Placenta-Related Parameters at Delivery in Relation to Folic Acid Supplementation in Different Pregnancies
Abstract
1. Introduction
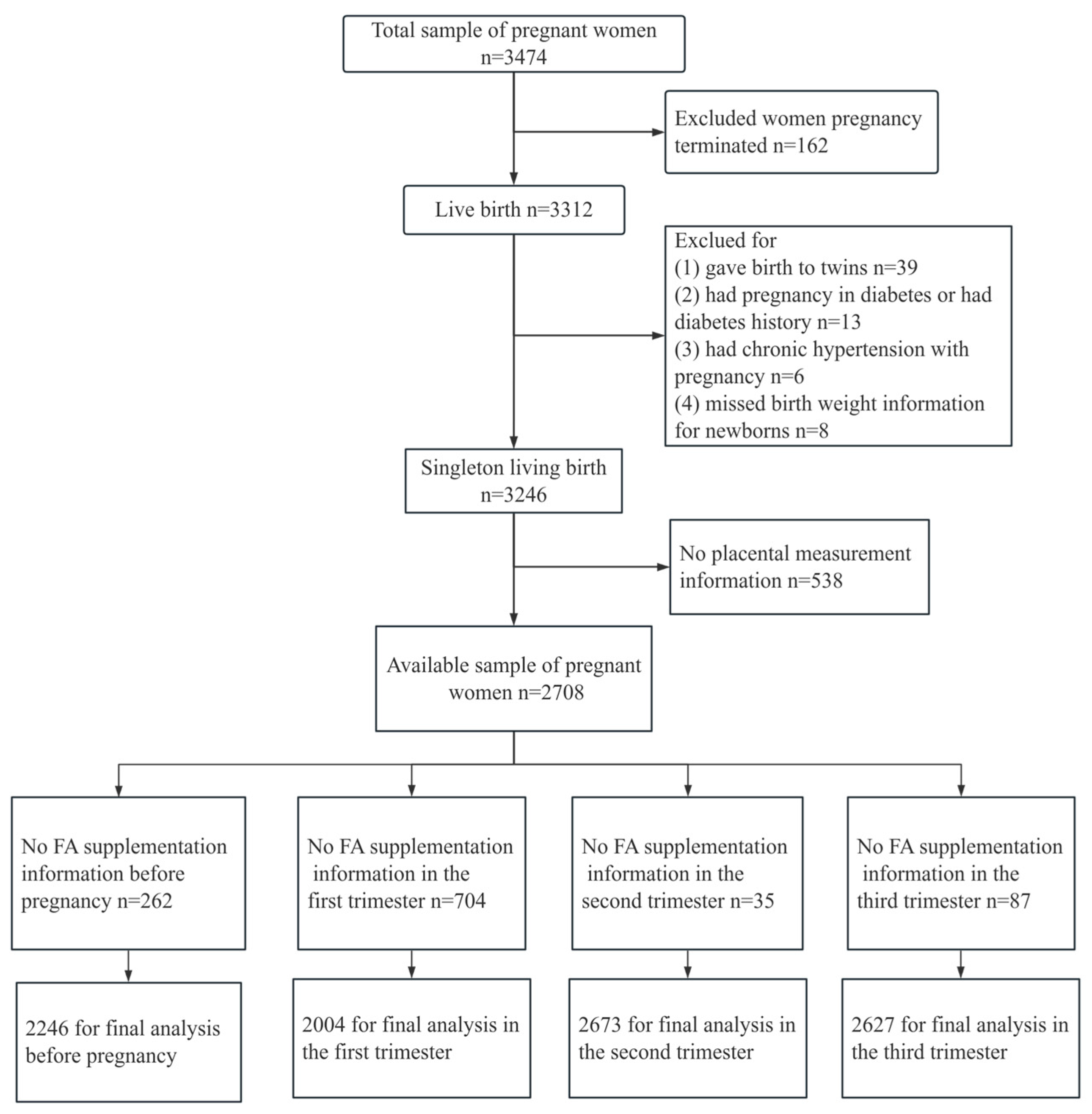
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Folic Acid Supplementation
2.4. Measurements of Placental Size and Shape
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Folic Acid Supplementation in Different Pregnancies
3.3. Placenta-Related Parameters Compared According to FA Use in Different Pregnancies
3.4. Association of FA Supplementation in Different Pregnancies with Placenta-Related Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alcazar Magana, A.; Reed, R.L.; Koluda, R.; Miranda, C.L.; Maier, C.S.; Stevens, J.F. Vitamin C Activates the Folate-Mediated One-Carbon Cycle in C2C12 Myoblasts. Antioxidants 2020, 9, 217. [Google Scholar] [CrossRef]
- Sijilmassi, O.; López-Alonso, J.M.; Sevilla, A.D.R.; González, J.M.; Asensio, M.d.C.B. Biometric Alterations of Mouse Embryonic Eye Structures Due to Short-Term Folic Acid Deficiency. Curr. Eye Res. 2018, 44, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Picciano, M.F. Folate and Human Reproduction. Am. J. Clin. Nutr. 2006, 83, 993–1016. [Google Scholar] [CrossRef]
- Antony, A.C. In Utero Physiology: Role of Folic Acid in Nutrient Delivery and Fetal Development. Am. J. Clin. Nutr. 2007, 85, 598S–603S. [Google Scholar] [CrossRef]
- MRC Vitamin Study Research Group. Prevention of Neural Tube Defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137. [Google Scholar] [CrossRef]
- Houk, V.N.; Oakley, G.P.; Erickson, J.D.; Mulinare, J.; James, L.M. Recommendations for the Use of Folic Acid to Reduce the Number of Cases of Spina Bifida and Other Neural Tube Defects. MMWR Recomm. Rep. 1992, 41, 1–7. [Google Scholar]
- McNulty, B.; McNulty, H.; Marshall, B.; Ward, M.; Molloy, A.M.; Scott, J.M.; Dornan, J.; Pentieva, K. Impact of Continuing Folic Acid after the First Trimester of Pregnancy: Findings of a Randomized Trial of Folic Acid Supplementation in the Second and Third Trimesters. Am. J. Clin. Nutr. 2013, 98, 92–98. [Google Scholar] [CrossRef]
- Wilson, R.D.; O’Connor, D.L. Guideline No. 427: Folic Acid and Multivitamin Supplementation for Prevention of Folic Acid-Sensitive Congenital Anomalies. J. Obstet. Gynaecol. Can. 2022, 44, 707–719. [Google Scholar] [CrossRef]
- Barker, D.J.P.; Osmond, C.; Thornburg, K.L.; Kajantie, E.; Eriksson, J.G. The Shape of the Placental Surface at Birth and Colorectal Cancer in Later Life. Am. J. Hum. Biol. 2013, 25, 566–568. [Google Scholar] [CrossRef]
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and Function of the Normal Human Placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental Origins of Chronic Disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.; Bulmer, J.N.; Innes, B.A.; Broughton Pipkin, F. Possible Roles for Folic Acid in the Regulation of Trophoblast Invasion and Placental Development in Normal Early Human Pregnancy. Biol. Reprod. 2011, 84, 148–1153. [Google Scholar] [CrossRef]
- Padmanabhan, N.; Watson, E.D. Lessons from the One-Carbon Metabolism: Passing It along to the next Generation. Reprod. BioMed. Online 2013, 27, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Chmurzynska, A.; Seremak-Mrozikiewicz, A.; Malinowska, A.M.; Różycka, A.; Radziejewska, A.; KurzawiŃska, G.; Barlik, M.; Wolski, H.; Drews, K. Associations between Folate and Choline Intake, Homocysteine Metabolism, and Genetic Polymorphism of MTHFR, BHMT and PEMT in Healthy Pregnant Polish Women. Nutr. Diet. 2020, 77, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Di Simone, N.; Riccardi, P.; Maggiano, N.; Piacentani, A.; D′Asta, M.; Capelli, A.; Caruso, A. Effect of Folic Acid on Homocysteine-Induced Trophoblast Apoptosis. Mol. Hum. Reprod. 2004, 10, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhou, L.F.; Xiang, X.L.; Wang, K.; Zhou, Q.; Duan, T. Folic Acid Attenuates Dexamethasone-Induced Placental Growth Restriction. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1130–1140. [Google Scholar] [PubMed]
- Fekete, K.; Berti, C.; Trovato, M.; Lohner, S.; Dullemeijer, C.; Souverein, O.W.; Cetin, I.; Decsi, T. Effect of Folate Intake on Health Outcomes in Pregnancy: A Systematic Review and Meta-Analysis on Birth Weight, Placental Weight and Length of Gestation. Nutr. J. 2012, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Rosario, F.J.; Nathanielsz, P.W.; Powell, T.L.; Jansson, T. Maternal Folate Deficiency Causes Inhibition of mTOR Signaling, down-Regulation of Placental Amino Acid Transporters and Fetal Growth Restriction in Mice. Sci. Rep. 2017, 7, 3982. [Google Scholar] [CrossRef] [PubMed]
- Eskild, A.; Romundstad, P.R.; Vatten, L.J. Placental Weight and Birthweight: Does the Association Differ between Pregnancies with and without Preeclampsia? Am. J. Obs. Gynecol. 2009, 201, 595.e1–595.e5. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2006, 29 (Suppl. S1), S43–S48. [Google Scholar] [CrossRef]
- Basevi, V.; Di Mario, S.; Morciano, C.; Nonino, F.; Magrini, N. Comment on: American Diabetes Association. Standards of Medical Care in Diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61. Diabetes Care 2011, 34, e53. [Google Scholar] [CrossRef]
- Baker, B.C.; Hayes, D.J.; Jones, R.L. Effects of Micronutrients on Placental Function: Evidence from Clinical Studies to Animal Models. Reproduction 2018, 156, R69–R82. [Google Scholar] [CrossRef]
- Shah, T.; Mishra, S.; More, A.; Otiv, S.; Apte, K.; Joshi, K. Combination of Vitamin B12 Active Forms Improved Fetal Growth in Wistar Rats through Up-Regulation of Placental miR-16 and miR-21 Levels. Life Sci. 2017, 191, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Rolschau, J.; Date, J.; Kristoffersen, K. Folic Acid Supplement and Intrauterine Growth. Acta Obstet. Gynecol. Scand. 1979, 58, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, L.; Rajalakshmi, K. Effect of Folic Acid Supplement on Birth Weights of Infants. Am. J. Obstet. Gynecol. 1975, 122, 332–336. [Google Scholar] [CrossRef]
- Bergen, N.; Jaddoe, V.; Timmermans, S.; Hofman, A.; Lindemans, J.; Russcher, H.; Raat, H.; Steegers-Theunissen, R.; Steegers, E. Homocysteine and Folate Concentrations in Early Pregnancy and the Risk of Adverse Pregnancy Outcomes: The Generation R Study. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 739–751. [Google Scholar] [CrossRef]
- Bruce, A.W. Generating Different Genetic Expression Patterns in the Early Embryo: Insights from the Mouse Model. Reprod. Biomed. Online 2013, 27, 586–592. [Google Scholar] [CrossRef]
- Faulk, C.; Dolinoy, D.C. Timing Is Everything: The When and How of Environmentally Induced Changes in the Epigenome of Animals. Epigenetics 2011, 6, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Van Otterdijk, S.D.; Klett, H.; Boerries, M.; Michels, K.B. The Impact of Pre-Pregnancy Folic Acid Intake on Placental DNA Methylation in a Fortified Cohort. FASEB J. 2023, 37, e22698. [Google Scholar] [CrossRef]
- Kim, J.-M.; Hong, K.; Lee, J.H.; Lee, S.; Chang, N. Effect of Folate Deficiency on Placental DNA Methylation in Hyperhomocysteinemic Rats. J. Nutr. Biochem. 2009, 20, 172–176. [Google Scholar] [CrossRef]
- Banister, C.E.; Koestler, D.C.; Maccani, M.A.; Padbury, J.F.; Houseman, E.A.; Marsit, C.J. Infant Growth Restriction Is Associated with Distinct Patterns of DNA Methylation in Human Placentas. Epigenetics 2011, 6, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.F.; Middleton, L.Y.M.; Zhu, Y.; Benke, K.S.; Feinberg, J.I.; Croen, L.A.; Hertz-Picciotto, I.; Newschaffer, C.J.; LaSalle, J.M.; Fallin, D.; et al. Prenatal Vitamin Intake in First Month of Pregnancy and DNA Methylation in Cord Blood and Placenta in Two Prospective Cohorts. Epigenetics Chromatin 2022, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Jaddoe, V.W.V.; Hofman, A.; Steegers-Theunissen, R.P.M.; Steegers, E.A.P. Periconception Folic Acid Supplementation, Fetal Growth and the Risks of Low Birth Weight and Preterm Birth: The Generation R Study. Br. J. Nutr. 2009, 102, 777–785. [Google Scholar] [CrossRef]
- Reynolds, L.P.; McLean, K.J.; McCarthy, K.L.; Diniz, W.J.; Menezes, A.C.B.; Forcherio, J.C.; Scott, R.R.; Borowicz, P.P.; Ward, A.K.; Dahlen, C.R.; et al. Nutritional Regulation of Embryonic Survival, Growth, and Development. Adv. Exp. Med. Biol. 2022, 1354, 63–76. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Robertson, W.; Brosens, I.; Dixon, G. Review Article: Trophoblast Invasion and the Establishment of Haemochorial Placentation in Man and Laboratory Animals. Placenta 1981, 2, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Rahat, B.; Hamid, A.; Bagga, R.; Kaur, J. Folic Acid Levels During Pregnancy Regulate Trophoblast Invasive Behavior and the Possible Development of Preeclampsia. Front. Nutr. 2022, 9, 847136. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Miao, Y.; Wei, H.; Peng, J.; Zhou, Y. Diallyl Trisulfide Promotes Placental Angiogenesis by Regulating Lipid Metabolism and Alleviating Inflammatory Responses in Obese Pregnant Mice. Nutrients 2022, 14, 2230. [Google Scholar] [CrossRef]
- Luan, Y.; Leclerc, D.; Cosín-Tomás, M.; Malysheva, O.V.; Wasek, B.; Bottiglieri, T.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Altered Methyl Metabolism and in Sex-Specific Placental Transcription Changes. Mol. Nutr. Food Res. 2021, 65, 2100197. [Google Scholar] [CrossRef]
- Zhou, R.; Zhe, L.; Chen, F.; Gao, T.; Zhang, X.; Huang, L.; Zhuo, Y.; Xu, S.; Lin, Y.; Feng, B.; et al. Maternal Folic Acid and Vitamin B12 Supplementation during Medium to Late Gestation Promotes Fetal Development via Improving Placental Antioxidant Capacity, Angiogenesis and Amino Acid Transport. J. Sci. Food Agric. 2024, 104, 2832–2841. [Google Scholar] [CrossRef]
- Reynolds, E. Vitamin B12, Folic Acid, and the Nervous System. Lancet Neurol. 2006, 5, 949–960. [Google Scholar] [CrossRef]
- Tsen, C.-M.; Hsieh, C.-C.; Yen, C.-H.; Lau, Y.-T. Homocysteine Altered ROS Generation and NO Accumulation in Endothelial Cells. Chin. J. Physiol. 2003, 46, 129–136. [Google Scholar] [PubMed]
- Eskes, T.K. Clotting Disorders and Placental Abruption: Homocysteine—A New Risk Factor. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 95, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.H.; Taljaard, M.; MacFarlane, A.J.; Gaudet, L.M.; Smith, G.N.; Rodger, M.; Rennicks White, R.; Walker, M.C.; Wen, S.W. The Role of Maternal Homocysteine Concentration in Placenta-Mediated Complications: Findings from the Ottawa and Kingston Birth Cohort. BMC Pregnancy Childbirth 2019, 19, 75. [Google Scholar] [CrossRef]
- Lestou, V.S.; Kalousek, D.K. Confined Placental Mosaicism and Intrauterine Fetal Growth. Arch. Dis. Child. Fetal Neonatal Ed. 1998, 79, F223–F226. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ge, X.; Zhu, B.; Xuan, Y.; Huang, K.; Rutayisire, E.; Mao, L.; Huang, S.; Yan, S.; Tao, F. Maternal Continuing Folic Acid Supplementation after the First Trimester of Pregnancy Increased the Risk of Large-for-Gestational-Age Birth: A Population-Based Birth Cohort Study. Nutrients 2016, 8, 493. [Google Scholar] [CrossRef]
- Cunningham, F. Fetal Growth Disorders. In Williams Obstetrics; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Freedman, A.A.; Hogue, C.J.; Marsit, C.J.; Rajakumar, A.; Smith, A.K.; Goldenberg, R.L.; Dudley, D.J.; Saade, G.R.; Silver, R.M.; Gibbins, K.J.; et al. Associations Between the Features of Gross Placental Morphology and Birthweight. Pediatr. Dev. Pathol. 2019, 22, 194–204. [Google Scholar] [CrossRef]
| Maternal Characteristics | Mean ± SD b or n (%) |
|---|---|
| Age (y) | 26.3 ± 3.8 |
| ≤24 | 802 (29.6) |
| 25~29 | 1436 (53.0) |
| ≥30 | 470 (17.4) |
| Race | |
| Han ethnicity | 2671 (98.6) |
| Others | 37 (1.4) |
| Residence | |
| Urban | 2107 (77.8) |
| Suburb | 376 (13.9) |
| Rural | 225 (8.3) |
| Education | |
| Junior high school and below | 529 (19.5) |
| High school or college | 1460 (54.0) |
| Bachelor’s degree or above | 719 (26.5) |
| Monthly income | |
| <2500 | 698 (25.8) |
| 2500–4000 | 1174 (43.3) |
| ≥4000 | 836 (30.9) |
| BMI a (kg/m2) | 20.8 ± 2.8 |
| <18.5 | 526 (19.4) |
| 18.5–23.9 | 1862 (68.8) |
| ≥24 | 320 (11.8) |
| Parity | |
| Primipara | 2438 (90.0) |
| Multipara | 270 (10.0) |
| Smoking | |
| Yes | 107 (4.0) |
| No | 2601 (96.0) |
| Drinking | |
| Yes | 216 (8.0) |
| No | 2492 (92.0) |
| FA Use | Pre-Pregnancy n = 2446 | First Trimester n = 2004 | Second Trimester n = 2673 | Third Trimester n = 2627 |
|---|---|---|---|---|
| Yes | 653 (26.7) | 1638 (81.7) | 239 (8.9) | 372 (14.2) |
| No | 1793 (73.3) | 366 (18.3) | 2434 (91.1) | 2255 (85.8) |
| Pregnancy | Length (cm) | Width (cm) | Area (cm2) | Thickness (cm) | Amniotic Fluid Volume (mL) |
|---|---|---|---|---|---|
| Before conception | |||||
| Nonusers | 18.86 ± 2.11 | 16.50 ± 1.92 | 245.90 ± 48.70 | 2.33 ± 0.44 | 397.57 ± 157.20 |
| FA users | 19.03 ± 2.07 | 16.74 ± 1.88 | 252.10 ± 50.71 | 2.37 ± 0.46 | 410.67 ± 161.24 |
| t | −1.835 | −2.758 | −2.753 | −1.886 | −1.867 |
| p-value | 0.067 | 0.006 ** | 0.006 ** | 0.060 | 0.062 |
| First trimester | |||||
| Nonusers | 18.90 ± 2.14 | 16.56 ± 1.92 | 247.37 ± 49.20 | 2.30 ± 0.45 | 396.05 ± 155.60 |
| FA users | 18.84 ± 2.04 | 16.59 ± 1.89 | 247.17 ± 49.33 | 2.34 ± 0.45 | 397.69 ± 155.65 |
| t | 0.558 | −0.341 | 0.072 | −1.451 | −0.191 |
| p-value | 0.577 | 0.733 | 0.943 | 0.147 | 0.849 |
| Second trimester | |||||
| Nonusers | 18.89 ± 2.09 | 16.56 ± 1.88 | 247.33 ± 48.80 | 2.34 ± 0.45 | 403.73 ± 159.39 |
| FA users | 19.03 ± 2.01 | 16.67 ± 1.93 | 250.69 ± 48.20 | 2.40 ± 0.46 | 401.58 ± 161.58 |
| t | −1.001 | −0.827 | −1.017 | −2.007 | 0.205 |
| p-value | 0.317 | 0.408 | 0.309 | 0.045 ** | 0.838 |
| Third trimester | |||||
| Nonusers | 18.91 ± 2.10 | 16.55 ± 1.90 | 247.32 ± 49.01 | 2.339 ± 0.44 | 403.49 ± 159.31 |
| FA users | 19.00 ± 2.00 | 16.75 ± 1.90 | 251.44 ± 48.79 | 2.393 ± 0.48 | 403.21 ± 160.77 |
| t | −0.731 | −1.858 | −1.503 | −2.155 | 0.032 |
| p-value | 0.465 | 0.063 | 0.133 | 0.031 ** | 0.974 |
| Pregnancy | Length (cm) | Width (cm) | Area (cm2) | Thickness (cm) | Amniotic Fluid Volume (mL) |
|---|---|---|---|---|---|
| model Ⅰ | |||||
| Before conception | |||||
| β | 0.199 | 0.231 | 6.408 | 0.043 | 12.750 |
| 95%CI | (0.010, 0.389) | (0.059, 0.404) | (1.361, 9.181) | (0.003, 0.083) | (−1.089, 26.589) |
| p-value | 0.039 ** | 0.009 ** | 0.005 ** | 0.035 ** | 0.071 |
| First trimester | |||||
| β | −0.034 | 0.000 | −0.382 | 0.041 | 2.290 |
| 95%CI | (−0.268, 0.200) | (−0.214, 0.215) | (−5.972, 5.207) | (−0.011, 0.092) | (−14.699, 19.280) |
| p-value | 0.776 | 0.997 | 0.893 | 0.121 | 0.792 |
| Second trimester | |||||
| β | 0.172 | 0.113 | 3.897 | 0.063 | −4.540 |
| 95%CI | (−0.106, 0.450) | (−0.138, 0.364) | (−2.584, 10.379) | (0.003, 0.123) | (−25.378, 16.299) |
| p-value | 0.224 | 0.377 | 0.309 | 0.039 ** | 0.669 |
| Third trimester | |||||
| β | 0.066 | 0.185 | 3.607 | 0.052 | −1.163 |
| 95%CI | (−0.165, 0.296) | (−0.025, 0.395) | (−1.786, 9.001) | (0.003, 0.102) | (−18.496, 16.170) |
| p-value | 0.577 | 0.083 | 0.190 | 0.039 ** | 0.895 |
| Model Ⅱ | |||||
| Before conception | |||||
| β | 0.206 | 0.239 | 6.783 | 0.031 | 10.070 |
| 95%CI | (0.017, 0.395) | (0.067, 0.412) | (2.348, 11.217) | (−0.009, 0.071) | (−3.859, 23.998) |
| p-value | 0.032 ** | 0.007 ** | 0.003 ** | 0.129 | 0.156 |
| First trimester | |||||
| β | −0.033 | 0.011 | −0.174 | 0.051 | 4.306 |
| 95%CI | (−0.267, 0.201) | (−0.205, 0.227) | (−5.785, 5.438) | (0.000, 0.103) | (−12.773, 21.385) |
| p-value | 0.784 | 0.918 | 0.952 | 0.051 | 0.621 |
| Second trimester | |||||
| β | 0.149 | 0.066 | 2.555 | 0.050 | −3.018 |
| 95%CI | (−0.129, 0.427) | (−0.189, 0.321) | (−3.993, 9.103) | (−0.011, 0.110) | (−25.248, 19.211) |
| p-value | 0.293 | 0.610 | 0.444 | 0.108 | 0.790 |
| Third trimester | |||||
| β | 0.094 | 0.193 | 4.402 | 0.052 | 7.371 |
| 95%CI | (−0.135, 0.323) | (−0.017, 0.402) | (−0.970, 9.775) | (0.002, 0.101) | (−10.842, 25.584) |
| p-value | 0.422 | 0.071 | 0.108 | 0.042 ** | 0.428 |
| Pregnancy | Length (cm) | Width (cm) | Area (cm2) | Thickness (cm) | Amniotic Fluid Volume (mL) |
|---|---|---|---|---|---|
| Before conception | |||||
| β | 0.193 | 0.241 | 6.398 | 0.040 | 13.012 |
| 95%CI | (−0.017, 0.404) | (0.052, 0.429) | (1.407, 11.389) | (−0.011, 0.091) | (−4.092, 30.12) |
| p-value | 0.071 | 0.013 ** | 0.012 ** | 0.128 | 0.136 |
| First trimester | |||||
| β | 0.021 | −0.042 | −0.541 | 0.061 | 17.687 |
| 95%CI | (−0.236, 0.278) | (−0.284, 0.201) | (−6.736, 5.654) | (0.004, 0.117) | (−0.550, 35.92) |
| p-value | 0.873 | 0.737 | 0.864 | 0.036 ** | 0.057 |
| Second trimester | |||||
| β | 0.283 | −0.006 | 3.507 | 0.066 | −6.043 |
| 95%CI | (−0.027, 0.591) | (−0.343, 0.330) | (−4.563, 11.578) | (0.004, 0.129) | (−29.186, 17.10) |
| p-value | 0.073 | 0.970 | 0.394 | 0.038 ** | 0.609 |
| Third trimester | |||||
| β | 0.120 | 0.172 | 4.096 | 0.052 | 6.115 |
| 95%CI | (−0.115, 0.355) | (−0.047, 0.392) | (−1.578, 9.770) | (−0.004, 0.109) | (−13.361, 25.59) |
| p-value | 0.316 | 0.124 | 0.157 | 0.067 | 0.538 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Yang, M.; Ren, S.; Ge, Z.; Cao, Y.; Qin, X.; Sheng, J.; Wang, S. Placenta-Related Parameters at Delivery in Relation to Folic Acid Supplementation in Different Pregnancies. Nutrients 2024, 16, 1729. https://doi.org/10.3390/nu16111729
Ren Y, Yang M, Ren S, Ge Z, Cao Y, Qin X, Sheng J, Wang S. Placenta-Related Parameters at Delivery in Relation to Folic Acid Supplementation in Different Pregnancies. Nutrients. 2024; 16(11):1729. https://doi.org/10.3390/nu16111729
Chicago/Turabian StyleRen, Yating, Maoyuan Yang, Siyi Ren, Zhihao Ge, Yu Cao, Xinsheng Qin, Jie Sheng, and Sufang Wang. 2024. "Placenta-Related Parameters at Delivery in Relation to Folic Acid Supplementation in Different Pregnancies" Nutrients 16, no. 11: 1729. https://doi.org/10.3390/nu16111729
APA StyleRen, Y., Yang, M., Ren, S., Ge, Z., Cao, Y., Qin, X., Sheng, J., & Wang, S. (2024). Placenta-Related Parameters at Delivery in Relation to Folic Acid Supplementation in Different Pregnancies. Nutrients, 16(11), 1729. https://doi.org/10.3390/nu16111729






