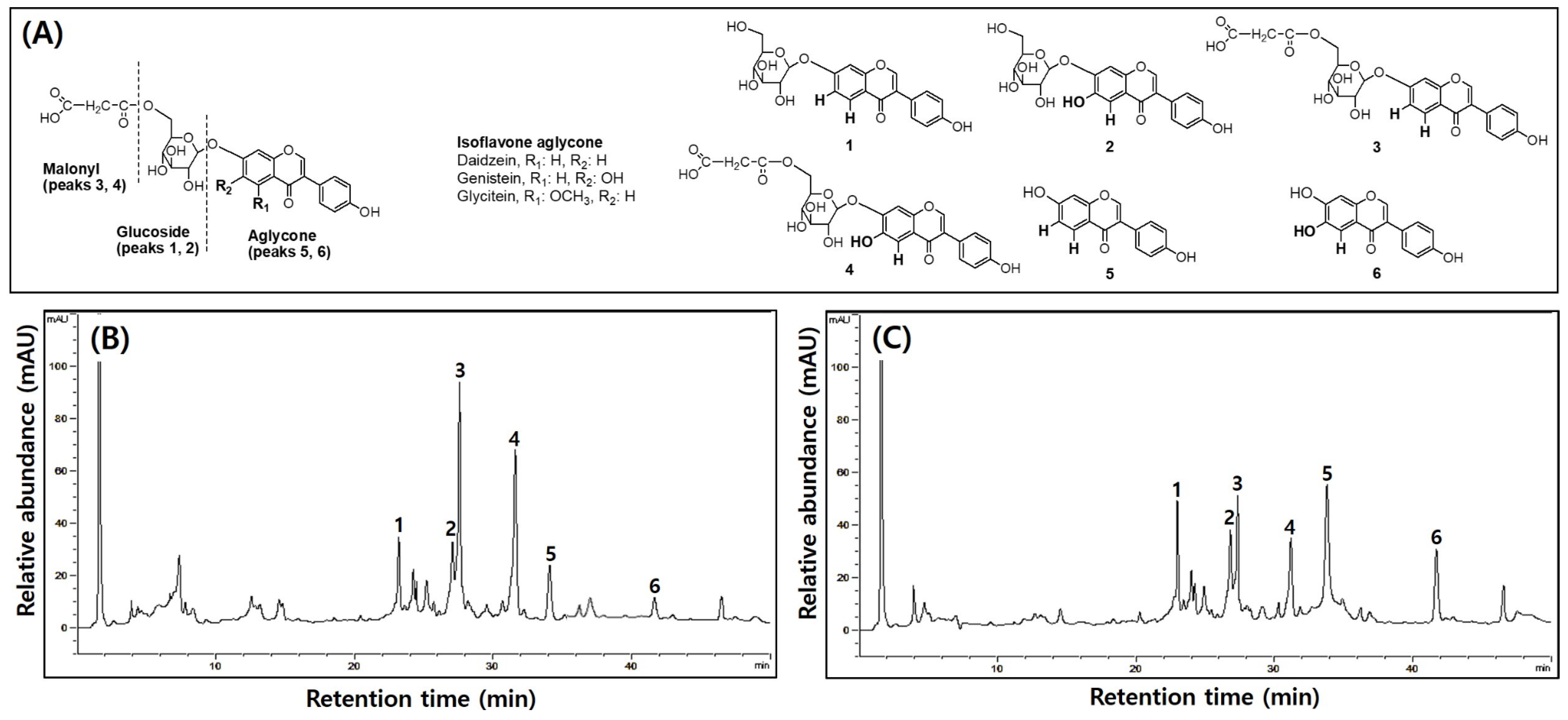
2.7. Analysis of TPCs, TFCs, and Isoflavone Derivatives
2.8. Analysis of Anti-Obesity Effects
2.8.1. Laboratory Animal Specifications
2.8.2. Composition of the Experimental Group, Experimental Diet, and Feeding Amount of the Sample
2.8.3. Dietary Intake, Dietary Efficiency, and Body Weight Measurements
2.8.4. Analysis of Epididymal Fat and Serum Biomarkers
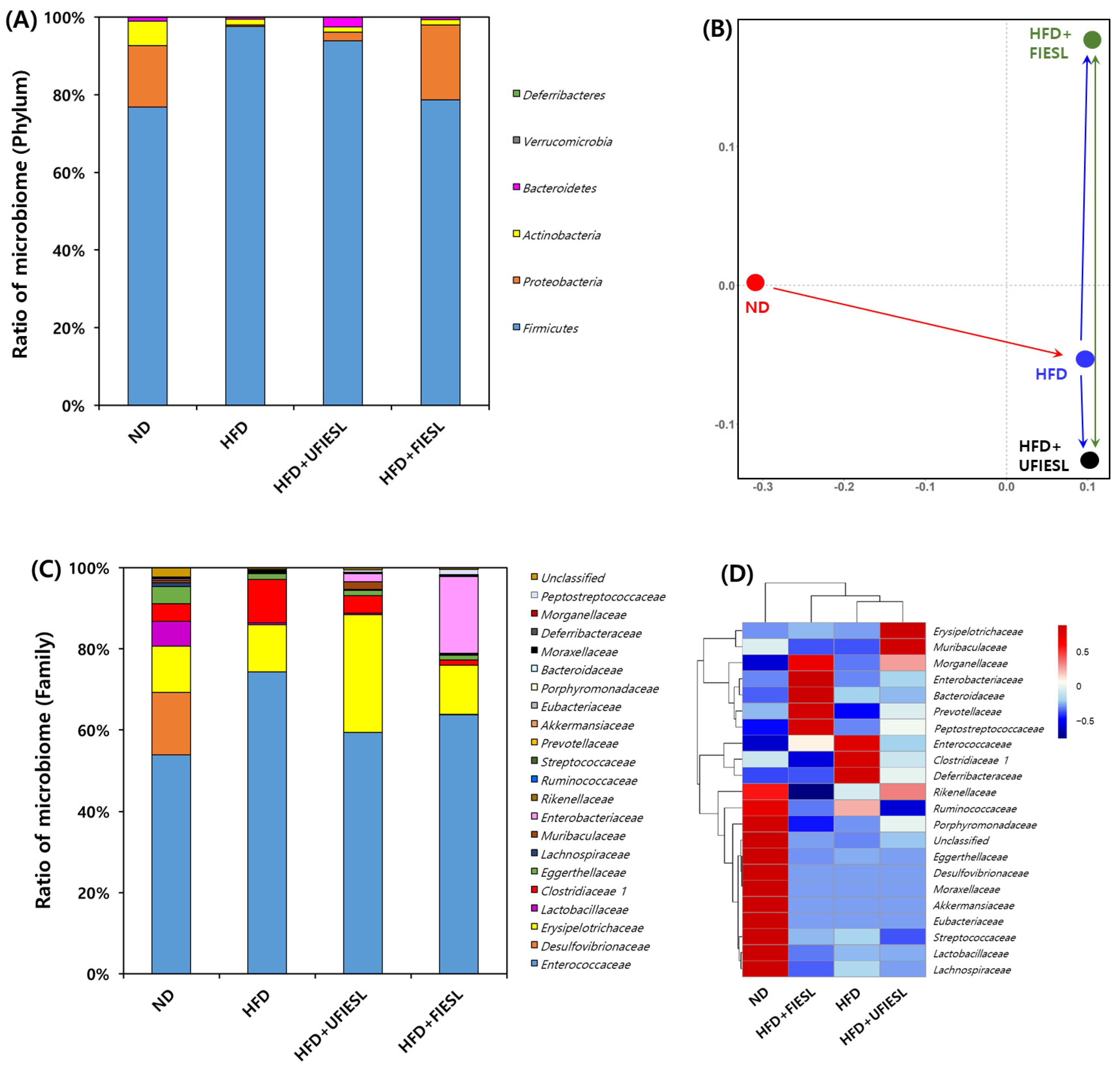
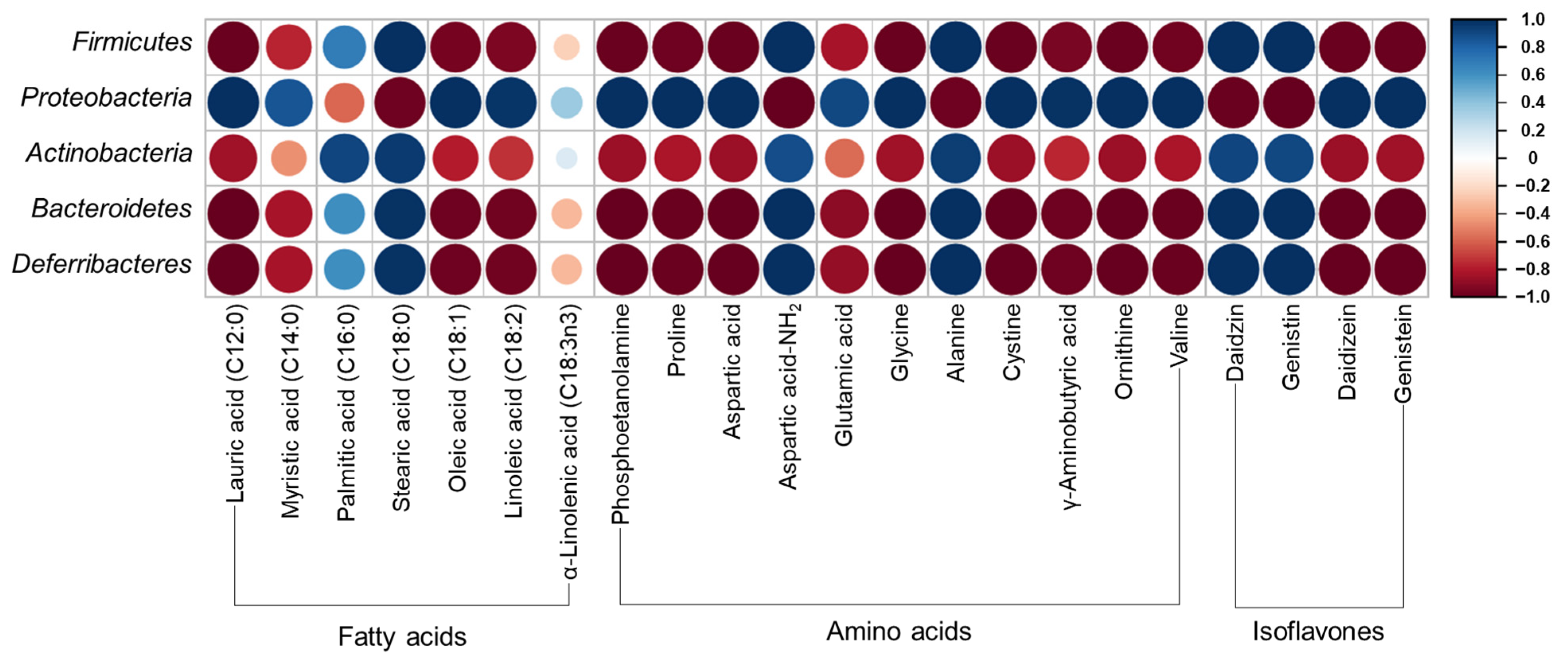
2.9. Analysis of Gut Microbiota
2.10. Statistical Analysis
3. Results and Discussion
3.1. Comparison of the Physicochemical Properties and Viable Cell Numbers in UFIESLs and FIESLs
3.2. Fatty Acid Composition in UFIESLs and FIESLs
3.3. Free Amino Acid Composition in UFIESLs and FIESLs
3.4. Comparison of TPCs, TFCs, and Isoflavone Compositions in UFIESLs and FIESLs
3.5. Comparison of Digestive Enzyme Inhibitory Activities in UFIESLs and FIESLs
3.6. Anti-Obesity Effects in UFIESLs and FIESLs
3.7. Gut Microbiota Distribution in UFIESLs and FIESLs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, H.H.; Ryu, H.W.; Lee, K.J.; Jeong, I.Y.; Kim, D.S.; Oh, S.R. Metabolomics investigation of flavonoid synthesis in soybean leaves depending on the growth stage. Metabolomics 2014, 10, 833–841. [Google Scholar] [CrossRef]
- Zang, Y.; Zhang, L.; Igarashi, K.; Yu, C. The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food Funct. 2015, 6, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.; Ju, J. Glycine max Merr. leaf extract possesses anti-oxidant properties, decreases inflammatory mediator production in murine macrophages, and inhibits growth, migration, and adhesion in human cancer cells. Food Sci. Biotechnol. 2017, 26, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.J.; Song, Y.H.; Curtis-Long, M.J.; Kim, D.W.; Woo, S.G.; Lee, Y.B.; Uddin, Z.; Kim, C.Y.; Park, K.H. Ethylene induced a high accumulation of dietary isoflavones and expression of isoflavonoid biosynthetic genes in soybean (Glycine max) leaves. J. Agric. Food Chem. 2016, 64, 7315–7324. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Cho, D.Y.; Jang, K.J.; Lee, J.H.; Jung, J.G.; Kim, M.J.; Jeong, J.B.; Haque, M.A.; Cho, K.M. Changes of γ-Aminobutyric Acid, Phytoestrogens, and Biofunctional Properties of the Isoflavone-Enriched Soybean (Glycine max) Leaves during Solid Lactic Acid Fermentation. Fermentation 2022, 8, 525. [Google Scholar] [CrossRef]
- Matthias, B. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Chen, Q.; Chen, Z.; He, T.; Zhang, L.; Huang, Q.; Liu, W.; Zeng, X.; Zhang, Y. Anti-obesity effects of mulberry leaf extracts on female high-fat diet-induced obesity: Modulation of white adipose tissue, gut microbiota, and metabolic markers. Food Res. Int. 2024, 177, 113875. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.Y.; Zhao, C.N.; Xu, X.Y.; Tang, G.Y.; Corke, H.; Gan, R.Y.; Li, H.B. Dietary plants, gut microbiota, and obesity: Effects and mechanisms. Trends Food Sci. Technol. 2019, 92, 194–204. [Google Scholar] [CrossRef]
- Postler, T.S.; Ghosh, S. Understanding the holobiont: How microbial metabolites affect human health and shape the immune system. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef]
- Cho, D.-Y.; Lee, H.-Y.; Jeong, J.-B.; Lee, J.-H.; Lee, G.-Y.; Jang, M.-Y.; Lee, J.-H.; Lee, J.-H.; Haque, M.A.; Cho, K.-M. Comprehensive Changes in Nutrient Constituents and Antioxidant Activity during Food Processing of Isoflavone-Enriched Soybean Leaf by Mycelia of Tricholoma matsutake. Fermentation 2023, 9, 677. [Google Scholar] [CrossRef]
- Hwang, C.E.; Kim, S.C.; Lee, H.Y.; Suh, H.K.; Cho, K.M.; Lee, J.H. Enhancement of isoflavone aglycone, amino acid, and CLA contents in fermented soybean yogurts using different strains: Screening of antioxidant and digestive enzyme inhibition properties. Food Chem. 2021, 340, 128199. [Google Scholar] [CrossRef]
- Foodsafetykorea. Available online: https://www.foodsafetykorea.go.kr/portal/board/boardDetail.do (accessed on 3 May 2024).
- Frings, C.S.; Fendley, T.W.; Dunn, R.T.; Queen, C.A. Improved determination of total serum lipids by the sulfo-phospho-vanillin reaction. Clin. Chem. 1972, 18, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: Chichester, UK, 1991; pp. 115–175. [Google Scholar]
- Yu, Y.; Lee, C.; Kim, J.; Hwang, S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng. 2005, 89, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package ‘corrplot’. Statistician 2017, 56, e24. [Google Scholar]
- Fu, R.; Guan, M.; Wang, G.; Pan, L.; Li, Y. Optimised fermentation of bamboo waste using three types lactic acid bacteria and forming mechanism of binderless particleboard. Ind. Crop. Prod. 2023, 196, 116522. [Google Scholar] [CrossRef]
- da Silva Sabo, S.; Vitolo, M.; González, J.M.D.; de Souza Oliveira, R.P. Overview of Lactobacillus plantarum as a promising bacteriocin producer among lactic acid bacteria. Food Res. Int. 2014, 64, 527–536. [Google Scholar] [CrossRef]
- Marnpae, M.; Chusak, C.; Balmori, V.; Kamonsuwan, K.; Dahlan, W.; Nhujak, T.; Hamid, N.; Adisakwattana, S. Probiotic Gac fruit beverage fermented with Lactobacillus paracasei: Physiochemical properties, phytochemicals, antioxidant activities, functional properties, and volatile flavor compounds. LWT 2022, 169, 113986. [Google Scholar] [CrossRef]
- Nagarajan, D.; Oktarina, N.; Chen, P.T.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Fermentative lactic acid production from seaweed hydrolysate using Lactobacillus sp. and Weissella sp. Bioresour. Technol. 2022, 344, 126166. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Guo, X.; Guo, R.; Zhu, L.; Qiu, X.; Yu, X.; Chai, J.; Gu, C.; Feng, Z. A novel strategy for improving the antioxidant, iridoid, and flavor properties of Noni (Morinda citrifolia L.) fruit juice by lactic acid bacteria fermentation. LWT 2023, 184, 115075. [Google Scholar] [CrossRef]
- Fiorino, G.M.; Tlais, A.Z.A.; Losito, I.; Filannino, P.; Gobbetti, M.; Di Cagno, R. Triacylglycerols hydrolysis and hydroxy-and epoxy-fatty acids release during lactic fermentation of plant matrices: An extensive study showing inter-and intra-species capabilities of lactic acid bacteria. Food Chem. 2023, 412, 135552. [Google Scholar] [CrossRef]
- Collins, Y.F.; McSweeney, P.L.; Wilkinson, M.G. Lipolysis and free fatty acid catabolism in cheese: A review of current knowledge. Int. Dairy J. 2003, 13, 841–866. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, J.; Liu, T.; Wang, J.; Cai, H.; Zhang, X.; Xia, D.Q.H.; Feng, F.; Tang, J. Differential modulations of lauric acid and its glycerides on high fat diet-induced metabolic disorders and gut microbiota dysbiosis. Food Res. Int. 2022, 157, 111437. [Google Scholar] [CrossRef]
- Hwang, H.; Lee, J.-H. Characterization of Arginine Catabolism by Lactic Acid Bacteria Isolated from Kimchi. Molecules 2018, 23, 3049. [Google Scholar] [CrossRef]
- Noens, E.E.; Lolkema, J.S. Convergent evolution of the arginine deiminase pathway: The ArcD and ArcE arginine/ornithine exchangers. Microbiologyopen 2017, 6, e00412. [Google Scholar] [CrossRef]
- de Souza, E.L.; de Oliveira, K.Á.; de Oliveira, M.E. Influence of lactic acid bacteria metabolites on physical and chemical food properties. Curr. Opin. Food Sci. 2023, 49, 100981. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Wen, G.; Zhou, J. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef]
- De Montijo-Prieto, S.; Razola-Díaz, M.d.C.; Barbieri, F.; Tabanelli, G.; Gardini, F.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Impact of Lactic Acid Bacteria Fermentation on Phenolic Compounds and Antioxidant Activity of Avocado Leaf Extracts. Antioxidants 2023, 12, 298. [Google Scholar] [CrossRef]
- Ali Asgar, M.D. Anti-diabetic potential of phenolic compounds: A review. Int. J. Food Prop. 2013, 16, 91–103. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; Rosa, L.A.d.l.; Vargas-Requena, C.L.; Bustos-Jaimes, I.; Alvarez-Parrilla, A.E. Polyphenolic Compounds and Digestive Enzymes: In Vitro Non-Covalent Interactions. Molecules 2017, 22, 669. [Google Scholar] [CrossRef]
- Ma, G.; Chai, X.; Hou, G.; Zhao, F.; Meng, Q. Phytochemistry, bioactivities and future prospects of mulberry leaves: A review. Food Chem. 2022, 372, 131335. [Google Scholar] [CrossRef]
- Li, H.; Kang, J.H.; Han, J.M.; Cho, M.H.; Chung, Y.J.; Park, K.H.; Shin, D.-H.; Park, H.-Y.; Choi, M.-S.; Jeong, T.S. Anti-obesity effects of soy leaf via regulation of adipogenic transcription factors and fat oxidation in diet-induced obese mice and 3T3-L1 adipocytes. J. Med. Food 2015, 18, 899–908. [Google Scholar] [CrossRef]
- Choi, M.S.; Ryu, R.; Seo, Y.R.; Jeong, T.S.; Shin, D.H.; Park, Y.B.; Kim, S.R.; Jung, U.J. The beneficial effect of soybean (Glycine max (L.) Merr.) leaf extracts in adults with prediabetes: A randomized placebo controlled trial. Food Funct. 2014, 5, 1621–1630. [Google Scholar] [CrossRef]
- Xie, C.L.; Kang, S.S.; Cho, K.M.; Park, K.H.; Lee, D.H. Isoflavone-enriched soybean (Glycine max) leaves prevents ovariectomy-induced obesity by enhancing fatty acid oxidation. J. Funct. Foods 2018, 43, 165–172. [Google Scholar] [CrossRef]
- Lee, M.R.; Kim, J.E.; Park, J.W.; Kang, M.J.; Choi, H.J.; Bae, S.J.; Choi, Y.W.; Kim, K.M.; Hong, J.T.; Hwang, D.Y. Fermented mulberry (Morus alba) leaves suppress high fat diet-induced hepatic steatosis through amelioration of the inflammatory response and autophagy pathway. BMC Complement. Med. Ther. 2020, 20, 283. [Google Scholar] [CrossRef]
- Son, H.K.; Shin, H.W.; Jang, E.S.; Moon, B.S.; Lee, C.H.; Lee, J.J. Gochujang prepared using rice and wheat koji partially alleviates high-fat diet-induced obesity in rats. Food Sci. Nutr. 2020, 8, 1562–1574. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, X.; Dong, Y.; Shi, L.; Xu, T.; Wu, F. The anti-obesity effect of fermented barley extracts with Lactobacillus plantarum dy-1 and Saccharomyces cerevisiae in diet-induced obese rats. Food Funct. 2017, 8, 1132–1143. [Google Scholar] [CrossRef]
- Zhao, D.; Cao, J.; Jin, H.; Shan, Y.; Fang, J.; Liu, F. Beneficial impacts of fermented celery (Apium graveolens L.) juice on obesity prevention and gut microbiota modulation in high-fat diet fed mice. Food Funct. 2021, 12, 9151–9164. [Google Scholar] [CrossRef]
- Wang, Y.; Han, C.; Cheng, J.; Wang, Z.; Liu, L.; Huang, H.; Liang, Q.; Liu, R.; Ran, B.; Li, W. Fermented Cerasus humilis fruits protect against high-fat diet induced hyperlipidemia which is associated with alteration of gut microbiota. J. Sci. Food Agric. 2023, 103, 2554–2563. [Google Scholar] [CrossRef]
- Xiao, X.; Li, S.; Zhou, X.; Li, M.; Zhang, Y.; Ye, H. The anti-obesogenic effects and underpinning mechanisms of fermented plant-based foods: A review. Trends Food Sci. Technol. 2023, 136, 1–10. [Google Scholar] [CrossRef]
- Ismail, N.A.; Ragab, S.H.; Abd ElBaky, A.; Shoeib, A.R.; Alhosary, Y.; Fekry, D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch. Med. Sci. 2011, 7, 501–507. [Google Scholar] [CrossRef]
- Johnson, E.L.; Heaver, S.L.; Walters, W.A.; Ley, R.E. Microbiome and metabolic disease: Revisiting the bacterial phylum Bacteroidetes. J. Mol. Med. 2017, 95, 1–8. [Google Scholar] [CrossRef]
- Lau, E.; Marques, C.; Pestana, D.; Santoalha, M.; Carvalho, D.; Freitas, P.; Calhau, C. The role of I-FABP as a biomarker of intestinal barrier dysfunction driven by gut microbiota changes in obesity. Nutr. Metab. 2016, 13, 31. [Google Scholar] [CrossRef]
- Shang, Y.; Khafipour, E.; Derakhshani, H.; Sarna, L.K.; Woo, C.W.; Siow, Y.L.; Karmin, O. Short term high fat diet induces obesity-enhancing changes in mouse gut microbiota that are partially reversed by cessation of the high fat diet. Lipids 2017, 52, 499–511. [Google Scholar] [CrossRef]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The Role of Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef]

| Ingredients | Groups (g/100 g Diet) | |
|---|---|---|
| ND | HFD | |
| Corn starch | 15 | 15 |
| Casein | 20 | 20 |
| Cellulose | 5 | 5 |
| Sucrose | 50 | 15 |
| Vitamin mix a | 1 | 1 |
| Mineral mix b | 3.5 | 3.5 |
| L-Cysteine | 0.3 | 0.3 |
| Choline bitartrate | 0.2 | 0.2 |
| Soybean oil | 5 | 5 |
| Lard | - | 35 |
| Groups a | Sample | Daily Dosage |
|---|---|---|
| HFD + UFIESL | Powder of unfermented IESLs | Sample concentration included isoflavone 27 mg/day/60 kg body weight |
| HFD + FIESL | Powder of fermented IESLs | Sample concentration included isoflavone 27 mg/day/60 kg body weight |
| Item a | Samples b | |
|---|---|---|
| UFIESLs | FIESLs | |
| Physicochemical properties | ||
| pH | 4.00 ± 0.20 a | 3.82 ± 0.14 a |
| Acidity (%, as lactic acid) | 0.17 ± 0.00 a | 0.19 ± 0.01 a |
| Reducing sugar (mg/g) | 1.82 ± 0.04 b | 2.75 ± 0.12 a |
| Soluble protein (mg/g) | 7.01 ± 0.28 a | 7.31 ± 0.29 a |
| Viable cell numbers (log CFU/g) | ||
| Lactiplantibacillus plantarum P1201 | nm c | 4.59 ± 0.15 a |
| Levilactobacillus brevis BMK184 | nm | 6.58 ± 0.18 a |
| Total | nm | 11.17 ± 0.23 a |
| Contents (mg/100 g) a | Samples b | |
|---|---|---|
| UFIESLs | FIESLs | |
| Saturated fatty acids (SFAs) | ||
| Lauric acid (C12:0) | 4.90 ± 0.15 b | 8.20 ± 0.29 a |
| Myristic acid (C14:0) | 8.80 ± 0.14 a | 9.10 ± 0.31 a |
| Palmitic acid (C16:0) | 228.80 ± 5.83 a | 225.20 ± 13.61 a |
| Stearic acid (C18:0) | 84.30 ± 3.44 a | 76.00 ± 1.11 b |
| Arachidic acid (C20:0) | 6.60 ± 0.18 a | 6.60 ± 0.27 a |
| Behenic acid (C22:0) | 8.80 ± 0.46 a | 8.90 ± 0.36 a |
| Lignoceric acid (C24:0) | 7.10 ± 0.24 a | 7.10 ± 0.22 a |
| Total | 349.3 ± 5.88 a | 341.1 ± 18.06 a |
| Unsaturated fatty acids (USFAs) | ||
| Palmitoleic acid (C16:1) | 2.40 ± 0.10 a | 2.80 ± 0.14 a |
| Oleic acid (C18:1) | 295.10 ± 9.58 b | 337.90 ± 7.10 a |
| Linoleic acid (C18:2) | 1573.70 ± 33.35 b | 1720.00 ± 117.84 a |
| γ-Linolenic acid (C18:3n6) | 2.20 ± 0.05 a | 2.20 ± 0.08 a |
| α-Linolenic acid (C18:3n3) | 294.80 ± 6.06 a | 297.20 ± 6.66 a |
| Eicosadienoic acid (C20:2) | 2.60 ± 0.09 ab | 3.00 ± 0.09 a |
| Total | 2170.80 ± 28.17 a | 2363.10 ± 114.94 a |
| Total fatty acids (TFAs) | 2520.10 ± 26.23 a | 2704.20 ± 101.14 a |
| Contents (mg/100 g) a | Samples b | |
|---|---|---|
| UFIESLs | FIESLs | |
| Non-essential amino acids (NEAAs) | ||
| Phosphoetanolamine | nd c | 11.37 ± 0.59 a |
| Proline | 113.92 ± 7.19 b | 144.41 ± 9.13 a |
| Hydroxyproline | 4.96 ± 0.26 a | nd |
| Aspartic acid | 99.04 ± 5.68 b | 394.85 ± 13.18 a |
| Serine | 38.64 ± 2.38 b | 50.56 ± 1.22 a |
| Aspartic acid-NH2 | 383.74 ± 20.33 a | 179.94 ± 8.08 b |
| Glutamic acid | 73.60 ± 1.82 a | 76.85 ± 2.44 a |
| Aminoadipic acid | 11.56 ± 0.51 b | 20.46 ± 1.11 a |
| Glycine | 9.03 ± 0.56 b | 19.89 ± 1.12 a |
| Alanine | 65.86 ± 2.64 a | 57.97 ± 3.60 a |
| Citrulline | 1.32 ± 0.04 a | nd |
| α-aminobutyric acid | 14.98 ± 0.56 a | 15.37 ± 0.84 a |
| Cystine | nd | 6.95 ± 0.33 a |
| Tyrosine | 23.60 ± 1.68 a | 1.99 ± 0.13 b |
| β-alanine | 12.62 ± 0.48 a | 18.48 ± 1.46 a |
| β-aminoisobutyric acid | 7.43 ± 0.25 a | 7.10 ± 0.25 a |
| γ-aminobutyric acid | 112.84 ± 5.20 a | 126.01 ± 5.75 a |
| Aminoethanol | 5.04 ± 0.23 a | 6.60 ± 0.15 a |
| Hydroxylysine | 1.25 ± 0.08 a | nd |
| Ornithine | 0.56 ± 0.03 b | 75.40 ± 2.29 a |
| Arginine | 84.18 ± 3.94 a | 0.73 ± 0.05 b |
| Total | 1064.17 ± 15.43 b | 1214.93 ± 23.72 a |
| Essential amino acids (EAAs) | ||
| Threonine | 26.35 ± 1.00 b | 40.95 ± 1.55 a |
| Valine | 66.00 ± 2.38 b | 80.06 ± 4.23 a |
| Methionine | 3.43 ± 0.14 a | 4.09 ± 0.18 a |
| Isoleucine | 41.73 ± 1.85 b | 54.27 ± 1.87 a |
| Leucine | 36.39 ± 1.16 b | 45.75 ± 1.78 a |
| Phenylalanine | 39.44 ± 2.50 b | 53.12 ± 1.88 a |
| Lysine | 20.28 ± 0.75 a | 21.75 ± 1.67 a |
| Histidine | 18.64 ± 0.93 a | 22.80 ± 0.81 a |
| Total | 252.26 ± 4.57 b | 322.79 ± 3.28 a |
| Total amino acids (TAAs) | 1316.43 ± 11.75 b | 1537.72 ± 23.56 a |
| Ammonia | 13.01 ± 0.81 b | 46.04 ± 1.41 a |
| Contents (mg/g) a | Samples b | |
|---|---|---|
| UFIESLs | FIESLs | |
| Total phenolic contents (TPCs) | 2.21 ± 0.08 b | 2.74 ± 0.16 a |
| Total flavonoid contents (TFCs) | 2.18 ± 0.08 b | 2.87 ± 0.06 a |
| Total isoflavone contents (TICs) | 2.64 ± 0.04 a | 2.35 ± 0.02 b |
| Daidzin | 0.75 ± 0.05 a | 0.60 ± 0.01 b |
| Genistin | 0.44 ± 0.01 a | 0.30 ± 0.01 b |
| Malnolyl-β-daidzin | 0.73 ± 0.04 a | 0.56 ± 0.02 b |
| Malnolyl-β-genistin | 0.56 ± 0.02 a | 0.42 ± 0.01 b |
| Daidizein | 0.10 ± 0.01 b | 0.35 ± 0.01 a |
| Genistein | 0.06 ± 0.00 b | 0.12 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-Y.; Cho, D.-Y.; Jeong, J.-B.; Lee, J.-H.; Lee, G.-Y.; Jang, M.-Y.; Lee, J.-H.; Cho, K.-M. Chemical Compositions before and after Lactic Acid Fermentation of Isoflavone-Enriched Soybean Leaves and Their Anti-Obesity and Gut Microbiota Distribution Effects. Nutrients 2024, 16, 1693. https://doi.org/10.3390/nu16111693
Lee H-Y, Cho D-Y, Jeong J-B, Lee J-H, Lee G-Y, Jang M-Y, Lee J-H, Cho K-M. Chemical Compositions before and after Lactic Acid Fermentation of Isoflavone-Enriched Soybean Leaves and Their Anti-Obesity and Gut Microbiota Distribution Effects. Nutrients. 2024; 16(11):1693. https://doi.org/10.3390/nu16111693
Chicago/Turabian StyleLee, Hee-Yul, Du-Yong Cho, Jong-Bin Jeong, Ji-Ho Lee, Ga-Young Lee, Mu-Yeun Jang, Jin-Hwan Lee, and Kye-Man Cho. 2024. "Chemical Compositions before and after Lactic Acid Fermentation of Isoflavone-Enriched Soybean Leaves and Their Anti-Obesity and Gut Microbiota Distribution Effects" Nutrients 16, no. 11: 1693. https://doi.org/10.3390/nu16111693





