Exploring the Role of Lactoferrin in Managing Allergic Airway Diseases among Children: Unrevealing a Potential Breakthrough
Abstract
:1. Introduction
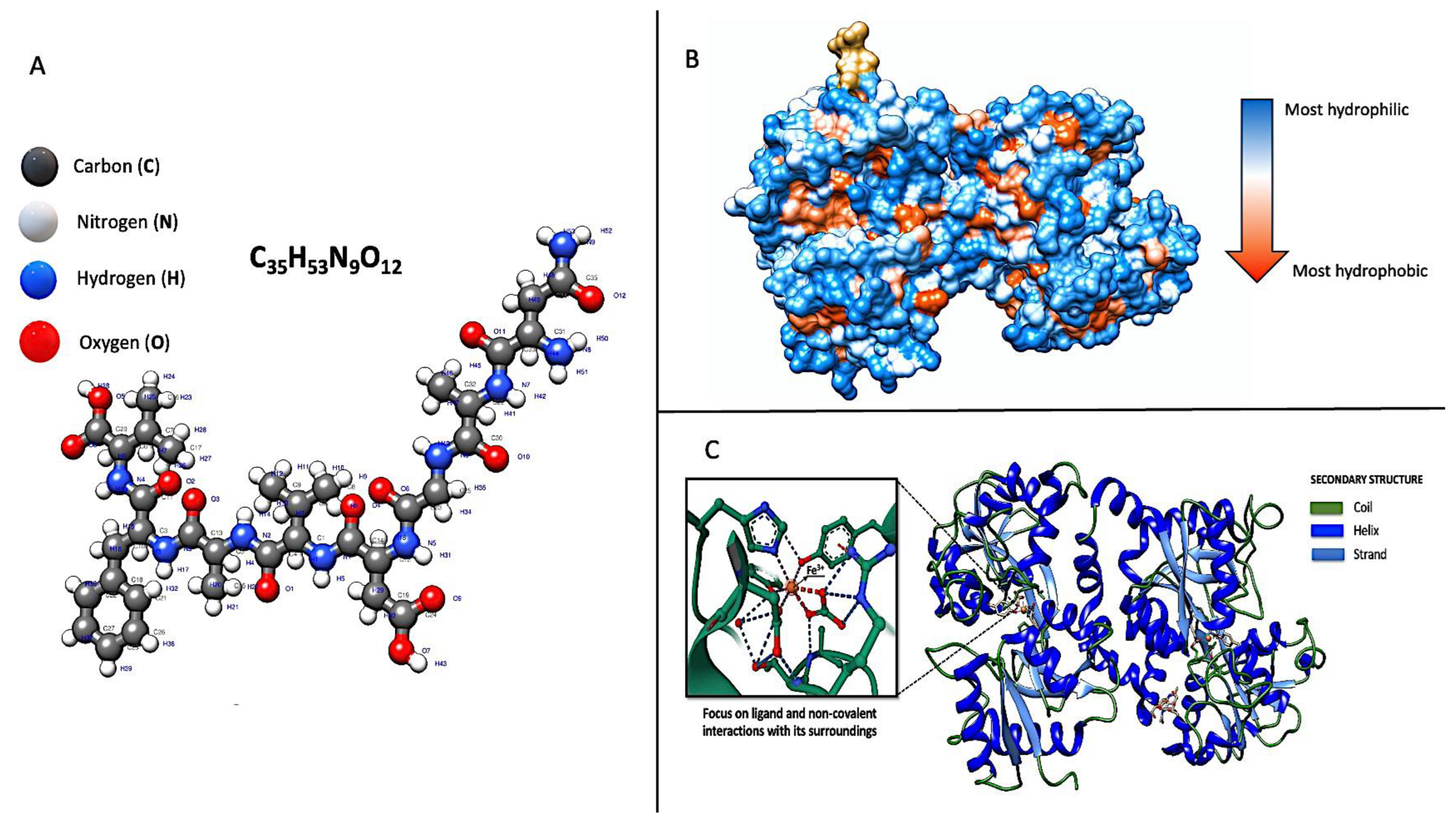
2. Genetics and Molecular Structure
3. Lactoferrin Receptors
4. Lactoferrin as “Middleman” of Innate and Adaptive Immunity
5. Lactoferrin’s Protective Effects against Oxidative Damage
6. Lactoferrin Clinical Application in Allergic Airway Diseases
6.1. Allergic Rhinitis
6.1.1. Materials and Methods
6.1.2. Results
6.2. Allergic Asthma
6.2.1. Materials and Methods
6.2.2. Results
6.3. Discussion
7. Lactoferrin in Human Milk: Concentration Variability, Impact on Infant Health, and Implications for Allergies
8. Limitation of Lactoferrin, Lactoferrin Analogues, and Future Perspective
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Margrethe, S.; Sorensen, S.P.L. The Proteins in Whey. Compte Rendu Des Trav. Du Lab. De Carlsberg Ser. Chim. 1940, 23, 55–99. [Google Scholar]
- Groves, M.L. The Isolation of a Red Protein from Milk2. J. Am. Chem. Soc. 1960, 82, 3345–3350. [Google Scholar] [CrossRef]
- Blanc, B.; Isliker, H. Isolation and Characterization of the Red Siderophilic Protein from Maternal Milk: Lactotransferrin. Bull. Soc. Chim. Biol. 1961, 43, 929–943. [Google Scholar]
- Brock, J.H. Lactoferrin in Human Milk: Its Role in Iron Absorption and Protection against Enteric Infection in the Newborn Infant. Arch. Dis. Child. 1980, 55, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Mejía, F.; Godínez-Victoria, M.; Molotla-Torres, D.E.; Drago-Serrano, M.E. Lactoferrin as a Component of Pharmaceutical Preparations: An Experimental Focus. Pharmaceuticals 2023, 16, 214. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Kaczyńska, K.; Kleczkowska, P.; Bukowska-Ośko, I.; Kramkowski, K.; Sulejczak, D. The Lactoferrin Phenomenon—A Miracle Molecule. Molecules 2022, 27, 2941. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Mejía, F.; Vega-Bautista, A.; Molotla-Torres, D.E.; Aguirre-Garrido, J.F.; Drago-Serrano, M.E. Bovine Lactoferrin as a Modulator of Neuroendocrine Components of Stress. Curr. Mol. Pharmacol. 2021, 14, 1037–1045. [Google Scholar] [CrossRef]
- Shinjo, T.; Sakuraba, K.; Nakaniida, A.; Ishibashi, T.; Kobayashi, M.; Aono, Y.; Suzuki, Y. Oral Lactoferrin Influences Psychological Stress in Humans: A Single-Dose Administration Crossover Study. Biomed. Rep. 2018, 8, 426. [Google Scholar] [CrossRef]
- Kamemori, N.; Takeuchi, T.; Hayashida, K.; Harada, E. Suppressive Effects of Milk-Derived Lactoferrin on Psychological Stress in Adult Rats. Brain Res. 2004, 1029, 34–40. [Google Scholar] [CrossRef]
- Bukowska-Ośko, I.; Sulejczak, D.; Kaczyńska, K.; Kleczkowska, P.; Kramkowski, K.; Popiel, M.; Wietrak, E.; Kowalczyk, P. Lactoferrin as a Human Genome “Guardian”—An Overall Point of View. Int. J. Mol. Sci. 2022, 23, 5248. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Conte, M.P.; Campione, E.; Bianchi, L.; Valenti, P. An Overview on in Vitro and in Vivo Antiviral Activity of Lactoferrin: Its Efficacy against SARS-CoV-2 Infection. BioMetals 2023, 36, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin Against SARS-CoV-2: In Vitro and In Silico Evidences. Front. Pharmacol. 2021, 12, 666600. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence. Int. J. Environ. Res. Public. Health 2021, 18, 10985. [Google Scholar] [CrossRef] [PubMed]
- Miotto, M.; Di Rienzo, L.; Bò, L.; Boffi, A.; Ruocco, G.; Milanetti, E. Molecular Mechanisms Behind Anti SARS-CoV-2 Action of Lactoferrin. Front. Mol. Biosci. 2021, 8, 607443. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in Aseptic and Septic Inflammation. Molecules 2019, 24, 1323. [Google Scholar] [CrossRef] [PubMed]
- Zimecki, M.; Artym, J.; Kocięba, M.; Duk, M.; Kruzel, M. The Effect of Carbohydrate Moiety Structure on the Immunoregulatory Activity of Lactoferrin in Vitro. Cell. Mol. Biol. Lett. 2014, 19, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Interactions of Lactoferrin with Cells Involved in Immune function. Biochem. Cell Biol. 2006, 84, 282–290. [Google Scholar] [CrossRef]
- He, Y.; Lawlor, N.T.; Newburg, D.S. Human Milk Components Modulate Toll-Like Receptor–Mediated Inflammation. Adv. Nutr. 2016, 7, 102–111. [Google Scholar] [CrossRef]
- Danilov, A.V.; Korshunov, N.I.; Danilova, T.G.; Mikhailov, V.P.; Tsyganova, L.A. AB0022 Lactoferrin—A Potent Anti-Inflammatory Agent for Treatment of Adjuvant Arthritis. Ann. Rheum. Dis. 2001, 60, A366–A367. [Google Scholar] [CrossRef]
- Rajvanshi, G.; Gupta, R. Lactoferrin for the Treatment of Rheumatoid Arthritis. Res. Rev. J. Dairy Sci. Technol. 2019, 8, 6–9. [Google Scholar]
- Yanagisawa, S.; Nagasaki, K.; Chea, C.; Ando, T.; Ayuningtyas, N.F.; Inubushi, T.; Ishikado, A.; Imanaka, H.; Sugiyama, E.; Takahashi, I.; et al. Oral Administration of Bovine Lactoferrin Suppresses the Progression of Rheumatoid Arthritis in an SKG Mouse Model. PLoS ONE 2022, 17, e0263254. [Google Scholar] [CrossRef]
- Actor, J.; Hwang, S.-A.; Kruzel, M. Lactoferrin as a Natural Immune Modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ren, Y.; Lu, Q.; Wang, K.; Wu, Y.; Wang, Y.; Zhang, Y.; Cui, X.; Yang, Z.; Chen, Z. Lactoferrin: A Glycoprotein That Plays an Active Role in Human Health. Front. Nutr. 2023, 9, 1018336. [Google Scholar] [CrossRef] [PubMed]
- Nanini, H.F.; Bernardazzi, C.; Castro, F.; Souza, H.S.P. de Damage-Associated Molecular Patterns in Inflammatory Bowel Disease: From Biomarkers to Therapeutic Targets. World J. Gastroenterol. 2018, 24, 4622. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Feng, G.; Zhang, X.; Hu, Q.; Sun, S.; Sun, J.; Sun, Y.; Wang, R.; Zhang, Y.; Wang, P.; et al. The Functional Role of Lactoferrin in Intestine Mucosal Immune System and Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 759507. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Ianiro, G.; Lepanto, M.S.; Rosa, L.; Valenti, P.; Bonaccorsi di Patti, M.C.; Musci, G. Lactoferrin in the Prevention and Treatment of Intestinal Inflammatory Pathologies Associated with Colorectal Cancer Development. Cancers 2020, 12, 3806. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Poli, A.; Agache, I.; Bianchini, R.; Bax, H.J.; Castells, M.; Crescioli, S.; Dombrowicz, D.; Ferastraoaru, D.; Fiebiger, E.; et al. AllergoOncology: Danger Signals in Allergology and Oncology: A European Academy of Allergy and Clinical Immunology (EAACI) Position Paper. Allergy 2022, 77, 2594–2617. [Google Scholar] [CrossRef] [PubMed]
- Schirmbeck, G.H.; Sizonenko, S.; Sanches, E.F. Neuroprotective Role of Lactoferrin during Early Brain Development and Injury through Lifespan. Nutrients 2022, 14, 2923. [Google Scholar] [CrossRef]
- Ahmed, K.; Saikat, A.; Moni, A.; Kakon, S.; Islam, M.; Uddin, M. Lactoferrin: Potential Functions, Pharmacological Insights, and Therapeutic Promises. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 223. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Abdelmoneem, M.A.; Hassanin, I.A.; Abd Elwakil, M.M.; Elnaggar, M.A.; Mokhtar, S.; Fang, J.-Y.; Elkhodairy, K.A. Lactoferrin, a Multi-Functional Glycoprotein: Active Therapeutic, Drug Nanocarrier & Targeting Ligand. Biomaterials 2020, 263, 120355. [Google Scholar] [CrossRef] [PubMed]
- Telang, S. Lactoferrin: A Critical Player in Neonatal Host Defense. Nutrients 2018, 10, 1228. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, C.; Berselli, E.; Blanco-Llamero, C.; Fathi, F.; Oliveira, M.B.P.P.; Krambeck, K.; Souto, E.B. Biomedical and Nutritional Applications of Lactoferrin. Int. J. Pept. Res. Ther. 2023, 29, 71. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 131676698, Lactoferrin (322–329) (human). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lactoferrin-_322-329_-_human (accessed on 28 February 2024).
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualisation system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N.; Baker, H.M. Lactoferrin: Molecular Structure, Binding Properties and Dynamics of Lactoferrin. Cell. Mol. Life Sci. 2005, 62, 2531–2539. [Google Scholar] [CrossRef]
- Fu, J.; Yang, L.; Tan, D.; Liu, L. Iron Transport Mechanism of Lactoferrin and Its Application in Food Processing. Food Sci. Technol. 2023, 43, e121122. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Z. Formation of Lactoferrin/Sodium Caseinate Complexes and Their Adsorption Behaviour at the Air/Water Interface. Food Chem. 2017, 232, 697–703. [Google Scholar] [CrossRef]
- Halabi, A.; Croguennec, T.; Bouhallab, S.; Dupont, D.; Deglaire, A. Modification of Protein Structures by Altering the Whey Protein Profile and Heat Treatment Affects In Vitro Static Digestion of Model Infant Milk Formulas. Food Funct. 2020, 11, 6933–6945. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, Z. Interaction between Lactoferrin and Whey Proteins and Its Influence on the Heat-Induced Gelation of Whey Proteins. Food Chem. 2018, 252, 92–98. [Google Scholar] [CrossRef]
- Goulding, D.A.; Vidal, K.; Bovetto, L.; O’Regan, J.; O’Brien, N.M.; O’Mahony, J.A. The Impact of Thermal Processing on the Simulated Infant Gastrointestinal Digestion, Bactericidal and Anti-Inflammatory Activity of Bovine Lactoferrin–An in Vitro Study. Food Chem. 2021, 362, 130142. [Google Scholar] [CrossRef]
- Bengoechea, C.; Jones, O.G.; Guerrero, A.; McClements, D.J. Formation and Characterization of Lactoferrin/Pectin Electrostatic Complexes: Impact of Composition, pH and Thermal Treatment. Food Hydrocoll. 2011, 25, 1227–1232. [Google Scholar] [CrossRef]
- Ueno, H.M.; Ueda, N.; Morita, M.; Kakehi, Y.; Kobayashi, T. Thermal Stability of the Iron–Lactoferrin Complex in Aqueous Solution Is Improved by Soluble Soybean Polysaccharide. Food Biophys. 2012, 7, 183–189. [Google Scholar] [CrossRef]
- Liu, F.; Wang, D.; Ma, C.; Gao, Y. Conjugation of Polyphenols Prevents Lactoferrin from Thermal Aggregation at Neutral pH. Food Hydrocoll. 2016, 58, 49–59. [Google Scholar] [CrossRef]
- Franco, I.; Pérez, M.D.; Conesa, C.; Calvo, M.; Sánchez, L. Effect of Technological Treatments on Bovine Lactoferrin: An Overview. Food Res. Int. 2018, 106, 173–182. [Google Scholar] [CrossRef]
- Morel, J.; Md Zain, S.N.; Archer, R. Comparison of Drying Techniques for Bovine Lactoferrin: Iron Binding and Antimicrobial Properties of Dried Lactoferrin. Int. Dairy J. 2022, 124, 105142. [Google Scholar] [CrossRef]
- Rastogi, N.; Singh, A.; Singh, P.K.; Tyagi, T.K.; Pandey, S.; Shin, K.; Kaur, P.; Sharma, S.; Singh, T.P. Structure of Iron Saturated C-lobe of Bovine Lactoferrin at pH 6.8 Indicates a Weakening of Iron Coordination. Proteins Struct. Funct. Bioinform. 2016, 84, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A. I Glicani, Molecole a Base Di Carboidrati, Responsabili Della Socialità Delle Cellule. Chim. E Ind. Online 2018, 6, 32. [Google Scholar] [CrossRef]
- Zlatina, K.; Galuska, S.P. The N-Glycans of Lactoferrin: More than Just a Sweet Decoration. Biochem. Cell Biol. 2021, 99, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Karav, S.; German, J.; Rouquié, C.; Le Parc, A.; Barile, D. Studying Lactoferrin N-Glycosylation. Int. J. Mol. Sci. 2017, 18, 870. [Google Scholar] [CrossRef]
- Shoji, H.; Oguchi, S.; Shinohara, K.; Shimizu, T.; Yamashiro, Y. Effects of Iron-Unsaturated Human Lactoferrin on Hydrogen Peroxide-Induced Oxidative Damage in Intestinal Epithelial Cells. Pediatr. Res. 2007, 61, 89–92. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The Multifaceted Functions of Neutrophils. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 181–218. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Iron and Infection. Int. J. Hematol. 2018, 107, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Tantilipikorn, P. The Relationship between Allergic Rhinitis and Viral Infections. Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 249. [Google Scholar] [CrossRef]
- Fine, D.H. Lactoferrin: A Roadmap to the Borderland between Caries and Periodontal Disease. J. Dent. Res. 2015, 94, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Gruden, Š.; Poklar Ulrih, N. Diverse Mechanisms of Antimicrobial Activities of Lactoferrins, Lactoferricins, and Other Lactoferrin-Derived Peptides. Int. J. Mol. Sci. 2021, 22, 11264. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.; Markowitz, K.; Velliyagounder, K. Effect of Human Lactoferrin on Candida Albicans Infection and Host Response Interactions in Experimental Oral Candidiasis in Mice. Arch. Oral Biol. 2022, 137, 105399. [Google Scholar] [CrossRef] [PubMed]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory Effects of Lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral Properties of Lactoferrin—A Natural Immunity Molecule. Molecules 2011, 16, 6992–7018. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N.; Anderson, B.F.; Baker, H.M.; Day, C.L.; Haridas, M.; Norris, G.E.; Rumball, S.V.; Smith, C.A.; Thomas, D.H. Three-Dimensional Structure of Lactoferrin in Various Functional States. In Lactoferrin: Structure and Function; Hutchens, T.W., Rumball, S.V., Lönnerdal, B., Eds.; Advances in Experimental Medicine and Biology; Springer US: Boston, MA, USA, 1994; pp. 1–12. ISBN 978-1-4615-2548-6. [Google Scholar]
- Ianiro, G.; Niro, A.; Rosa, L.; Valenti, P.; Musci, G.; Cutone, A. To Boost or to Reset: The Role of Lactoferrin in Energy Metabolism. Int. J. Mol. Sci. 2023, 24, 15925. [Google Scholar] [CrossRef]
- Zhao, H.-J.; Zhao, X.-H. Modulatory Effect of the Supplemented Copper Ion on In Vitro Activity of Bovine Lactoferrin to Murine Splenocytes and RAW264.7 Macrophages. Biol. Trace Elem. Res. 2019, 189, 519–528. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, H.-J.; Huang, L.-Y.; Song, C.-L.; Li, H.-Q.; Zhao, X.-H. Low-Level Cu-Fortification of Bovine Lactoferrin: Focus on Its Effect on in Vitro Anti-Inflammatory Activity in LPS-Stimulated Macrophages. Curr. Res. Food Sci. 2023, 6, 100520. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Morikawa, H.; Kamon, H.; Iguchi, M.; Hojyo, S.; Fukada, T.; Yamashita, S.; Kaisho, T.; Akira, S.; Murakami, M.; et al. Toll-like Receptor–Mediated Regulation of Zinc Homeostasis Influences Dendritic Cell Function. Nat. Immunol. 2006, 7, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Truong-Tran, A.Q.; Ruffin, R.E.; Foster, P.S.; Koskinen, A.M.; Coyle, P.; Philcox, J.C.; Rofe, A.M.; Zalewski, P.D. Altered Zinc Homeostasis and Caspase-3 Activity in Murine Allergic Airway Inflammation. Am. J. Respir. Cell Mol. Biol. 2002, 27, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Ramezanpour, M.; Cooksley, C.; Lee, T.J.; Jeong, B.; Kao, S.; Suzuki, T.; Psaltis, A.J.; Nakamaru, Y.; Homma, A.; et al. Zinc-Depletion Associates with Tissue Eosinophilia and Collagen Depletion in Chronic Rhinosinusitis. Rhinol. J. 2020, 58, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Suzuki, T.; Watanabe, M.; Hatakeyama, S.; Kimura, S.; Nakazono, A.; Honma, A.; Nakamaru, Y.; Vreugde, S.; Homma, A. Role of Intracellular Zinc in Molecular and Cellular Function in Allergic Inflammatory Diseases. Allergol. Int. 2021, 70, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, A.; Pryshchepa, O.; Som, N.N.; Śpiewak, P.; Gołębiowski, A.; Rafińska, K.; Dobrucka, R.; Kurzydłowski, K.; Buszewski, B.; Pomastowski, P. Study on the Zinc Ions Binding to Human Lactoferrin. J. Mol. Struct. 2023, 1282, 135149. [Google Scholar] [CrossRef]
- Zhao, H.-J.; Zhao, X.-H. Effect of the Zn Supplementation on Immuno-Modulatory Activities of Bovine Lactoferrin in the Murine Splenocytes and RAW264.7 Macrophages. Biol. Trace Elem. Res. 2019, 192, 287–296. [Google Scholar] [CrossRef]
- Zupin, L.; Polesello, V.; Coelho, A.V.C.; Boniotto, M.; Arraes, L.C.; Segat/, L.; Crovella, S. Lactotransferrin Gene Functional Polymorphisms Do Not Influence Susceptibility to Human Immunodeficiency Virus-1 Mother-to-Child Transmission in Different Ethnic Groups. Mem. Inst. Oswaldo Cruz 2015, 110, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Velliyagounder, K.; Kaplan, J.B.; Furgang, D.; Legarda, D.; Diamond, G.; Parkin, R.E.; Fine, D.H. One of Two Human Lactoferrin Variants Exhibits Increased Antibacterial and Transcriptional Activation Activities and Is Associated with Localized Juvenile Periodontitis. Infect. Immun. 2003, 71, 6141–6147. [Google Scholar] [CrossRef]
- Jordan, W.J.; Eskdale, J.; Lennon, G.P.; Pestoff, R.; Wu, L.; Fine, D.H.; Gallagher, G. A Non-Conservative, Coding Single-Nucleotide Polymorphism in the N-Terminal Region of Lactoferrin Is Associated with Aggressive Periodontitis in an African-American, but Not a Caucasian Population. Genes Immun. 2005, 6, 632–635. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Juo, S.-H.; Ho, Y.-P.; Ho, K.-Y.; Yang, Y.-H.; Tsai, C.-C. Association between Lactoferrin Gene Polymorphisms and Aggressive Periodontitis among Taiwanese Patients. J. Periodontal Res. 2009, 44, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Toruner, G.A.; Velliyagounder, K.; Sampathkumar, V.; Godboley, D.; Furgang, D. A Lactotransferrin Single Nucleotide Polymorphism Demonstrates Biological Activity That Can Reduce Susceptibility to Caries. Infect. Immun. 2013, 81, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, L.F.; Pecharki, G.D.; Brancher, J.A.; Cordeiro Junior, C.A.; Medeiros, K.G.D.S.; Antunes, A.A.; Arruda, E.S.; Werneck, R.I.; Azevedo, L.R.D.; Mazur, R.F.; et al. Analysis of the Association between Lactotransferrin (LTF) Gene Polymorphism and Dental Caries. J. Appl. Oral Sci. 2010, 18, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhou, Y.; Li, X.; Yi, H. The Relationship of Haplotype in Lactotransferrin and Its Expression Levels in Chinese Han Ovarian Cancer. Acta Biochim. Biophys. Sin. 2011, 43, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, W.; Zheng, D.; Peng, S.; Xiong, W.; Ma, J.; Zeng, Z.; Wu, M.; Zhou, M.; Xiang, J.; et al. Risk of Nasopharyngeal Carcinoma Associated with Polymorphic Lactotransferrin Haplotypes. Med. Oncol. Northwood Lond. Engl. 2012, 29, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Videm, V.; Dahl, H.; Wålberg, L.E.; Wiseth, R. Functional Polymorphisms in the LTF Gene and Risk of Coronary Artery Stenosis. Hum. Immunol. 2012, 73, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Panella, T.J.; Liu, Y.H.; Huang, A.T.; Teng, C.T. Polymorphism and Altered Methylation of the Lactoferrin Gene in Normal Leukocytes, Leukemic Cells, and Breast Cancer. Cancer Res. 1991, 51, 3037–3043. [Google Scholar]
- Liu, L.-H.E.; Gladwell, W.; Teng, C.T. Detection of Exon Polymorphisms in the Human Lactoferrin Gene. Biochem. Cell Biol. Biochim. Biol. Cell. 2002, 80, 17–22. [Google Scholar] [CrossRef]
- Choi, G.-S.; Shin, S.-Y.; Kim, J.-H.; Lee, H.-Y.; Palikhe, N.S.; Ye, Y.-M.; Kim, S.-H.; Park, H.-S. Serum Lactoferrin Level as a Serologic Biomarker for Allergic Rhinitis. Clin. Exp. Allergy 2010, 40, 403–410. [Google Scholar] [CrossRef]
- Kaczyńska, K.; Jampolska, M.; Wojciechowski, P.; Sulejczak, D.; Andrzejewski, K.; Zając, D. Potential of Lactoferrin in the Treatment of Lung Diseases. Pharmaceuticals 2023, 16, 192. [Google Scholar] [CrossRef]
- Fernández-Delgado, L.; Vega-Rioja, A.; Ventura, I.; Chamorro, C.; Aroca, R.; Prados, M.; Bobadilla, P.; Rodríguez, D.; Palacios, R.; Monteseirín, J. Allergens Induce the Release of Lactoferrin by Neutrophils from Asthmatic Patients. PLoS ONE 2015, 10, e0141278. [Google Scholar] [CrossRef]
- Zorina, V.N.; Burdina, A.V.; Korotkiy, N.G.; Shkolnikova, T.V.; Zorin, N.A. Possible role of lactoferrin in the pathogenesis of atopic dermatitis. Immunopathol. Allergol. Infectology 2012, 4, 11–15. [Google Scholar]
- Tong, P.L.; West, N.P.; Cox, A.J.; Gebski, V.J.; Watts, A.M.; Dodds, A.; De St Groth, B.F.; Cripps, A.W.; Shumack, S. Oral Supplementation with Bovine Whey-Derived Ig-Rich Fraction and Lactoferrin Improves SCORAD and DLQI in Atopic Dermatitis. J. Dermatol. Sci. 2017, 85, 143–146. [Google Scholar] [CrossRef]
- Hamilos, D.L. Drivers of Chronic Rhinosinusitis: Inflammation versus Infection. J. Allergy Clin. Immunol. 2015, 136, 1454–1459. [Google Scholar] [CrossRef]
- Dinarte, V.R.P.; Santos, A.R.D.D.; Araújo, L.F.D.; Reis, M.G.A.D.; Tamashiro, E.; Valera, F.C.P.; Silva Júnior, W.A.D.; Anselmo-Lima, W.T. Polymorphisms in Chronic Rhinosinusitis with Nasal Polyps–A Systematic Review. Braz. J. Otorhinolaryngol. 2017, 83, 705–711. [Google Scholar] [CrossRef]
- Capponi, M.; Gori, A.; De Castro, G.; Ciprandi, G.; Anania, C.; Brindisi, G.; Tosca, M.; Cinicola, B.L.; Salvatori, A.; Loffredo, L.; et al. (R)Evolution in Allergic Rhinitis Add-On Therapy: From Probiotics to Postbiotics and Parabiotics. J. Clin. Med. 2022, 11, 5154. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Lactoferrin: Mammalian Lactoferrin Receptors: Structure and Function. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Uehori, J.; Matsumoto, M.; Suzuki, Y.; Matsuhisa, A.; Toyoshima, K.; Seya, T. Human Intelectin Is a Novel Soluble Lectin That Recognizes Galactofuranose in Carbohydrate Chains of Bacterial Cell Wall. J. Biol. Chem. 2001, 276, 23456–23463. [Google Scholar] [CrossRef]
- Wrackmeyer, U.; Hansen, G.H.; Seya, T.; Danielsen, E.M. Intelectin: A Novel Lipid Raft-Associated Protein in the Enterocyte Brush Border. Biochemistry 2006, 45, 9188–9197. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, R.; Olivero, F.; Carpino, G.; Overi, D.; Rosa, L.; Lepanto, M.S.; Cutone, A.; Franchitto, A.; Alpini, G.; Onori, P.; et al. Role of Lactoferrin and Its Receptors on Biliary Epithelium. BioMetals 2018, 31, 369–379. [Google Scholar] [CrossRef]
- Yi, L.; Cheng, D.; Zhang, K.; Huo, X.; Mo, Y.; Shi, H.; Di, H.; Zou, Y.; Zhang, H.; Zhao, J.; et al. Intelectin Contributes to Allergen-Induced IL-25, IL-33, and TSLP Expression and Type 2 Response in Asthma and Atopic Dermatitis. Mucosal Immunol. 2017, 10, 1491–1503. [Google Scholar] [CrossRef]
- Pemberton, A.D.; Rose-Zerilli, M.J.; Holloway, J.W.; Gray, R.D.; Holgate, S.T. A Single-Nucleotide Polymorphism in Intelectin 1 Is Associated with Increased Asthma Risk. J. Allergy Clin. Immunol. 2008, 122, 1033–1034. [Google Scholar] [CrossRef]
- Kuperman, D.A.; Lewis, C.C.; Woodruff, P.G.; Rodriguez, M.W.; Yang, Y.H.; Dolganov, G.M.; Fahy, J.V.; Erle, D.J. Dissecting Asthma Using Focused Transgenic Modeling and Functional Genomics. J. Allergy Clin. Immunol. 2005, 116, 305–311. [Google Scholar] [CrossRef]
- Kerr, S.C.; Carrington, S.D.; Oscarson, S.; Gallagher, M.E.; Solon, M.; Yuan, S.; Ahn, J.N.; Dougherty, R.H.; Finkbeiner, W.E.; Peters, M.C.; et al. Intelectin-1 Is a Prominent Protein Constituent of Pathologic Mucus Associated with Eosinophilic Airway Inflammation in Asthma. Am. J. Respir. Crit. Care Med. 2014, 189, 1005–1007. [Google Scholar] [CrossRef]
- Gu, N.; Kang, G.; Jin, C.; Xu, Y.; Zhang, Z.; Erle, D.J.; Zhen, G. Intelectin Is Required for IL-13-Induced Monocyte Chemotactic Protein-1 and -3 Expression in Lung Epithelial Cells and Promotes Allergic Airway Inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 298, L290–L296. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, M.; Okoye, I.; Lacy, P. Epithelial Cell Alarmin Cytokines: Frontline Mediators of the Asthma Inflammatory Response. Front. Immunol. 2022, 13, 975914. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Walter Canonica, G.; Melén, E.; Palomares, O.; Scadding, G.K.; Togias, A.; et al. Allergic Rhinitis. Nat. Rev. Dis. Primer 2020, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Park, S.W.; Nguyenvu, L.T.; Rodriguez, M.W.; Barbeau, R.; Paquet, A.C.; Erle, D.J. IL-13 and Epidermal Growth Factor Receptor Have Critical but Distinct Roles in Epithelial Cell Mucin Production. Am. J. Respir. Cell Mol. Biol. 2007, 36, 244–253. [Google Scholar] [CrossRef]
- Voehringer, D.; Stanley, S.A.; Cox, J.S.; Completo, G.C.; Lowary, T.L.; Locksley, R.M. Nippostrongylus Brasiliensis: Identification of Intelectin-1 and -2 as Stat6-Dependent Genes Expressed in Lung and Intestine during Infection. Exp. Parasitol. 2007, 116, 458–466. [Google Scholar] [CrossRef]
- Lau, M.Y.Z.; Dharmage, S.C.; Burgess, J.A.; Lowe, A.J.; Lodge, C.J.; Campbell, B.; Matheson, M.C. CD14 Polymorphisms, Microbial Exposure and Allergic Diseases: A Systematic Review of Gene-Environment Interactions. Allergy 2014, 69, 1440–1453. [Google Scholar] [CrossRef]
- Baveye, S.; Elass, E.; Fernig, D.G.; Blanquart, C.; Mazurier, J.; Legrand, D. Human Lactoferrin Interacts with Soluble CD14 and Inhibits Expression of Endothelial Adhesion Molecules, E-Selectin and ICAM-1, Induced by the CD14-Lipopolysaccharide Complex. Infect. Immun. 2000, 68, 6519–6525. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.; Tang, N.; Wong, G.; Fok, T. CD14 and Toll-Like Receptors: Potential Contribution of Genetic Factors and Mechanisms to Inflammation and Allergy. Curr. Drug Target Inflamm. Allergy 2005, 4, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Baldini, M.; Vercelli, D.; Martinez, F.D. Review Article CD14: An Example of Gene by Environment Interaction in Allergic Disease. Allergy 2002, 57, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Baldini, M.; Carla Lohman, I.; Halonen, M.; Erickson, R.P.; Holt, P.G.; Martinez, F.D. A Polymorphism* in the 5 ′ Flanking Region of the CD14 Gene Is Associated with Circulating Soluble CD14 Levels and with Total Serum Immunoglobulin E. Am. J. Respir. Cell Mol. Biol. 1999, 20, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Koppelman, G.H.; Reijmerink, N.E.; Colin Stine, O.; Howard, T.D.; Whittaker, P.A.; Meyers, D.A.; Postma, D.S.; Bleecker, E.R. Association of a Promoter Polymorphism of the CD14 Gene and Atopy. Am. J. Respir. Crit. Care Med. 2001, 163, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Mao, X.; Baldini, M.; Roberts, M.; Adra, C.; Shirakawa, T.; Holt, P.; Martinez, F.; Hopkin, J. Serum Total IgE Levels and CD14 on Chromosome 5q31. Clin. Genet. 1999, 56, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Ober, C.; Tsalenko, A.; Parry, R.; Cox, N.J. A Second-Generation Genomewide Screen for Asthma-Susceptibility Alleles in a Founder Population. Am. J. Hum. Genet. 2000, 67, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Sengler, C.; Haider, A.; Sommerfeld, C.; Lau, S.; Baldini, M.; Martinez, F.; Wahn, U.; Nickel, R.; German Multicenter Allergy Study Group. Evaluation of the CD14 C-159 T Polymorphism in the German Multicenter Allergy Study Cohort. Clin. Exp. Allergy 2003, 33, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Blais, D.R.; Harrold, J.; Altosaar, I. Killing the Messenger in the Nick of Time: Persistence of Breast Milk sCD14 in the Neonatal Gastrointestinal Tract. Pediatr. Res. 2006, 59, 371–376. [Google Scholar] [CrossRef]
- Fikri, B.; Tani, Y.; Nagai, K.; Sahara, M.; Mitsuishi, C.; Togawa, Y.; Nakano, T.; Yamaide, F.; Ohno, H.; Shimojo, N. Soluble CD14 in Breast Milk and Its Relation to Atopic Manifestations in Early Infancy. Nutrients 2019, 11, 2118. [Google Scholar] [CrossRef]
- Legrand, D.; Elass, E.; Pierce, A.; Mazurier, J. Lactoferrin and Host Defence: An Overview of Its Immuno-Modulating and Anti-Inflammatory Properties. Biometals 2004, 17, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D. Overview of Lactoferrin as a Natural Immune Modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, H.; Xie, Y.; Wang, Y. Recombinant Expression of Porcine Lactoferrin Peptide LF-6 with Intein Technology and Its Immunomodulatory Function in ETEC K88-Infected Mice. Int. Immunopharmacol. 2016, 39, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, Ã.; Haukland, H.H.; Ulvatne, H.; Vorland, L.H. Anti-Complement Effects of Lactoferrin-Derived Peptides. FEMS Immunol. Med. Microbiol. 2004, 41, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Weiszhár, Z.; Bikov, A.; Gálffy, G.; Tamási, L.; Ungvári, I.; Szalai, C.; Losonczy, G.; Horváth, I. Elevated Complement Factor H Levels in Asthmatic Sputa. J. Clin. Immunol. 2013, 33, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Hoda, U.; Pavlidis, S.; Bansal, A.T.; Takahashi, K.; Hu, S.; Ng Kee Kwong, F.; Rossios, C.; Sun, K.; Bhavsar, P.; Loza, M.; et al. Clinical and Transcriptomic Features of Persistent Exacerbation-prone Severe Asthma in U-BIOPRED Cohort. Clin. Transl. Med. 2022, 12, e816. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, P.F.; Hallström, T.; Riesbeck, K. Human Complement Control and Complement Evasion by Pathogenic Microbes--Tipping the Balance. Mol. Immunol. 2013, 56, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Lactoferrin: Lactoferrin: A Modulator of Immune and Inflammatory Responses. Cell. Mol. Life Sci. 2005, 62, 2549. [Google Scholar] [CrossRef]
- Redwan, E.M.; Uversky, V.N.; El-Fakharany, E.M.; Al-Mehdar, H. Potential Lactoferrin Activity against Pathogenic Viruses. C. R. Biol. 2014, 337, 581–595. [Google Scholar] [CrossRef]
- Presti, S.; Manti, S.; Parisi, G.F.; Papale, M.; Barbagallo, I.A.; Li Volti, G.; Leonardi, S. Lactoferrin: Cytokine Modulation and Application in Clinical Practice. J. Clin. Med. 2021, 10, 5482. [Google Scholar] [CrossRef]
- de la Rosa, G.; Yang, D.; Tewary, P.; Varadhachary, A.; Oppenheim, J.J. Lactoferrin Acts as an Alarmin to Promote the Recruitment and Activation of APCs and Antigen-Specific Immune Responses. J. Immunol. Baltim. Md 1950 2008, 180, 6868–6876. [Google Scholar] [CrossRef]
- Spadaro, M.; Caorsi, C.; Ceruti, P.; Varadhachary, A.; Forni, G.; Pericle, F.; Giovarelli, M. Lactoferrin, a Major Defense Protein of Innate Immunity, Is a Novel Maturation Factor for Human Dendritic Cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 2747–2757. [Google Scholar] [CrossRef]
- Latorre, D.; Puddu, P.; Valenti, P.; Gessani, S. Reciprocal Interactions between Lactoferrin and Bacterial Endotoxins and Their Role in the Regulation of the Immune Response. Toxins 2010, 2, 54–68. [Google Scholar] [CrossRef]
- Wilk, K.M.; Hwang, S.-A.; Actor, J.K. Lactoferrin Modulation of Antigen-Presenting-Cell Response to BCG Infection. Postepy Hig. Med. Dosw. Online 2007, 61, 277–282. [Google Scholar]
- Legrand, D.; van Berkel, P.H.; Salmon, V.; van Veen, H.A.; Slomianny, M.C.; Nuijens, J.H.; Spik, G. The N-Terminal Arg2, Arg3 and Arg4 of Human Lactoferrin Interact with Sulphated Molecules but Not with the Receptor Present on Jurkat Human Lymphoblastic T-Cells. Biochem. J. 1997, 327 Pt 3, 841–846. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Sato, K.; Shinmoto, H.; Dosako, S. Role of Basic Residues of Human Lactoferrin in the Interaction with B Lymphocytes. Biosci. Biotechnol. Biochem. 2000, 64, 314–318. [Google Scholar] [CrossRef]
- Sfeir, R.M.; Dubarry, M.; Boyaka, P.N.; Rautureau, M.; Tomé, D. The Mode of Oral Bovine Lactoferrin Administration Influences Mucosal and Systemic Immune Responses in Mice. J. Nutr. 2004, 134, 403–409. [Google Scholar] [CrossRef]
- Zimecki, M.; Mazurier, J.; Spik, G.; Kapp, J.A. Lactoferrin Inhibits Proliferative Response and Cytokine Production of TH1 but Not TH2 Cell Lines. Arch. Immunol. Ther. Exp. 1996, 44, 51–56. [Google Scholar]
- Wang, S.B.; Deng, Y.Q.; Ren, J.; Xiao, B.K.; Chen, Z.; Tao, Z.Z. Lactoferrin Administration into the Nostril Alleviates Murine Allergic Rhinitis and Its Mechanisms. Scand. J. Immunol. 2013, 78, 507–515. [Google Scholar] [CrossRef]
- Bi, B.Y.; Lefebvre, A.M.; Duś, D.; Spik, G.; Mazurier, J. Effect of Lactoferrin on Proliferation and Differentiation of the Jurkat Human Lymphoblastic T Cell Line. Arch. Immunol. Ther. Exp. 1997, 45, 315–320. [Google Scholar]
- Davidson, L.A.; Lönnerdal, B. Persistence of Human Milk Proteins in the Breast-Fed Infant. Acta Paediatr. 1987, 76, 733–740. [Google Scholar] [CrossRef]
- Fischer, R.; Debbabi, H.; Blais, A.; Dubarry, M.; Rautureau, M.; Boyaka, P.N.; Tome, D. Uptake of Ingested Bovine Lactoferrin and Its Accumulation in Adult Mouse Tissues. Int. Immunopharmacol. 2007, 7, 1387–1393. [Google Scholar] [CrossRef]
- Han, M.; Lee, D.; Lee, S.H.; Kim, T.H. Oxidative Stress and Antioxidant Pathway in Allergic Rhinitis. Antioxidants 2021, 10, 1266. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Abubakar-Waziri, H.; Lakhdar, R.; Raby, K.; Dixey, P.; Adcock, I.M.; Mumby, S.; Bhavsar, P.K.; Chung, K.F. Molecular Mechanisms of Oxidative Stress in Asthma. Mol. Aspects Med. 2022, 85, 101026. [Google Scholar] [CrossRef]
- Khan, A.Q.; Agha, M.V.; Sheikhan, K.S.A.M.; Younis, S.M.; Tamimi, M.A.; Alam, M.; Ahmad, A.; Uddin, S.; Buddenkotte, J.; Steinhoff, M. Targeting Deregulated Oxidative Stress in Skin Inflammatory Diseases: An Update on Clinical Importance. Biomed. Pharmacother. 2022, 154, 113601. [Google Scholar] [CrossRef]
- Bowler, R.P.; Crapo, J.D. Oxidative Stress in Allergic Respiratory Diseases. J. Allergy Clin. Immunol. 2002, 110, 349–356. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Imase, M.; Oda, H.; Wakabayashi, H.; Ishii, K. Lactoferrin Directly Scavenges Hydroxyl Radicals and Undergoes Oxidative Self-Degradation: A Possible Role in Protection against Oxidative DNA Damage. Int. J. Mol. Sci. 2014, 15, 1003–1013. [Google Scholar] [CrossRef]
- Mulder, A.M.; Connellan, P.A.; Oliver, C.J.; Morris, C.A.; Stevenson, L.M. Bovine Lactoferrin Supplementation Supports Immune and Antioxidant Status in Healthy Human Males. Nutr. Res. 2008, 28, 583–589. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Z.; Wang, Y.; Zhang, L.; Chua, N.; Dai, L.; Chen, J.; Ho, C.L. Evaluation of the Anti-Inflammatory and Anti-Oxidative Effects of Therapeutic Human Lactoferrin Fragments. Front. Bioeng. Biotechnol. 2021, 9, 779018. [Google Scholar] [CrossRef]
- Passali, D.; Bellussi, L.M.; Gregori, D.; Lauriello, M.; Passali, F.M.; Passali, G.C.; Gip Stop Study Group. Nasal Obstruction as a Key Symptom in Allergic Rhinitis: Efficacy and Safety of a Medical Device in Children. Otolaryngol. Pol. 2012, 66, 249–253. [Google Scholar] [CrossRef]
- Passali, D.; Passali, F.M.; Loglisci, M.; Cambi, J.; Bellussi, L.M. Efficacy and Safety of a Medical Device in Reducing Nasal Obstruction in Allergic Children. Minerva Pediatr. 2015, 67, 239–243. [Google Scholar]
- Lin, C.-C.; Chuang, K.-C.; Chen, S.-W.; Chao, Y.-H.; Yen, C.-C.; Yang, S.-H.; Chen, W.; Chang, K.-H.; Chang, Y.-K.; Chen, C.-M. Lactoferrin Ameliorates Ovalbumin-Induced Asthma in Mice through Reducing Dendritic-Cell-Derived Th2 Cell Responses. Int. J. Mol. Sci. 2022, 23, 14185. [Google Scholar] [CrossRef]
- Kruzel, M. Iron-Mediated Dismutation of Superoxide Anion Augments Antigen-Induced Allergic Inflammation: Effect of Lactoferrin. Postepy Hig. Med. Dośw. (Online) 2007, 61, 268–276. [Google Scholar]
- Bournazou, I.; Mackenzie, K.J.; Duffin, R.; Rossi, A.G.; Gregory, C.D. Inhibition of Eosinophil Migration by Lactoferrin. Immunol. Cell Biol. 2010, 88, 220–223. [Google Scholar] [CrossRef]
- Human Lactoferrin Induces Asthmatic Symptoms in NC/Nga Mice–Nagaoka–2017–Physiological Reports–Wiley Online Library. Available online: https://physoc.onlinelibrary.wiley.com/doi/full/10.14814/phy2.13365 (accessed on 1 February 2024).
- Shinagawa, K.; Oshikata, C.; Kaneko, T.; Tsurikisawa, N. A Case of Lactoferrin-Induced Occupational Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 3600–3602. [Google Scholar] [CrossRef]
- Dasgupta, A.; Chakraborty, R.; Saha, B.; Suri, H.; Singh, P.; Raj, A.; Taneja, B.; Dash, D.; Sengupta, S.; Agrawal, A. Sputum Protein Biomarkers in Airway Diseases: A Pilot Study. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 2203–2215. [Google Scholar] [CrossRef]
- Haghi, M.; Windhab, N.; Hartwig, B.; Young, P.M.; Traini, D. Human Stimulus Factor Is a Promising Peptide for Delivery of Therapeutics. J. Pharm. Sci. 2019, 108, 1401–1403. [Google Scholar] [CrossRef]
- Phase II Clinical Trial of Lactoferrin in Asthma.|SBIR.Gov. Available online: https://www.sbir.gov/content/phase-ii-clinical-trial-lactoferrin-asthma-0 (accessed on 1 February 2024).
- Glynn, P.; Varadhachary, A. Oral Lactoferrin in the Treatment of Respiratory Disorders. U.S. Patents 7238661B2, 3 July 2007. [Google Scholar]
- Czosnykowska-Łukacka, M.; Orczyk-Pawiłowicz, M.; Broers, B.; Królak-Olejnik, B. Lactoferrin in Human Milk of Prolonged Lactation. Nutrients 2019, 11, 2350. [Google Scholar] [CrossRef]
- Lönnerdal, B. Infant Formula and Infant Nutrition: Bioactive Proteins of Human Milk and Implications for Composition of Infant Formulas. Am. J. Clin. Nutr. 2014, 99, 712S–717S. [Google Scholar] [CrossRef]
- Marshall, K. Therapeutic Applications of Whey Protein. Altern. Med. Rev. J. Clin. Ther. 2004, 9, 136–156. [Google Scholar]
- Rai, D.; Adelman, A.S.; Zhuang, W.; Rai, G.P.; Boettcher, J.; Lönnerdal, B. Longitudinal Changes in Lactoferrin Concentrations in Human Milk: A Global Systematic Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jiang, R.; Chen, Q.; Wang, J.; Duan, Y.; Pang, X.; Jiang, S.; Bi, Y.; Zhang, H.; Lönnerdal, B.; et al. Concentration of Lactoferrin in Human Milk and Its Variation during Lactation in Different Chinese Populations. Nutrients 2018, 10, 1235. [Google Scholar] [CrossRef]
- Haschke, F.; Haiden, N.; Thakkar, S.K. Nutritive and Bioactive Proteins in Breastmilk. Ann. Nutr. Metab. 2016, 69 (Suppl. 2), 17–26. [Google Scholar] [CrossRef]
- Garcia-Rodenas, C.L.; De Castro, C.A.; Jenni, R.; Thakkar, S.K.; Beauport, L.; Tolsa, J.-F.; Fischer-Fumeaux, C.J.; Affolter, M. Temporal Changes of Major Protein Concentrations in Preterm and Term Human Milk. A Prospective Cohort Study. Clin. Nutr. Edinb. Scotl. 2019, 38, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- Affolter, M.; Garcia-Rodenas, C.L.; Vinyes-Pares, G.; Jenni, R.; Roggero, I.; Avanti-Nigro, O.; de Castro, C.A.; Zhao, A.; Zhang, Y.; Wang, P.; et al. Temporal Changes of Protein Composition in Breast Milk of Chinese Urban Mothers and Impact of Caesarean Section Delivery. Nutrients 2016, 8, 504. [Google Scholar] [CrossRef] [PubMed]
- Trend, S.; Strunk, T.; Lloyd, M.L.; Kok, C.H.; Metcalfe, J.; Geddes, D.T.; Lai, C.T.; Richmond, P.; Doherty, D.A.; Simmer, K.; et al. Levels of Innate Immune Factors in Preterm and Term Mothers’ Breast Milk during the 1st Month Postpartum. Br. J. Nutr. 2016, 115, 1178–1193. [Google Scholar] [CrossRef]
- Albenzio, M.; Santillo, A.; Stolfi, I.; Manzoni, P.; Iliceto, A.; Rinaldi, M.; Magaldi, R. Lactoferrin Levels in Human Milk after Preterm and Term Delivery. Am. J. Perinatol. 2016, 33, 1085–1089. [Google Scholar] [CrossRef]
- Polonkai, E.; Gyimesi, E.; Kovács, I.; Csillag, A.; Balla, G.; Rajnavölgyi, É.; Bácsi, A.; Sipka, S. A Possible Role of Elevated Breast Milk Lactoferrin and the Cytokine IL-17 Levels in Predicting Early Allergy in Infants: A Pilot Study. Acta Aliment. 2016, 45, 157–162. [Google Scholar] [CrossRef]
- Manzoni, P. Clinical Benefits of Lactoferrin for Infants and Children. J. Pediatr. 2016, 173, S43–S52. [Google Scholar] [CrossRef]
- Buccigrossi, V.; de Marco, G.; Bruzzese, E.; Ombrato, L.; Bracale, I.; Polito, G.; Guarino, A. Lactoferrin Induces Concentration-Dependent Functional Modulation of Intestinal Proliferation and Differentiation. Pediatr. Res. 2007, 61, 410–414. [Google Scholar] [CrossRef]
- Niewiem, M.; Grzybowska-Chlebowczyk, U. Intestinal Barrier Permeability in Allergic Diseases. Nutrients 2022, 14, 1893. [Google Scholar] [CrossRef] [PubMed]
- Van Elburg, R.M.; Heymans, H.S.A.; De Monchy, J.G.R. Effect of Disodiumcromoglycate on Intestinal Permeability Changes and Clinical Response during Cow’s Milk Challenge. Pediatr. Allergy Immunol. 1993, 4, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Schrander, J.J.P.; Unsalan-Hooyen, R.W.M.; Forget, P.P.; Jansen, J. [51Cr]EDTA Intestinal Permeability in Children with Cow’s Milk Intolerance. J. Pediatr. Gastroenterol. Nutr. 1990, 10, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, R.; Atherton, D.J.; Levinsky, R.J. Differences between Normal and Milk Allergic Subjects in Their Immune Responses after Milk Ingestion. Arch. Dis. Child. 1983, 58, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Heyman, M.; Desjeux, J.F. Cytokine-Induced Alteration of the Epithelial Barrier to Food Antigens in Disease. Ann. N. Y. Acad. Sci. 2000, 915, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Salinas, E.; Reyes-Pavón, D.; Cortes-Perez, N.G.; Torres-Maravilla, E.; Bitzer-Quintero, O.K.; Langella, P.; Bermúdez-Humarán, L.G. Bioactive Compounds in Food as a Current Therapeutic Approach to Maintain a Healthy Intestinal Epithelium. Microorganisms 2021, 9, 1634. [Google Scholar] [CrossRef]
- Jackson, P.G.; Baker, R.W.R.; Lessof, M.H.; Ferrett, J.; Macdonald, D.M. Intestinal Permeability in Patients with Eczema and Food Allergy. Lancet 1981, 317, 1285–1286. [Google Scholar] [CrossRef] [PubMed]
- Benard, A.; Desreumeaux, P.; Huglo, D.; Hoorelbeke, A.; Tonnel, A.-B.; Wallaert, B. Increased Intestinal Permeability in Bronchial Asthma. J. Allergy Clin. Immunol. 1996, 97, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Conesa, C.; Bellés, A.; Grasa, L.; Sánchez, L. The Role of Lactoferrin in Intestinal Health. Pharmaceutics 2023, 15, 1569. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, Function, Denaturation and Digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef]
- Liu, H.; Boggs, I.; Weeks, M.; Li, Q.; Wu, H.; Harris, P.; Ma, Y.; Day, L. Kinetic Modelling of the Heat Stability of Bovine Lactoferrin in Raw Whole Milk. J. Food Eng. 2020, 280, 109977. [Google Scholar] [CrossRef]
- Lei, D.; Ma, X. Effect of Enzymatic Glycosylation on the Structure and Properties of Wheat Gluten Protein Fibers. J. Eng. Fibers Fabr. 2021, 16, 155892502110003. [Google Scholar] [CrossRef]
- van Veen, H.A.; Geerts, M.E.J.; van Berkel, P.H.C.; Nuijens, J.H. The Role of N-Linked Glycosylation in the Protection of Human and Bovine Lactoferrin against Tryptic Proteolysis. Eur. J. Biochem. 2004, 271, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Troost, F.J.; Saris, W.H.M.; Brummer, R.-J.M. Orally Ingested Human Lactoferrin Is Digested and Secreted in the Upper Gastrointestinal Tract in Vivo in Women with Ileostomies. J. Nutr. 2002, 132, 2597–2600. [Google Scholar] [CrossRef] [PubMed]
- Troost, F.J.; Steijns, J.; Saris, W.H.; Brummer, R.J. Gastric Digestion of Bovine Lactoferrin in Vivo in Adults. J. Nutr. 2001, 131, 2101–2104. [Google Scholar] [CrossRef]
- Gisbert, J.P.; McNicholl, A.G.; Gomollon, F. Questions and Answers on the Role of Fecal Lactoferrin as a Biological Marker in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2009, 15, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Spik, G.; Brunet, B.; Mazurier-Dehaine, C.; Fontaine, G.; Montreuil, J. Characterization and Properties of the Human and Bovine Lactotransferrins Extracted from the Faeces of Newborn Infants. Acta Paediatr. Scand. 1982, 71, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K. Potent Antibacterial Peptides Generated by Pepsin Digestion of Bovine Lactoferrin. J. Dairy Sci. 1991, 74, 4137–4142. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K.; Tomita, M. Identification of the Bactericidal Domain of Lactoferrin. Biochim. Biophys. Acta BBA Protein Struct. Mol. Enzymol. 1992, 1121, 130–136. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, C.; Zhang, J. Lactoferrin and Its Detection Methods: A Review. Nutrients 2021, 13, 2492. [Google Scholar] [CrossRef]


| Reference | First Author | Publication Year | Study Model | LF Type | Administration Route | Effects |
|---|---|---|---|---|---|---|
| [130] | Passali D | 2015 | Human, in vivo | Associated with carboximetil b-glucan, D-panthenol, and dipotassium glycyrrhizinate. | Intranasal spray. Two puffs into each nostril two times a day over the course of 4 weeks | The improvement of AR symptoms evaluated with VAS (nasal obstruction, sneezing, watery eyes, rhinorrhea, and overall symptom burden). The improvement of AAR and MCTt. |
| [118] | Wang SB | 2013 | Murine, in vivo | Recombinant human | Intranasal instillation 100 μg LF | Decrease in eosinophils, goblet cells, and granulocytes. The downregulation of Th2-related cytokines and transcription factors (IL-5 and GATA-3), Th17-related cytokines and transcription factors (IL-17 and ROR-c). |
| [129] | Passali D | 2012 | Human, in vivo | Associated with carboximetil b-glucan, D-panthenol, and dipotassium glycyrrhizinate. | Intranasal spray. Two puffs into each nostril two times a day over the course of 4 weeks | The improvement of AR symptoms evaluated with VAS (nasal obstruction, sneezing, watery eyes, rhinorrhea, and overall symptom burden). |
| [65] | Choi GS | 2010 | Human, in vivo | Endogenous | Nasal lavage fluid serum | LF expression was upregulated after NPT. Serum LF level is associated with the phenotype of Dpt-sensitive AR. Serum LF level in combination with the serum Dpt-specific IgE level, may be a marker for early detection of AR. |
| References | First Author | Publication Year | Study Model | LF Type | Administration Route | Effects |
|---|---|---|---|---|---|---|
| [138] | Lin CC | 2022 | Murine, in vitro and in vivo | Unspecified | Oral administration 100 mg/kg or 300 mg/kg | The improvement of OVA-induced AHR and pulmonary inflammation and suppression of the production of OVA-induced Th2 Cytokines. Increase in anti-inflammatory cytokines in the BALF regulation of OVA-specific IgG1 and IgE secretion in the serum. Decrease in OVA-specific Th2 responses in the spleen. The downregulation of the surface molecules CD80 and CD86 in DCs in the spleen of OVA-treated mice. Decrease in the capacity of DCs to stimulate OVA-specific Th2-cell responses in vitro. |
| [144] | Dasgupta A | 2021 | Human, in vivo | Endogenous | Sputum | LF biomarkers for frequent exacerbators. |
| [142] | Shinagawa K | 2020 | Human, in vivo | Bovine | Inhalation | Occupational asthma. |
| [145] | Haghi M | 2019 | Human, in vitro | Peptide derived from the N-terminal domain of human lactoferrin | Transwell passage | Cell-penetrating peptide for delivery therapeutics across respiratory epithelia. |
| [144] | Nagaoka K | 2017 | Murine, in vivo | Human | Intranasal inoculation | The induction of airway inflammation: increased AHR, eosinophils in BALF, serum LF-specific IgG levels, and mRNA levels of IL-13, eotaxin 1, and eotaxin 2. |
| [141] | Bournazou I | 2010 | Human, in vitro | Human milk-derived and neutrophil-derived | Added to the upper chamber along with eosinophils | The control of eosinophil infiltration in atopic inflammatory conditions. |
| [140] | Chodaczek G | 2007 | Murine, in vivo | Human milk-derived. Apo-LF (100 μg) and holo.LF (100 μg) | Intranasal | Diminishing the effect of oxidative stress in allergic airway inflammation. |
| [139] | Kruzel ML | 2006 | Human, in vitro; murine, in vivo | Human milk-derived. Apo-LF (100 μg) and holo.LF (100 μg) | Intranasal | Lowering RWE-induced increase in ROS levels in bronchial epithelial cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gori, A.; Brindisi, G.; Daglia, M.; Giudice, M.M.d.; Dinardo, G.; Di Minno, A.; Drago, L.; Indolfi, C.; Naso, M.; Trincianti, C.; et al. Exploring the Role of Lactoferrin in Managing Allergic Airway Diseases among Children: Unrevealing a Potential Breakthrough. Nutrients 2024, 16, 1906. https://doi.org/10.3390/nu16121906
Gori A, Brindisi G, Daglia M, Giudice MMd, Dinardo G, Di Minno A, Drago L, Indolfi C, Naso M, Trincianti C, et al. Exploring the Role of Lactoferrin in Managing Allergic Airway Diseases among Children: Unrevealing a Potential Breakthrough. Nutrients. 2024; 16(12):1906. https://doi.org/10.3390/nu16121906
Chicago/Turabian StyleGori, Alessandra, Giulia Brindisi, Maria Daglia, Michele Miraglia del Giudice, Giulio Dinardo, Alessandro Di Minno, Lorenzo Drago, Cristiana Indolfi, Matteo Naso, Chiara Trincianti, and et al. 2024. "Exploring the Role of Lactoferrin in Managing Allergic Airway Diseases among Children: Unrevealing a Potential Breakthrough" Nutrients 16, no. 12: 1906. https://doi.org/10.3390/nu16121906







