Exogenous Nucleotides Improve the Skin Aging of SAMP8 Mice by Modulating Autophagy through MAPKs and AMPK Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
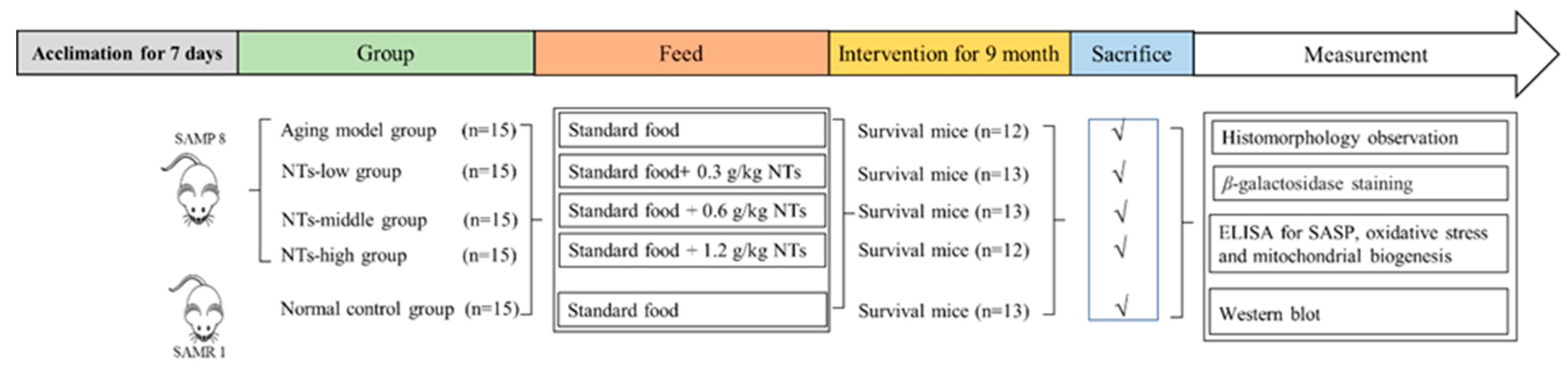
2.2. Animals and Treatment
2.3. Histomorphology Observation
2.3.1. Hematoxylin-Eosin Staining
2.3.2. Two-Photon Excitation Fluorescence Imaging
2.4. Senescence-Associated-β-Galactosidase Staining
2.5. ELISA Analysis
2.5.1. Senescence-Associated Secretory Phenotype (SASP) Analysis
2.5.2. The Oxidative Stress-Associated Biological Indicators Analysis
2.5.3. The Mitochondrial Function-Associated Biological Indicators Analysis
2.5.4. Hydroxyproline (Hyp) Content Analysis
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
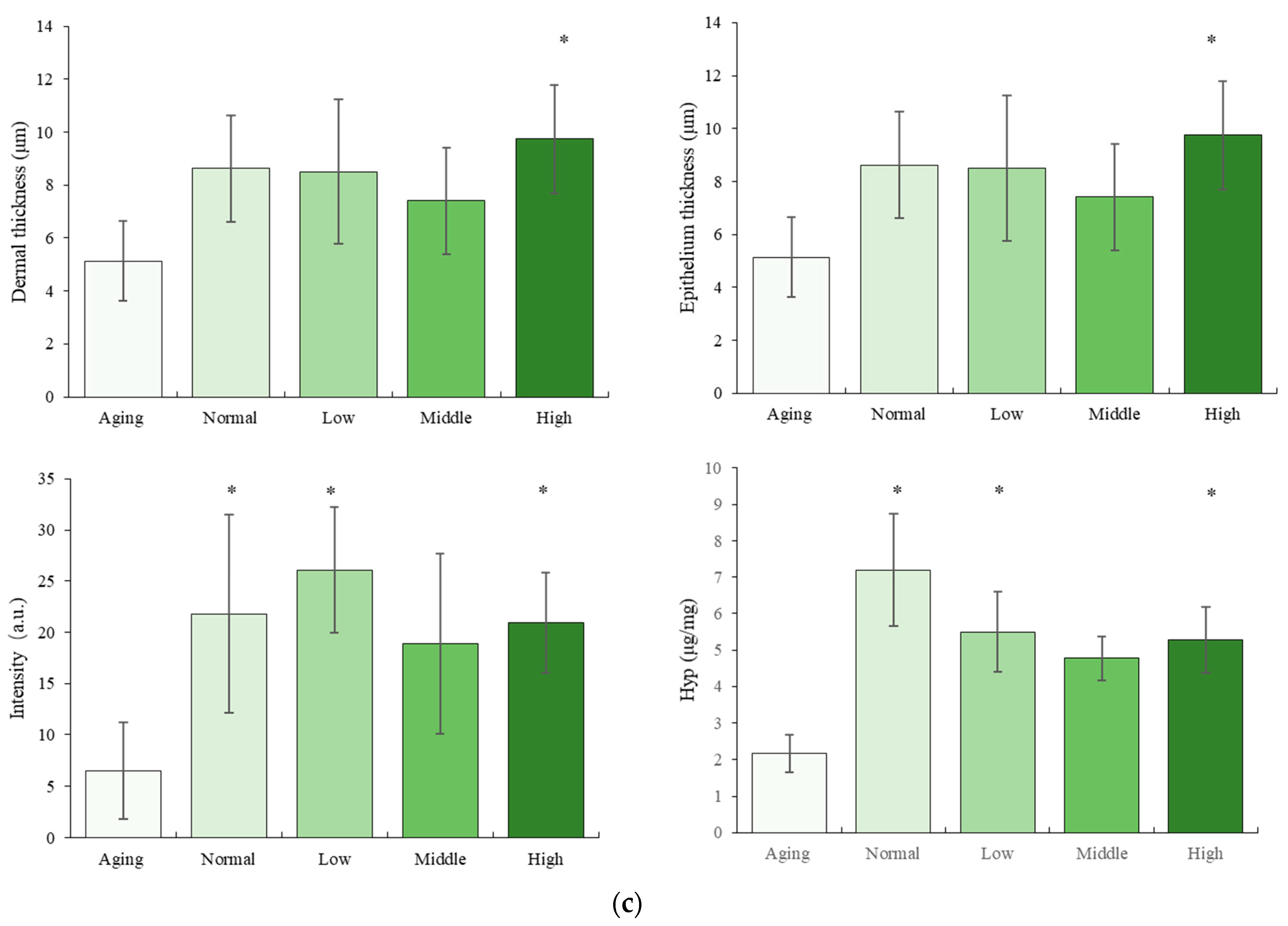
3.1. The Effect of NTs on the Morphology
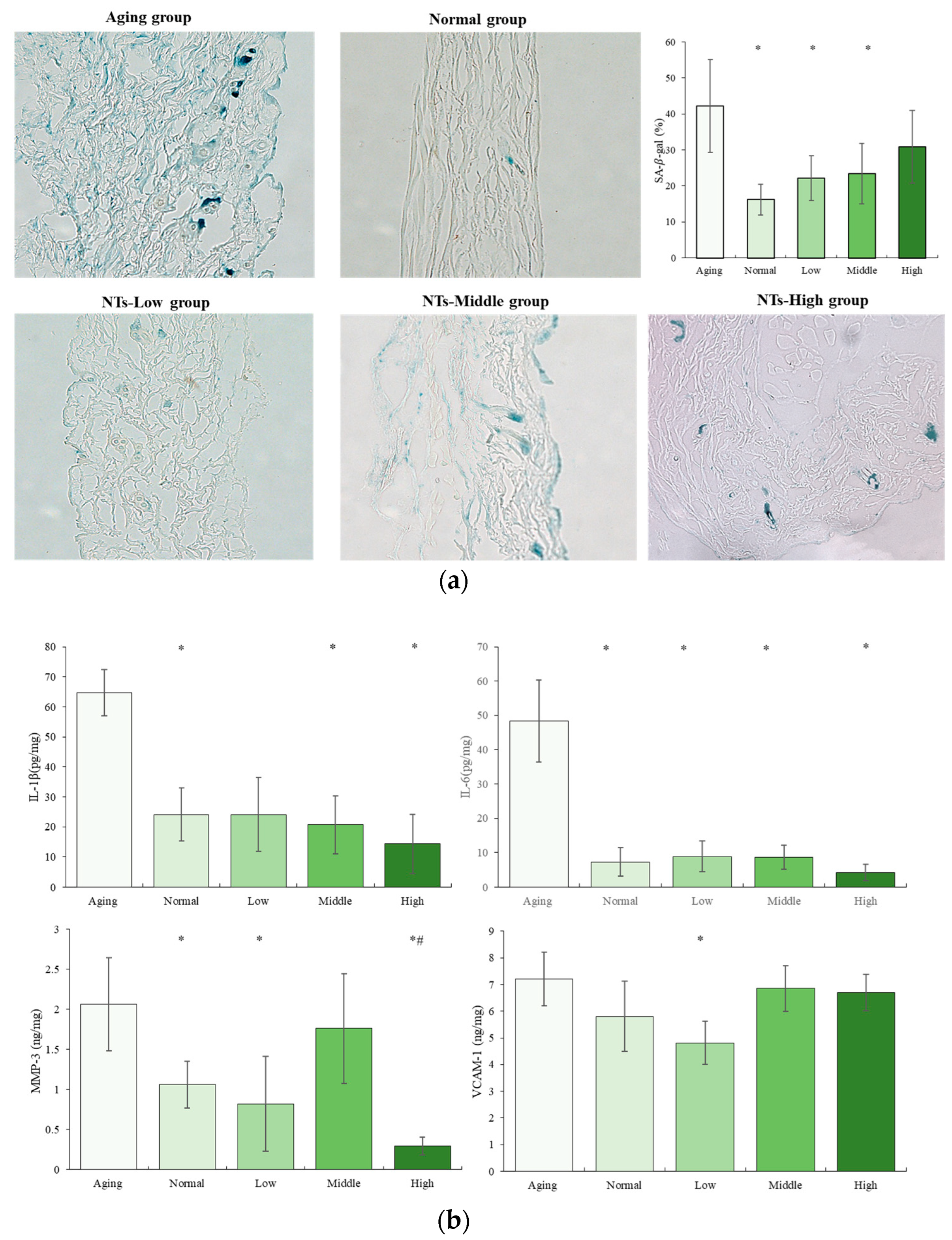
3.2. The Anti-Aging Effects of NTs
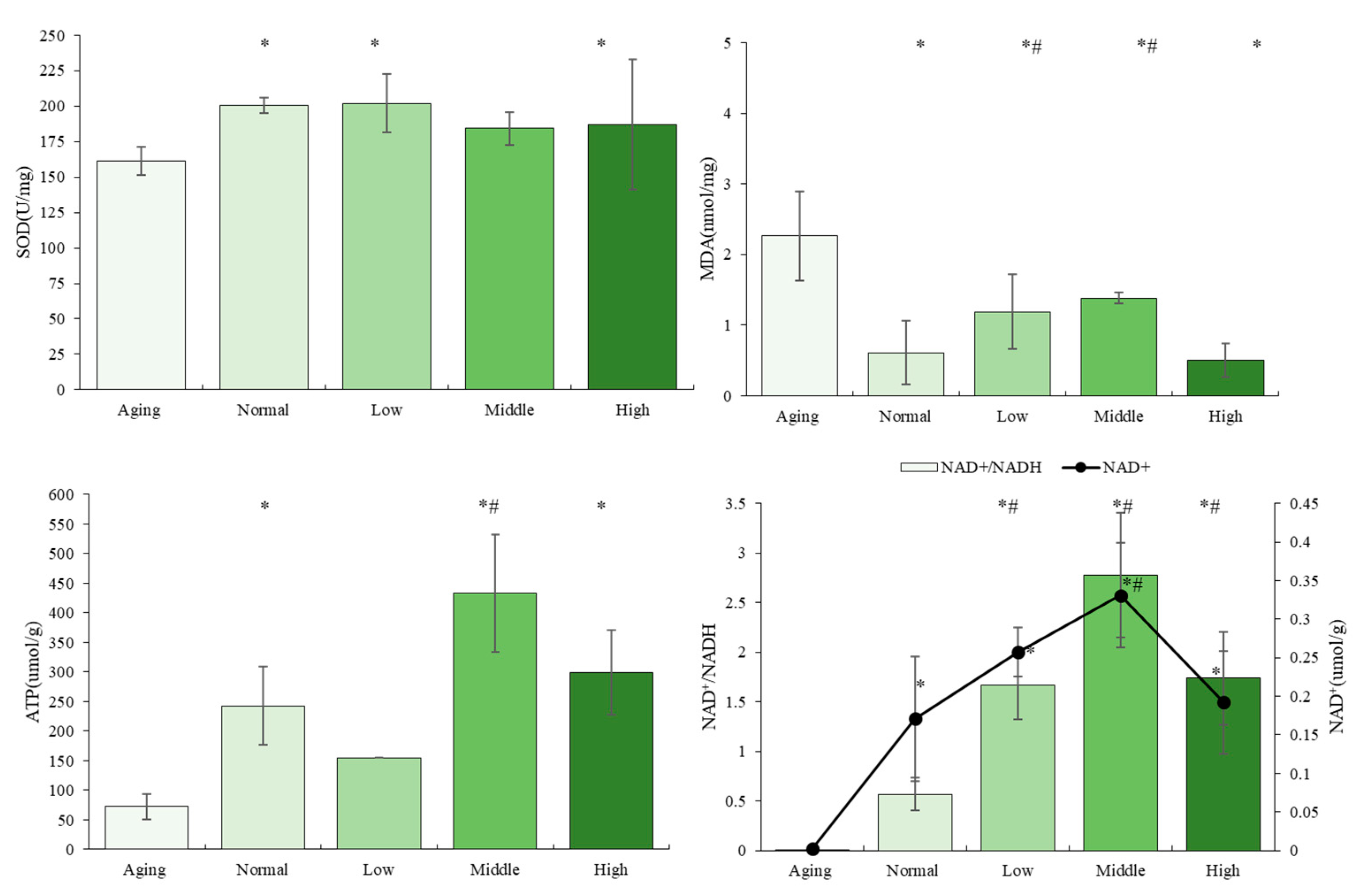
3.3. The Effects of NTs on Antioxidant Activity and Mitochondrial Biogenesis
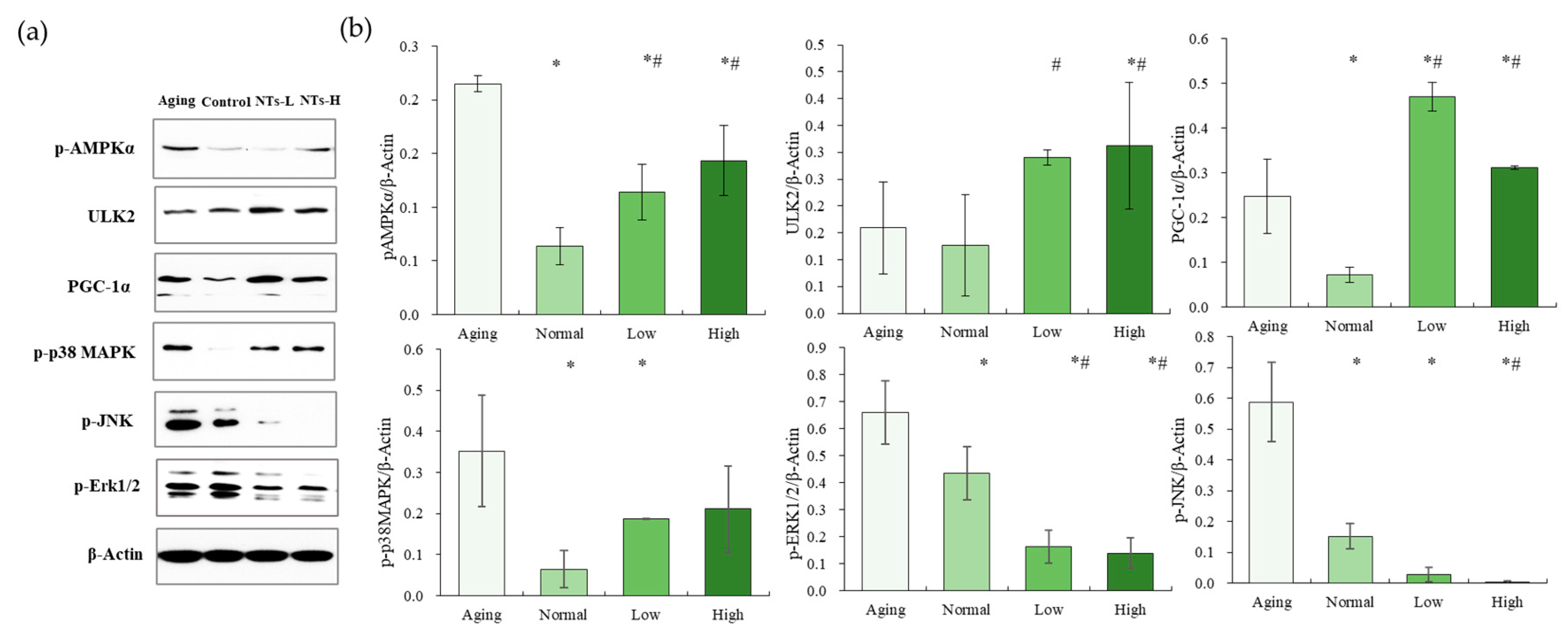
3.4. The NTs Improve Aging and May Activate Autophagy
3.5. NTs Activate the AMPK Pathway and Inhibit the MAPK Pathway
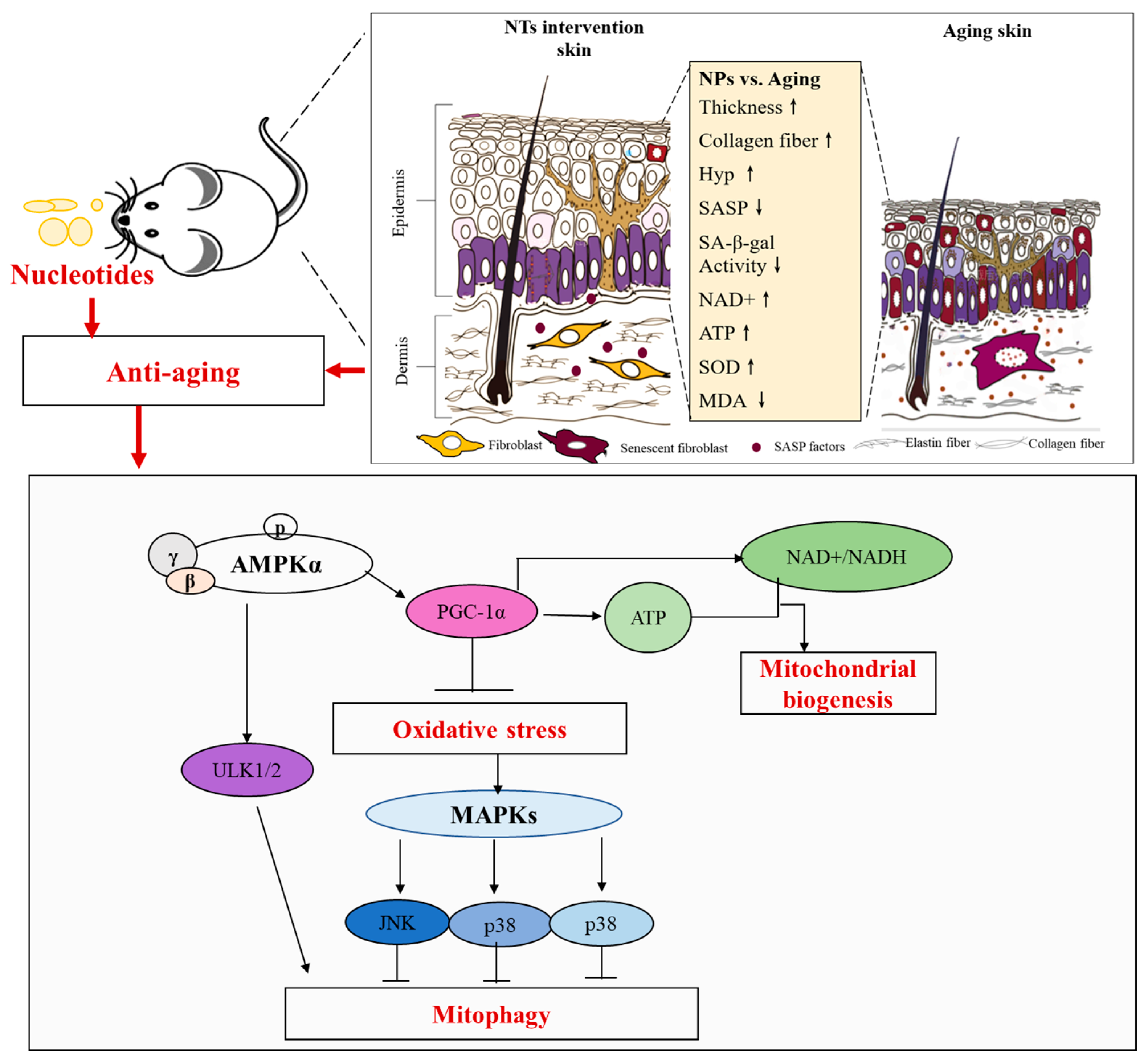
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blume-Peytavi, U.; Kottner, J.; Sterry, W.; Hodin, M.W.; Griffiths, T.W.; Watson, R.E.; Hay, R.J.; Griffiths, C.E. Age-Associated Skin Conditions and Diseases: Current Perspectives and Future option. Gerontologist 2016, 56, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.L.; Pitcher, L.E.; Niedernhofer, L.J.; Robbins, P.D. Targeting Cellular Senescence with Senotherapeutics: Development of New Approaches for Skin Care. Plast. Reconstr. Surg. 2022, 150, 12S–19S. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin senescence: Mechanisms and impact on whole-body aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Gunn, D.A.; De Craen, A.J.; Dick, J.L.; Tomlin, C.C.; Van Heemst, D.; Catt, S.D.; Griffiths, T.; Ogden, S.; Maier, A.B.; Murray, P.G.; et al. Facial appearance reflects human familial longevity and cardiovascular disease risk in healthy individuals. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Akerman, S.C.; Hossain, S.; Shobo, A.; Zhong, Y.; Jourdain, R.; Hancock, M.A.; George, K.; Breton, L.; Multhaup, G. Neurodegenerative Disease-Related Proteins within the Epidermal Layer of the Human Skin. J. Alzheimer’s Dis. 2019, 69, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Haiminen, N.; Carrieri, A.-P.; Hu, R.; Jiang, L.; Parida, L.; Russell, B.; Allaband, C.; Zarrinpar, A.; Vazquez-Baeza, Y.; et al. Human Skin, Oral, and Gut Microbiomes Predict Chronological Age. mSystems 2020, 5, e00630. [Google Scholar] [CrossRef] [PubMed]
- Gunn, D.A.; Larsen, L.A.; Lall, J.S.; Rexbye, H.; Christensen, K. Mortality is Written on the Face. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 72–77. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef]
- Papaccio, F.D.; Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef]
- Wang, C.H.; Wu, S.B.; Wu, Y.T.; Wei, Y.H. Oxidative stress response elicited by mitochondrial dysfunction: Implication in the pathophysiology of aging. Exp. Biol. Med. 2013, 238, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Jing, R.; Guo, K.; Zhong, Y.; Wang, L.; Zhao, J.; Gao, B.; Ye, Z.; Chen, Y.; Li, X.; Xu, N.; et al. Protective effects of fucoidan purified from Undaria pinnatifida against UV-irradiated skin photoaging. Ann. Transl. Med. 2021, 9, 1185. [Google Scholar] [CrossRef]
- Guo, K.; Liu, R.; Jing, R.; Wang, L.; Li, X.; Zhang, K.; Fu, M.; Ye, J.; Hu, Z.; Zhao, W.; et al. Cryptotanshinone protects skin cells from ultraviolet radiation-induced photoaging via its antioxidant effect and by reducing mitochondrial dysfunction and inhibiting apoptosis. Front. Pharmacol. 2022, 13, 1036013. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, X.; Li, M.; Yu, X.; Huang, F.; Wang, Y.; Yan, Y.; Zhang, H.; Shi, Y.; He, X. The role of mitochondrial quality surveillance in skin aging: Focus on mitochondrial dynamics, biogenesis and mitophagy. Ageing Res. Rev. 2023, 87, 101917. [Google Scholar] [CrossRef]
- Jeong, D.; Qomaladewi, N.P.; Lee, J.; Park, S.H.; Cho, J.Y. The Role of Autophagy in Skin Fibroblasts, Keratinocytes, Melanocytes, and Epidermal Stem Cells. J. Investig. Dermatol. 2020, 140, 1691–1697. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, X.; Hao, D.; Zhou, M.; Li, X.; He, G.; Jiang, X. Insights into autophagy machinery in cells related to skin diseases and strategies for therapeutic modulation. Biomed. Pharmacother. 2019, 113, 108775. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Passos, J.F.; Khosla, S.; Tchkonia, T.; Kirkland, J.L. Reducing Senescent Cell Burden in Aging and Disease. Trends Mol. Med. 2020, 26, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. eBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef]
- Moiseeva, O.; Deschênes-Simard, X.; St-Germain, E.; Igelmann, S.; Huot, G.; Cadar, A.E.; Bourdeau, V.; Pollak, M.N.; Ferbeyre, G. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell 2013, 12, 489–498. [Google Scholar] [CrossRef]
- Pal, H.C.; Athar, M.; Elmets, C.A.; Afaq, F. Fisetin inhibits UVB-induced cutaneous inflammation and activation of PI3K/AKT/NFκB signaling pathways in SKH-1 hairless mice. Photochem. Photobiol. 2015, 91, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yu, Z.; Sunchu, B.; Shoaf, J.; Dang, I.; Zhao, S.; Caples, K.; Bradley, L.; Beaver, L.M.; Ho, E.; et al. Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell 2017, 16, 564–574. [Google Scholar] [CrossRef]
- Villanueva-Paz, M.; Povea-Cabello, S.; Villalón-García, I.; Álvarez-Córdoba, M.; Suárez-Rivero, J.M.; Talaverón-Rey, M.; Jackson, S.; Falcón-Moya, R.; Rodríguez-Moreno, A.; Sánchez-Alcázar, J.A. Parkin-mediated mitophagy and autophagy flux disruption in cellular models of MERRF syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165726. [Google Scholar] [CrossRef]
- Kim, D.; Yang, K.E.; Kim, D.W.; Hwang, H.Y.; Kim, J.; Choi, J.S.; Kwon, H.J. Activation of Ca2+-AMPK-mediated autophagy by ginsenoside Rg3 attenuates cellular senescence in human dermal fibroblasts. Clin. Transl. Med. 2021, 11, e521. [Google Scholar] [CrossRef] [PubMed]
- Oblong, J.E.; Bowman, A.; Rovito, H.A.; Jarrold, B.B.; Sherrill, J.D.; Black, M.R.; Nelson, G.; Kimball, A.B.; Birch-Machin, M.A. Metabolic dysfunction in human skin: Restoration of mitochondrial integrity and metabolic output by nicotinamide (niacinamide) in primary dermal fibroblasts from older aged donors. Aging Cell 2020, 19, e13248. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Hao, X.; Wen, J.; Zhang, S.; Zhao, B.; Miao, J. Grp94 Inhibitor HCP1 Inhibits Human Dermal Fibroblast Senescence. Genes 2022, 13, 1651. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Cagide, F.; Simões, J.; Pita, C.; Pereira, E.; Videira, A.J.C.; Soares, P.; Duarte, J.F.S.; Santos, A.M.S.; Oliveira, P.J.; et al. Targeting Hydroxybenzoic Acids to Mitochondria as a Strategy to Delay Skin Ageing: An In Vitro Approach. Molecules 2022, 27, 6183. [Google Scholar] [CrossRef] [PubMed]
- Lohakul, J.; Jeayeng, S.; Chaiprasongsuk, A.; Torregrossa, R.; Wood, M.E.; Saelim, M.; Thangboonjit, W.; Whiteman, M.; Panich, U. Mitochondria-Targeted Hydrogen Sulfide Delivery Molecules Protect Against UVA-Induced Photoaging in Human Dermal Fibroblasts, and in Mouse Skin In Vivo. Antioxid. Redox Signal 2022, 36, 1268–1288. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pozo, A.; Gil, A. Nucleotides as semiessential nutritional components. Br. J. Nutr. 2002, 87, S135–S137. [Google Scholar] [CrossRef]
- Lane, A.N.; Fan, T.W. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef]
- Ding, T.; Song, G.; Liu, X.; Xu, M.; Li, Y. Nucleotides as optimal candidates for essential nutrients in living organisms: A review. J. Funct. Foods 2021, 82, 104498. [Google Scholar] [CrossRef]
- Zhu, N.; Liu, X.; Xu, M.; Li, Y. Dietary Nucleotides Retard Oxidative Stress-Induced Senescence of Human Umbilical Vein Endothelial Cells. Nutrients 2021, 13, 3279. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Liu, R.; Xu, M.H.; Li, Y. Neuroprotective Actions of Different Exogenous Nucleotides in H2O2-Induced Cell Death in PC-12 Cells. Molecules 2023, 28, 1226. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, R.; Wei, C.; Xu, M.; Li, Y. Exogenous Nucleotides Improved the Oxidative Stress and Sirt-1 Protein Level of Brown Adipose Tissue on Senescence-Accelerated Mouse Prone-8 (SAMP8) Mice. Nutrients 2022, 14, 2796. [Google Scholar] [CrossRef] [PubMed]
- Palero, J.A.; De Bruijn, H.S.; Van der Ploeg van den Heuvel, A.; Sterenborg, H.J.; Gerritsen, H.C. Spectrally resolved multiphoton imaging of in vivo and excised mouse skin tissues. Biophys. J. 2007, 93, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Rico-Jimenez, J.J.; Zhang, C.; Alex, A.; Chaney, E.J.; Barkalifa, R.; Spillman, D.R.J.; Marjanovic, M.; Arp, Z.; Hood, S.R.; et al. Simultaneous label-free autofluorescence and multi-harmonic imaging reveals in vivo structural and metabolic changes in murine skin. Biomed. Opt. Express 2019, 10, 5431–5444. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Tresguerres, J.Á.F.; Fernández-Tresguerres, I.; Viña, J.; Rancan, L.; Paredes, S.D.; Linillos-Pradillo, B.; Vara, E. Effects of GH on the Aging Process in Several Organs: Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 7848. [Google Scholar] [CrossRef]
- Takeda, T. Senescence-accelerated mouse (SAM): A biogerontological resource in aging research. Neurobiol. Aging 1999, 20, 105–110. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Shi, J.S. SAMP8 Mice as a Model of Age-Related Cognition Decline with Underlying Mechanisms in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 75, 385–395. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Z.; Gao, P.; Fan, Q.; Zhang, T.; Cui, L.; Shi, L.; Liu, Z.; Yang, Z.; He, L.; et al. Daphnetin Alleviates Senile and Disuse Osteoporosis by Distinct Modulations of Bone Formation and Resorption. Antioxidants 2022, 11, 2365. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Ikarashi, N.; Tabata, K.; Miyazawa, A.; Kon, R.; Sakai, H.; Hosoe, T. Expression analysis of genes important for maintaining skin function in a senescence-accelerated mouse prone model. Geriatr. Gerontol. Int. 2023, 23, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Kazanci, A.; Kurus, M.; Atasever, A. Analyses of changes on skin by aging. Skin Res. Technol. 2016, 23, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The Role of Oxidative Stress in Cardiovascular Aging and Cardiovascular Diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, D.J.; Blin, M.G.; Song, J.; Wood, S.C.; Zhang, M.; Beard, D.A.; Goldstein, D.R. Age-Associated Mitochondrial Dysfunction Accelerates Atherogenesis. Circ. Res. 2020, 126, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Waldera Lupa, D.M.; Kalfalah, F.; Safferling, K.; Boukamp, P.; Poschmann, G.; Volpi, E.; Götz-Rösch, C.; Bernerd, F.; Haag, L.; Huebenthal, U.; et al. Characterization of Skin Aging-Associated Secreted Proteins (SAASP) Produced by Dermal Fibroblasts Isolated from Intrinsically Aged Human Skin. J. Investig. Dermatol. 2015, 135, 1954–1968. [Google Scholar] [CrossRef]
- Quan, T.; Fisher, G.J. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Qin, Z.; Fisher, G.J.; Voorhees, J.J.; Quan, T.J.; Jo, C.; Medicine, M. Actin cytoskeleton assembly regulates collagen production via TGF-β type II receptor in human skin fibroblasts. J. Cell Mol. Med. 2018, 22, 4085–4096. [Google Scholar] [CrossRef]
- Jung, K.; Linse, F.; Heller, R.; Moths, C.; Goebel, R.; Neumann, C. Adhesion molecules in atopic dermatitis: VCAM-1 and ICAM-1 expression is increased in healthy-appearing skin. Allergy 1996, 51, 452–460. [Google Scholar] [CrossRef]
- Spilioti, E.; Vargiami, M.; Letsiou, S.; Gardikis, K.; Sygouni, V.; Koutsoukos, P.; Chinou, I.; Kassi, E.; Moutsatsou, P. Biological properties of mud extracts derived from various spa resorts. Environ. Geochem. Health 2017, 39, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; Van de Sluis, B.; Kirkland, J.L.; Van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Bhatia-Dey, N.; Kanherkar, R.R.; Stair, S.E.; Makarev, E.O.; Csoka, A.B. Cellular senescence as the causal nexus of aging. Front. Genet. 2016, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, Y.; Guan, Y.; Wei, X.; Chen, S.; Li, X.; Li, Y.; Huang, Z.; Liu, S.; Li, G.; et al. The accelerated aging skin in rhino-like SHJHhr mice. Exp. Dermatol. 2022, 31, 1597–1606. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological functions of autophagy genes: A disease perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Song, X.; Narzt, M.S.; Nagelreiter, I.M.; Hohensinner, P.; Terlecki-Zaniewicz, L.; Tschachler, E.; Grillari, J.; Gruber, F. Autophagy deficient keratinocytes display increased DNA damage, senescence and aberrant lipid composition after oxidative stress in vitro and in vivo. Redox Biol. 2017, 11, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Chin, T.; Tan, C.Y.R.; Rovito, H.A.; Quek, L.S.; Oblong, J.E.; Bellanger, S. Nicotinamide Metabolism Modulates the Proliferation/Differentiation Balance and Senescence of Human Primary Keratinocytes. J. Investig. Dermatol. 2019, 139, 1638–1647. [Google Scholar] [CrossRef]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020, 11, 444. [Google Scholar] [CrossRef]
- Wilson, N.; Kataura, T.; Korsgen, M.E.; Sun, C.; Sarkar, S.; Korolchuk, V.I. The autophagy-NAD axis in longevity and disease. Trends Cell Biol. 2023, 33, 788–802. [Google Scholar] [CrossRef]
- Nadeeshani, H.; Li, J.; Ying, T.; Zhang, B.; Lu, J. Nicotinamide mononucleotide (NMN) as an anti-aging health product—Promises and safety concerns. J. Adv. Res. 2021, 37, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Rubinsztein, D.C.; Walker, D.W. Autophagy as a promoter of longevity: Insights from model organisms. Nat. Rev. Mol. Cell Biol. 2018, 19, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Hou, H.; Fan, Y.; Wang, S.; Chen, Q.; Si, L.; Li, B. Protective effect of gelatin peptides from pacific cod skin against photoaging by inhibiting the expression of MMPs via MAPK signaling pathway. J. Photochem. Photobiol. B 2016, 165, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Tan, J.; Yang, X.; Tan, H.; Xu, X.; You, M.; Qin, W.; Huang, L.; Li, S.; Mo, M.; et al. Polysaccharide Extracted from Laminaria japonica Delays Intrinsic Skin Aging in Mice. Evid. Based Complement. Alternat Med. 2016, 2016, 5137386. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, M.B.; Yun, J.G.; Hwang, J.K. Protective effects of standardized siegesbeckia glabrescens extract and its active compound kirenol against UVB-inducedphotoaging through inhibition of MAPK/NF-κb pathways. J. Microbiol. Biotechnol. 2017, 27, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Park, S.Y.; Hwang, E.; Zhang, M.; Seo, S.A.; Lin, P.; Yi, T.H. Thymusvulgaris alleviates UVB irradiation induced skin damage via inhibition of MAPK/AP-1and activation of Nrf2-ARE antioxidant system. J. Cell. Mol. Med. 2017, 21, 336–348. [Google Scholar] [CrossRef]
- Wen, W.; Chen, J.; Ding, L.; Luo, X.; Zheng, X.; Dai, Q.; Gu, Q.; Liu, C.; Liang, M.; Guo, X.; et al. Astragaloside exerts anti-photoaging effects in UVB-inducedpremature senescence of rat dermal fibroblasts through enhanced autophagy. Biochem. Biophys. 2018, 657, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qin, Y.; Liu, B.; Gao, M.; Li, A.; Li, X.; Gong, G. PGC-1alpha-Mediated Mitochondrial Quality Control: Molecular Mechanisms and Implications for Heart Failure. Front. Cell Dev. Biol. 2022, 10, 871357. [Google Scholar]
- Wong, W.; Crane, E.D.; Zhang, H.; Li, J.; Day, T.A.; Green, A.E.; Menzies, K.J.; Crane, J.D. PGC-1alpha controls epidermal stem cell fate and skin repair by sustaining NAD+ homeostasis during aging. Mol. Metab. 2022, 65, 101575. [Google Scholar] [CrossRef]
- Park, J.E.; Woo, S.W.; Kim, M.B.; Kim, C.; Hwang, J.K. Standardized kaempferia parviflora extract inhibits intrinsic aging process in human dermal fibroblasts and hairless mice by inhibiting cellular senescence and mitochondrial dysfunction. Evid. Based Complement. Altern. Med. 2017, 2017, 6861085. [Google Scholar] [CrossRef]
- Xiao, B.; Sanders, M.J.; Underwood, E.; Heath, R.; Mayer, F.V.; Carmena, D.; Jing, C.; Walker, P.A.; Eccleston, J.F.; Haire, L.F.; et al. Structure of mammalian AMPK and its regulation by ADP. Nature 2011, 472, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, au tophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Corona Velazquez, A.F.; Jackson, W.T. So Many Roads: The Multifaceted Regulation of Autophagy Induction. Mol. Cell Biol. 2018, 38, e00303-18. [Google Scholar] [CrossRef] [PubMed]
- Nakada, D.; Saunders, T.L.; Morrison, S.J. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature 2010, 468, 653–658. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, R.; Zhang, Y.; Liu, R.; Wei, C.; Wang, X.; Wu, X.; Yu, X.; Li, Z.; Mao, R.; Hu, J.; et al. Exogenous Nucleotides Improve the Skin Aging of SAMP8 Mice by Modulating Autophagy through MAPKs and AMPK Pathways. Nutrients 2024, 16, 1907. https://doi.org/10.3390/nu16121907
Fan R, Zhang Y, Liu R, Wei C, Wang X, Wu X, Yu X, Li Z, Mao R, Hu J, et al. Exogenous Nucleotides Improve the Skin Aging of SAMP8 Mice by Modulating Autophagy through MAPKs and AMPK Pathways. Nutrients. 2024; 16(12):1907. https://doi.org/10.3390/nu16121907
Chicago/Turabian StyleFan, Rui, Ying Zhang, Rui Liu, Chan Wei, Xiujuan Wang, Xin Wu, Xiaochen Yu, Zhen Li, Ruixue Mao, Jiani Hu, and et al. 2024. "Exogenous Nucleotides Improve the Skin Aging of SAMP8 Mice by Modulating Autophagy through MAPKs and AMPK Pathways" Nutrients 16, no. 12: 1907. https://doi.org/10.3390/nu16121907
APA StyleFan, R., Zhang, Y., Liu, R., Wei, C., Wang, X., Wu, X., Yu, X., Li, Z., Mao, R., Hu, J., Zhu, N., Liu, X., Li, Y., & Xu, M. (2024). Exogenous Nucleotides Improve the Skin Aging of SAMP8 Mice by Modulating Autophagy through MAPKs and AMPK Pathways. Nutrients, 16(12), 1907. https://doi.org/10.3390/nu16121907





