Changes in Fecal Short-Chain Fatty Acids in IBS Patients and Effects of Different Interventions: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration and Reporting Format
2.2. Information Sources and Search Strategy
2.3. Study Selection and Eligibility Criteria
2.4. Data Extraction
2.5. Quality Assessment of Included Studies
2.6. Statistical Analysis
2.7. Sensitivity Analysis and Publication Bias Assessment
3. Results
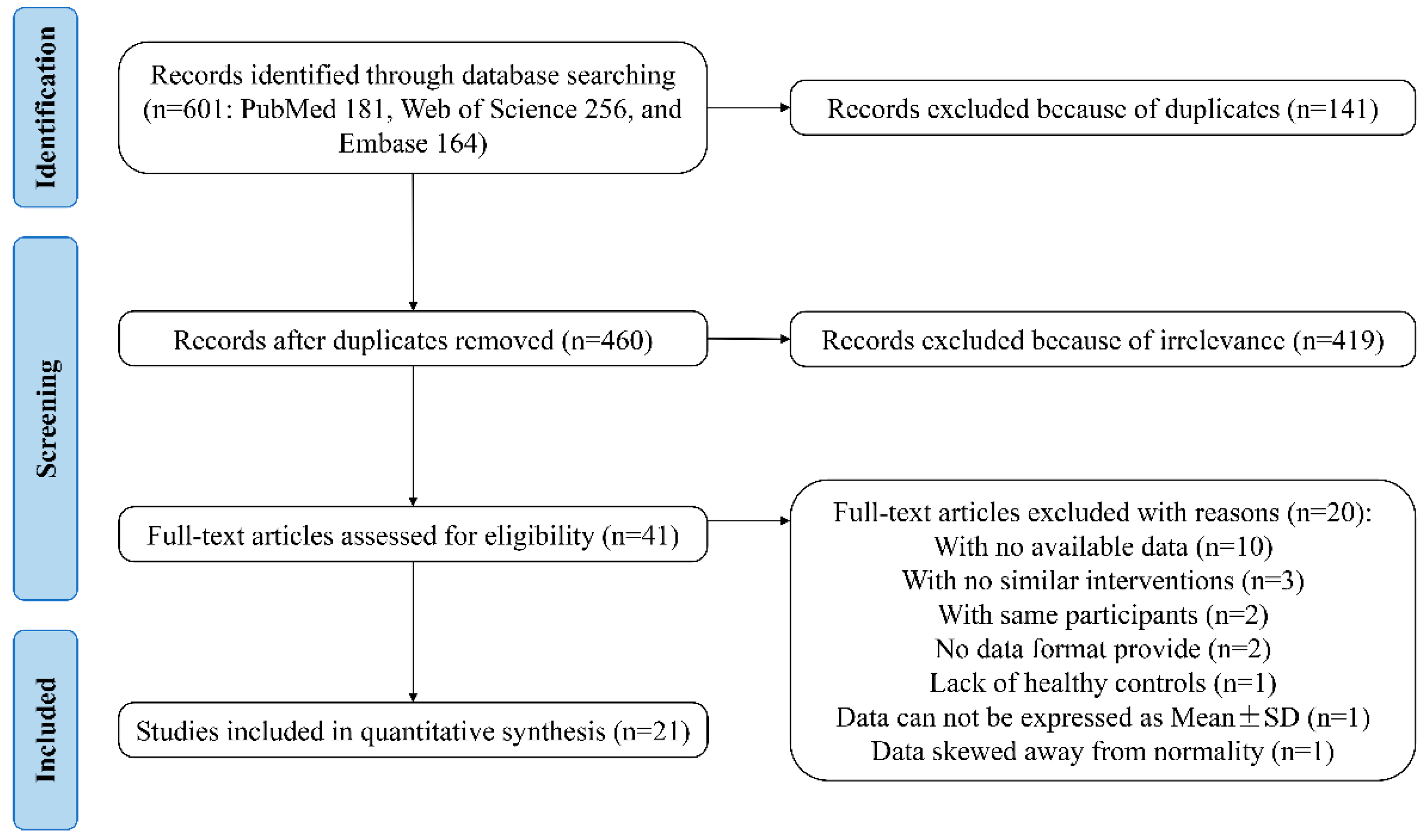
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality Assessment of Studies
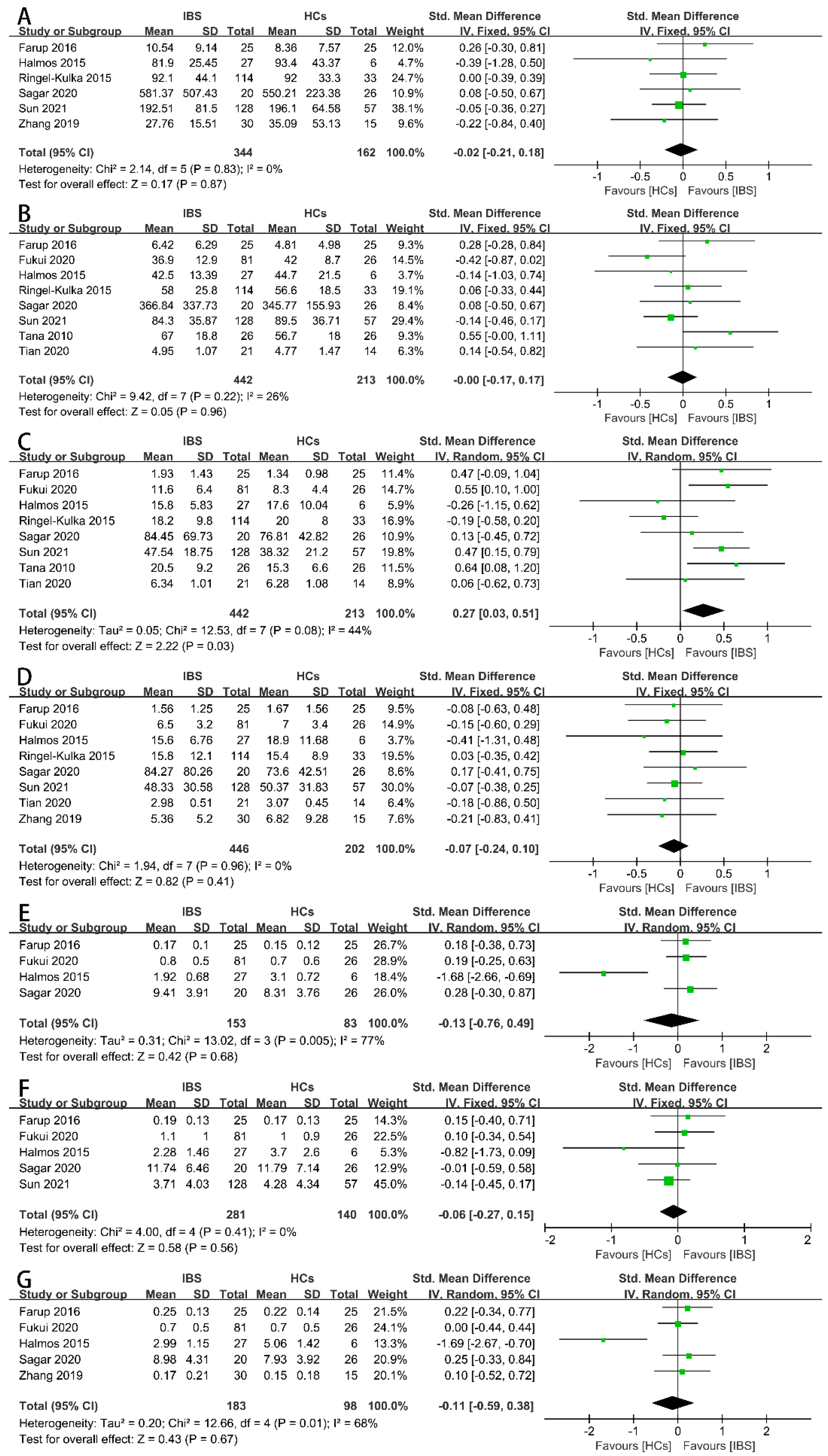
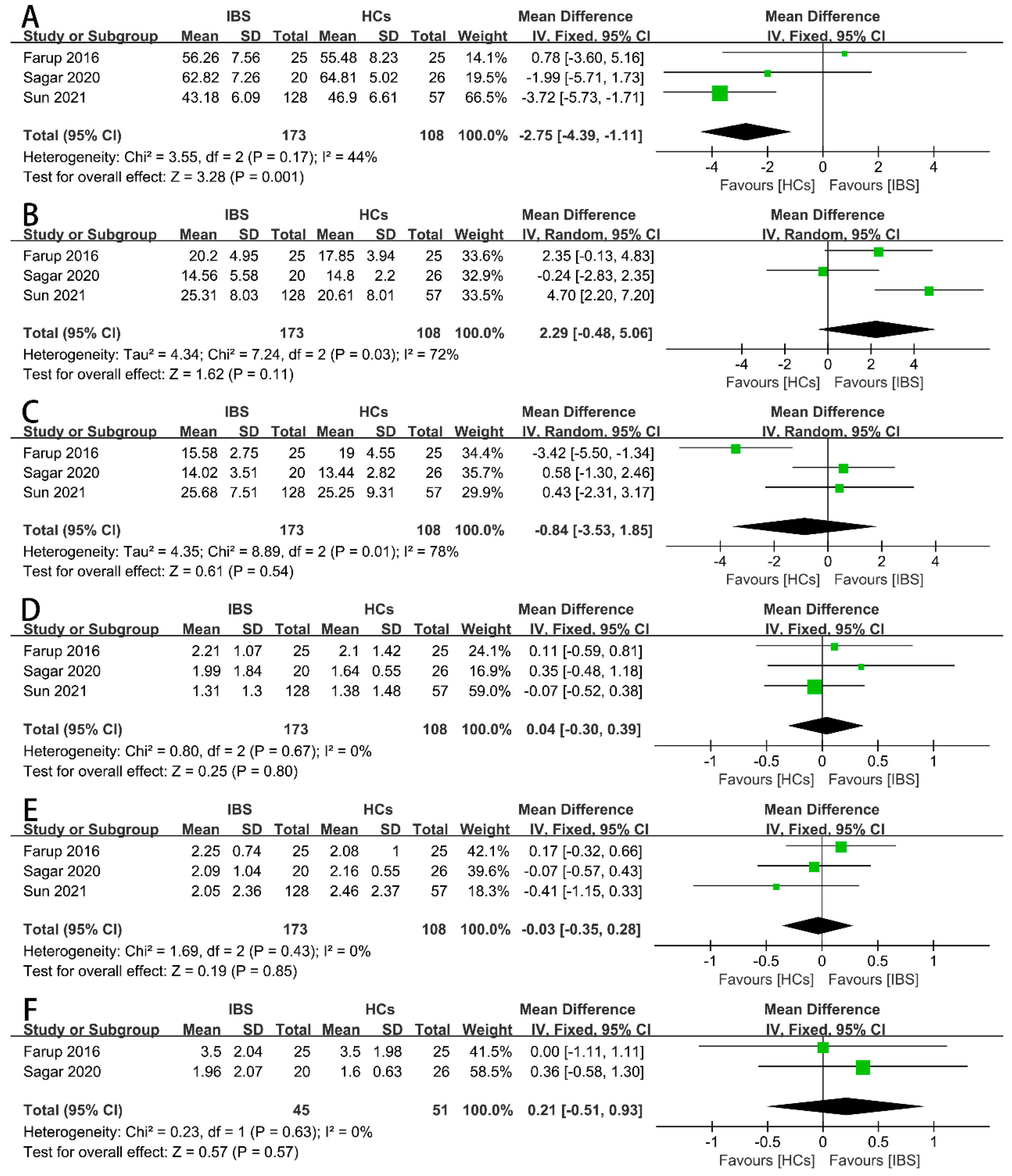
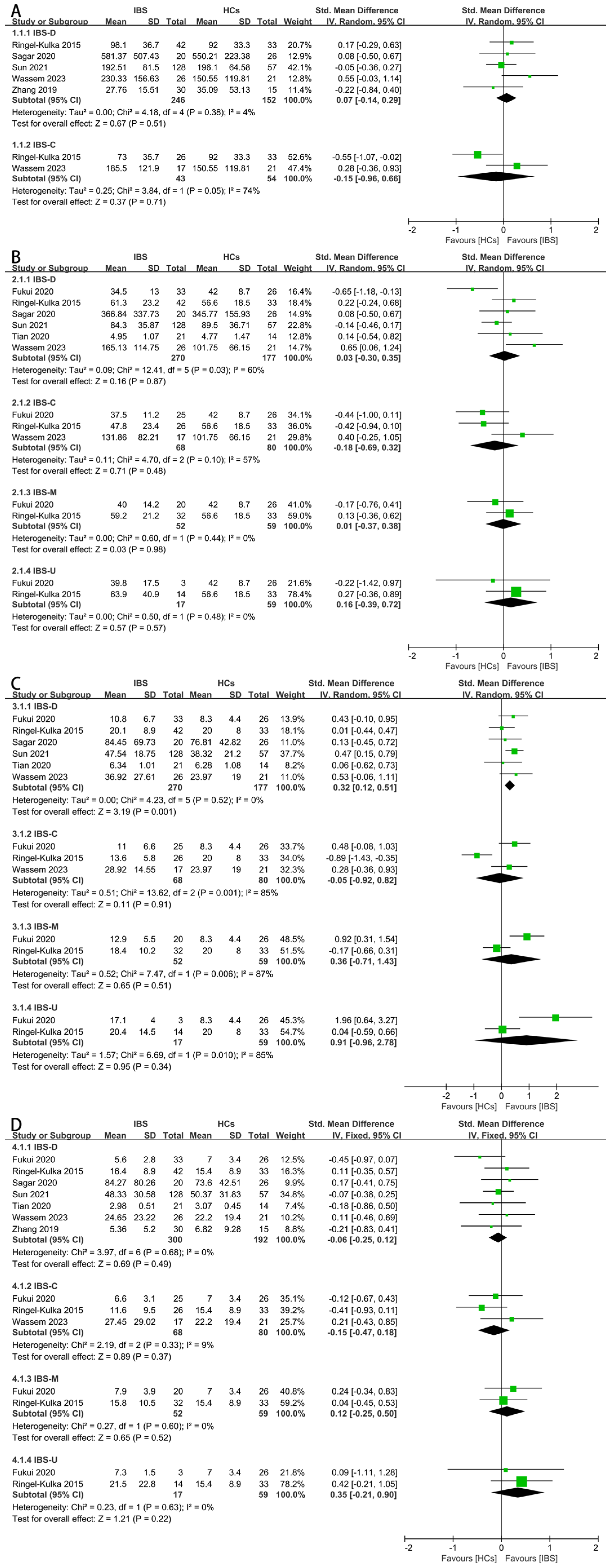
3.4. Results of Synthesis
3.5. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef]
- Zweig, A.; Schindler, V.; Becker, A.S.; van Maren, A.; Pohl, D. Higher prevalence of joint hypermobility in constipation predominant irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30, e13353. [Google Scholar] [CrossRef]
- Pimentel, M.; Lembo, A. Microbiome and Its Role in Irritable Bowel Syndrome. Dig. Dis. Sci. 2020, 65, 829–839. [Google Scholar] [CrossRef]
- Chey, W.D.; Kurlander, J.; Eswaran, S. Irritable bowel syndrome: A clinical review. JAMA 2015, 313, 949–958. [Google Scholar] [CrossRef]
- Mamieva, Z.; Poluektova, E.; Svistushkin, V.; Sobolev, V.; Shifrin, O.; Guarner, F.; Ivashkin, V. Antibiotics, gut microbiota, and irritable bowel syndrome: What are the relations? World J. Gastroenterol. 2022, 28, 1204–1219. [Google Scholar] [CrossRef]
- Dupont, H.L. Review article: Evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment. Pharmacol. Ther. 2014, 39, 1033–1042. [Google Scholar] [CrossRef]
- Kassinen, A.; Krogius-Kurikka, L.; Mäkivuokko, H.; Rinttilä, T.; Paulin, L.; Corander, J.; Malinen, E.; Apajalahti, J.; Palva, A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 2007, 133, 24–33. [Google Scholar] [CrossRef]
- Masoodi, I.; Alshanqeeti, A.S.; Alyamani, E.J.; AlLehibi, A.A.; Alqutub, A.N.; Alsayari, K.N.; Alomair, A.O. Microbial dysbiosis in irritable bowel syndrome: A single-center metagenomic study in Saudi Arabia. JGH Open 2020, 4, 649–655. [Google Scholar] [CrossRef]
- Carroll, I.M.; Ringel-Kulka, T.; Siddle, J.P.; Ringel, Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 2012, 24, 521–530.e248. [Google Scholar] [CrossRef]
- Ahluwalia, B.; Iribarren, C.; Magnusson, M.K.; Sundin, J.; Clevers, E.; Savolainen, O.; Ross, A.B.; Törnblom, H.; Simrén, M.; Öhman, L. A Distinct Faecal Microbiota and Metabolite Profile Linked to Bowel Habits in Patients with Irritable Bowel Syndrome. Cells 2021, 10, 1459. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Sciume, G.D.; Berti, G.; Lambiase, C.; Paglianiti, I.; Villanacci, V.; Rettura, F.; Grosso, A.; Ricchiuti, A.; Bortoli, N.; Usai Satta, P.; et al. Misinterpreting Diarrhea-Predominant Irritable Bowel Syndrome and Functional Diarrhea: Pathophysiological Highlights. J. Clin. Med. 2023, 12, 5787. [Google Scholar] [CrossRef]
- Tana, C.; Umesaki, Y.; Imaoka, A.; Handa, T.; Kanazawa, M.; Fukudo, S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol. Motil. 2010, 22, 512-e115. [Google Scholar] [CrossRef]
- Treem, W.R.; Ahsan, N.; Kastoff, G.; Hyams, J.S. Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: In vitro studies of carbohydrate fermentation. J. Pediatr. Gastroenterol. Nutr. 1996, 23, 280–286. [Google Scholar] [CrossRef]
- Tian, Z.; Zhuang, X.; Luo, M.; Yin, W.; Xiong, L. The propionic acid and butyric acid in serum but not in feces are increased in patients with diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2020, 20, 73. [Google Scholar] [CrossRef]
- Xie, C.R.; Tang, B.; Shi, Y.Z.; Peng, W.Y.; Ye, K.; Tao, Q.F.; Yu, S.G.; Zheng, H.; Chen, M. Low FODMAP Diet and Probiotics in Irritable Bowel Syndrome: A Systematic Review with Network Meta-analysis. Front. Pharmacol. 2022, 13, 853011. [Google Scholar] [CrossRef]
- Varjú, P.; Farkas, N.; Hegyi, P.; Garami, A.; Szabó, I.; Illés, A.; Solymár, M.; Vincze, Á.; Balaskó, M.; Pár, G.; et al. Low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet improves symptoms in adults suffering from irritable bowel syndrome (IBS) compared to standard IBS diet: A meta-analysis of clinical studies. PLoS ONE 2017, 12, e0182942. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, P.; Zhang, L.; Hou, X. A Low-FODMAP Diet Improves the Global Symptoms and Bowel Habits of Adult IBS Patients: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 683191. [Google Scholar] [CrossRef] [PubMed]
- Myneedu, K.; Deoker, A.; Schmulson, M.J.; Bashashati, M. Fecal microbiota transplantation in irritable bowel syndrome: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2019, 7, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, Ed000142. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D. The power of the standard test for the presence of heterogeneity in meta-analysis. Stat. Med. 2006, 25, 2688–2699. [Google Scholar] [CrossRef] [PubMed]
- Hustoft, T.N.; Hausken, T.; Ystad, S.O.; Valeur, J.; Brokstad, K.; Hatlebakk, J.G.; Lied, G.A. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2017, 29, e12969. [Google Scholar] [CrossRef] [PubMed]
- Gargari, G.; Taverniti, V.; Gardana, C.; Cremon, C.; Canducci, F.; Pagano, I.; Barbaro, M.R.; Bellacosa, L.; Castellazzi, A.M.; Valsecchi, C.; et al. Fecal Clostridiales distribution and short-chain fatty acids reflect bowel habits in irritable bowel syndrome. Environ. Microbiol. 2018, 20, 3201–3213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, L.; Wang, X.; Fox, M.; Luo, L.; Du, L.; Chen, B.; Chen, X.; He, H.; Zhu, S.; et al. Low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet compared with traditional dietary advice for diarrhea-predominant irritable bowel syndrome: A parallel-group, randomized controlled trial with analysis of clinical and microbiological factors associated with patient outcomes. Am. J. Clin. Nutr. 2021, 113, 1531–1545. [Google Scholar] [CrossRef]
- Lin, H.; Guo, Q.; Wen, Z.; Tan, S.; Chen, J.; Lin, L.; Chen, P.; He, J.; Wen, J.; Chen, Y. The multiple effects of fecal microbiota transplantation on diarrhea-predominant irritable bowel syndrome (IBS-D) patients with anxiety and depression behaviors. Microb. Cell Fact. 2021, 20, 233. [Google Scholar] [CrossRef]
- Fredericks, E.; Theunissen, R.; Roux, S. Short chain fatty acids and monocarboxylate transporters in irritable bowel syndrome. Turk. J. Gastroenterol. 2020, 31, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Dale, H.F.; Jensen, C.; Hausken, T.; Valeur, J.; Hoff, D.A.L.; Lied, G.A. Effects of a Cod Protein Hydrolysate Supplement on Symptoms, Gut Integrity Markers and Fecal Fermentation in Patients with Irritable Bowel Syndrome. Nutrients 2019, 11, 1635. [Google Scholar] [CrossRef] [PubMed]
- Acosta, A.; Camilleri, M.; Shin, A.; Linker Nord, S.; O’Neill, J.; Gray, A.V.; Lueke, A.J.; Donato, L.J.; Burton, D.D.; Szarka, L.A.; et al. Effects of Rifaximin on Transit, Permeability, Fecal Microbiome, and Organic Acid Excretion in Irritable Bowel Syndrome. Clin. Transl. Gastroenterol. 2016, 7, e173. [Google Scholar] [CrossRef] [PubMed]
- Remes-Troche, J.M.; Taboada-Liceaga, H.; Gill, S.; Amieva-Balmori, M.; Rossi, M.; Hernández-Ramírez, G.; García-Mazcorro, J.F.; Whelan, K. Nopal fiber (Opuntia ficus-indica) improves symptoms in irritable bowel syndrome in the short term: A randomized controlled trial. Neurogastroenterol. Motil. 2021, 33, e13986. [Google Scholar] [CrossRef] [PubMed]
- Cremon, C.; Guglielmetti, S.; Gargari, G.; Taverniti, V.; Castellazzi, A.M.; Valsecchi, C.; Tagliacarne, C.; Fiore, W.; Bellini, M.; Bertani, L.; et al. Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: A pilot randomized clinical trial. United Eur. Gastroenterol. J. 2018, 6, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Rossi, M.; Kaminski, T.; Dimidi, E.; Ralph, F.S.E.; Wilson, B.; Martin, L.D.; Louis, P.; Lomer, M.C.E.; Irving, P.M.; et al. Long-term personalized low FODMAP diet improves symptoms and maintains luminal Bifidobacteria abundance in irritable bowel syndrome. Neurogastroenterol. Motil. 2022, 34, e14241. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Valeur, J.; Hausken, T.; Gunnar Hatlebakk, J. Changes in fecal short-chain fatty acids following fecal microbiota transplantation in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2021, 33, e13983. [Google Scholar] [CrossRef]
- El-Salhy, M.; Casen, C.; Valeur, J.; Hausken, T.; Hatlebakk, J.G. Responses to faecal microbiota transplantation in female and male patients with irritable bowel syndrome. World J. Gastroenterol. 2021, 27, 2219–2237. [Google Scholar] [CrossRef]
- Mujagic, Z.; Jonkers, D.; Ludidi, S.; Keszthelyi, D.; Hesselink, M.A.; Weerts, Z.; Kievit, R.N.; Althof, J.F.; Leue, C.; Kruimel, J.W.; et al. Biomarkers for visceral hypersensitivity in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2017, 29, e13137. [Google Scholar] [CrossRef]
- Walton, C.; Fowler, D.P.; Turner, C.; Jia, W.; Whitehead, R.N.; Griffiths, L.; Dawson, C.; Waring, R.H.; Ramsden, D.B.; Cole, J.A.; et al. Analysis of volatile organic compounds of bacterial origin in chronic gastrointestinal diseases. Inflamm. Bowel Dis. 2013, 19, 2069–2078. [Google Scholar] [CrossRef]
- Xu, C.; Jia, Q.; Zhang, L.; Wang, Z.; Zhu, S.; Wang, X.; Liu, Y.; Li, M.; Zhang, J.; Wang, X.; et al. Multiomics Study of Gut Bacteria and Host Metabolism in Irritable Bowel Syndrome and Depression Patients. Front. Cell. Infect. Microbiol. 2020, 10, 580980. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, M.A.; Walton, B.A.; Brydon, W.G.; Anderson, J.R. Faecal weight, constituents, colonic motility, and lactose tolerance in the irritable bowel syndrome. Digestion 1984, 30, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, G.; Noor, S.O.; Ridgway, K.; Scovell, L.; Jamieson, C.; Johnson, I.T.; Colquhoun, I.J.; Kemsley, E.K.; Narbad, A. Metabolomics of fecal extracts detects altered metabolic activity of gut microbiota in ulcerative colitis and irritable bowel syndrome. J. Proteome Res. 2011, 10, 4208–4218. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yamashita, R.; Kawashima, J.; Mori, H.; Kurokawa, K.; Fukuda, S.; Gotoh, Y.; Nakamura, K.; Hayashi, T.; Kasahara, Y.; et al. Omics profiles of fecal and oral microbiota change in irritable bowel syndrome patients with diarrhea and symptom exacerbation. J. Gastroenterol. 2022, 57, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Rijnaarts, I.; Hermes, G.D.A.; de Roos, N.M.; Witteman, B.J.M.; de Wit, N.J.W.; Govers, C.; Smidt, H.; Zoetendal, E.G. Fecal Microbiota Signatures Are Not Consistently Related to Symptom Severity in Irritable Bowel Syndrome. Dig. Dis. Sci. 2022, 67, 5137–5148. [Google Scholar] [CrossRef]
- Farup, P.G.; Rudi, K.; Hestad, K. Faecal short-chain fatty acids—A diagnostic biomarker for irritable bowel syndrome? BMC Gastroenterol. 2016, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Nishida, A.; Matsuda, S.; Kira, F.; Watanabe, S.; Kuriyama, M.; Kawakami, K.; Aikawa, Y.; Oda, N.; Arai, K.; et al. Usefulness of Machine Learning-Based Gut Microbiome Analysis for Identifying Patients with Irritable Bowels Syndrome. J. Clin. Med. 2020, 9, 2403. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, P.B.; Andersen, J.R.; Arffmann, S.; Krag, E. Short-chain fatty acids and the irritable bowel syndrome: The effect of wheat bran. Scand. J. Gastroenterol. 1987, 22, 185–192. [Google Scholar] [CrossRef]
- Ringel-Kulka, T.; Choi, C.H.; Temas, D.; Kim, A.; Maier, D.M.; Scott, K.; Galanko, J.A.; Ringel, Y. Altered Colonic Bacterial Fermentation as a Potential Pathophysiological Factor in Irritable Bowel Syndrome. Am. J. Gastroenterol. 2015, 110, 1339–1346. [Google Scholar] [CrossRef]
- Sagar, N.M.; Duboc, H.; Kay, G.L.; Alam, M.T.; Wicaksono, A.N.; Covington, J.A.; Quince, C.; Kokkorou, M.; Svolos, V.; Palmieri, L.J.; et al. The pathophysiology of bile acid diarrhoea: Differences in the colonic microbiome, metabolome and bile acids. Sci. Rep. 2020, 10, 20436. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.H.; Liu, Z.J.; Zhang, L.; Wei, H.; Song, L.J.; Zhu, S.W.; He, M.B.; Duan, L.P. Sex-based differences in fecal short-chain fatty acid and gut microbiota in irritable bowel syndrome patients. J. Dig. Dis. 2021, 22, 246–255. [Google Scholar] [CrossRef]
- Vernia, P.; Latella, G.; Magliocca, F.M.; Mancuso, G.; Caprilli, R. Seeking clues for a positive diagnosis of the irritable bowel syndrome. Eur. J. Clin. Investig. 1987, 17, 189–193. [Google Scholar] [CrossRef]
- Waseem, M.R.; Shin, A.; Siwiec, R.; James-Stevenson, T.; Bohm, M.; Rogers, N.; Wo, J.; Waseem, L.; Gupta, A.; Jarrett, M.; et al. Associations of Fecal Short Chain Fatty Acids with Colonic Transit, Fecal Bile Acid, and Food Intake in Irritable Bowel Syndrome. Clin. Transl. Gastroenterol. 2023, 14, e00541. [Google Scholar] [CrossRef]
- Zhang, W.X.; Zhang, Y.; Qin, G.; Li, K.M.; Wei, W.; Li, S.Y.; Yao, S.K. Altered profiles of fecal metabolites correlate with visceral hypersensitivity and may contribute to symptom severity of diarrhea-predominant irritable bowel syndrome. World J. Gastroenterol. 2019, 25, 6416–6429. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Lomer, M.C.E.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable Carbohydrate Restriction Reduces Luminal Bifidobacteria and Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Codling, C.; O’Mahony, L.; Shanahan, F.; Quigley, E.M.; Marchesi, J.R. A molecular analysis of fecal and mucosal bacterial communities in irritable bowel syndrome. Dig. Dis. Sci. 2010, 55, 392–397. [Google Scholar] [CrossRef]
- Carroll, I.M.; Ringel-Kulka, T.; Keku, T.O.; Chang, Y.H.; Packey, C.D.; Sartor, R.B.; Ringel, Y. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G799–G807. [Google Scholar] [CrossRef]
- Jeffery, I.B.; Quigley, E.M.; Öhman, L.; Simrén, M.; O’Toole, P.W. The microbiota link to irritable bowel syndrome: An emerging story. Gut Microbes 2012, 3, 572–576. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, N.; Pluznick, J.L. From microbe to man: The role of microbial short chain fatty acid metabolites in host cell biology. Am. J. Physiol. Cell Physiol. 2014, 307, C979–C985. [Google Scholar] [CrossRef] [PubMed]
- Bashashati, M.; Rezaei, N.; Andrews, C.N.; Chen, C.Q.; Daryani, N.E.; Sharkey, K.A.; Storr, M.A. Cytokines and irritable bowel syndrome: Where do we stand? Cytokine 2012, 57, 201–209. [Google Scholar] [CrossRef]
- Miller, T.L.; Wolin, M.J. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl. Environ. Microbiol. 1996, 62, 1589–1592. [Google Scholar] [CrossRef] [PubMed]
- Załęski, A.; Banaszkiewicz, A.; Walkowiak, J. Butyric acid in irritable bowel syndrome. Prz. Gastroenterol. 2013, 8, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Malinen, E.; Rinttilä, T.; Kajander, K.; Mättö, J.; Kassinen, A.; Krogius, L.; Saarela, M.; Korpela, R.; Palva, A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am. J. Gastroenterol. 2005, 100, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Michl, T.; Jocic, M.; Heinemann, A.; Schuligoi, R.; Holzer, P. Vagal afferent signaling of a gastric mucosal acid insult to medullary, pontine, thalamic, hypothalamic and limbic, but not cortical, nuclei of the rat brain. Pain 2001, 92, 19–27. [Google Scholar] [CrossRef] [PubMed]
- De la Cuesta-Zuluaga, J.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2018, 11, 51. [Google Scholar] [CrossRef]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Luo, M.; Zhuang, X.; Tian, Z.; Xiong, L. Alterations in short-chain fatty acids and serotonin in irritable bowel syndrome: A systematic review and meta-analysis. BMC Gastroenterol. 2021, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Jia, Q.; Song, L.; Duan, L. Alterations in fecal short-chain fatty acids in patients with irritable bowel syndrome: A systematic review and meta-analysis. Medicine 2019, 98, e14513. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Tatewaki, M.; Yamada, T.; Fujimiya, M.; Mantyh, C.; Voss, M.; Eubanks, S.; Harris, M.; Pappas, T.N.; Takahashi, T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1269–R1276. [Google Scholar] [CrossRef] [PubMed]
- Nybacka, S.; Tornblom, H.; Josefsson, A.; Hreinsson, J.P.; Bohn, L.; Frandemark, A.; Weznaver, C.; Storsrud, S.; Simren, M. A low FODMAP diet plus traditional dietary advice versus a low-carbohydrate diet versus pharmacological treatment in irritable bowel syndrome (CARIBS): A single-centre, single-blind, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2024, 9, 507–520. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Yao, C.K.; Gill, P.A.; Thwaites, P.A.; Ardalan, Z.S.; McSweeney, C.S.; Denman, S.E.; Chrimes, A.F.; Muir, J.G.; Berean, K.J.; et al. Detection of changes in regional colonic fermentation in response to supplementing a low FODMAP diet with dietary fibres by hydrogen concentrations, but not by luminal pH. Aliment. Pharmacol. Ther. 2023, 58, 417–428. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Loughman, A.; Staudacher, H.M. Effects of a low FODMAP diet on the colonic microbiome in irritable bowel syndrome: A systematic review with meta-analysis. Am. J. Clin. Nutr. 2022, 116, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Eetemadi, A.; Tagkopoulos, I. Methane and fatty acid metabolism pathways are predictive of Low-FODMAP diet efficacy for patients with irritable bowel syndrome. Clin. Nutr. 2021, 40, 4414–4421. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Chen, V.L.; Steiner, C.A.; Berinstein, J.A.; Eswaran, S.; Waljee, A.K.; Higgins, P.D.R.; Owyang, C. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2019, 114, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lv, L.; Wang, C. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Meta-Analysis of Randomized Controlled Trials. Front. Cell. Infect. Microbiol. 2022, 12, 827395. [Google Scholar] [CrossRef]
- Ianiro, G.; Eusebi, L.H.; Black, C.J.; Gasbarrini, A.; Cammarota, G.; Ford, A.C. Systematic review with meta-analysis: Efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2019, 50, 240–248. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V.; Karakan, T.; Karatas, A. Strain-specific and outcome-specific efficacy of probiotics for the treatment of irritable bowel syndrome: A systematic review and meta-analysis. EClinicalMedicine 2021, 41, 101154. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, X.; Jiang, Z.; Ma, J.; Yang, D. Changes in Fecal Short-Chain Fatty Acids in IBS Patients and Effects of Different Interventions: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1727. https://doi.org/10.3390/nu16111727
Ju X, Jiang Z, Ma J, Yang D. Changes in Fecal Short-Chain Fatty Acids in IBS Patients and Effects of Different Interventions: A Systematic Review and Meta-Analysis. Nutrients. 2024; 16(11):1727. https://doi.org/10.3390/nu16111727
Chicago/Turabian StyleJu, Xuan, Zhenliang Jiang, Jiayin Ma, and Dong Yang. 2024. "Changes in Fecal Short-Chain Fatty Acids in IBS Patients and Effects of Different Interventions: A Systematic Review and Meta-Analysis" Nutrients 16, no. 11: 1727. https://doi.org/10.3390/nu16111727
APA StyleJu, X., Jiang, Z., Ma, J., & Yang, D. (2024). Changes in Fecal Short-Chain Fatty Acids in IBS Patients and Effects of Different Interventions: A Systematic Review and Meta-Analysis. Nutrients, 16(11), 1727. https://doi.org/10.3390/nu16111727






