Probiotic Supplementation in the Neonatal Age Group and the Risk of Hospitalisation in the First Two Years: A Data Linkage Study from Western Australia
Abstract
:1. Introduction
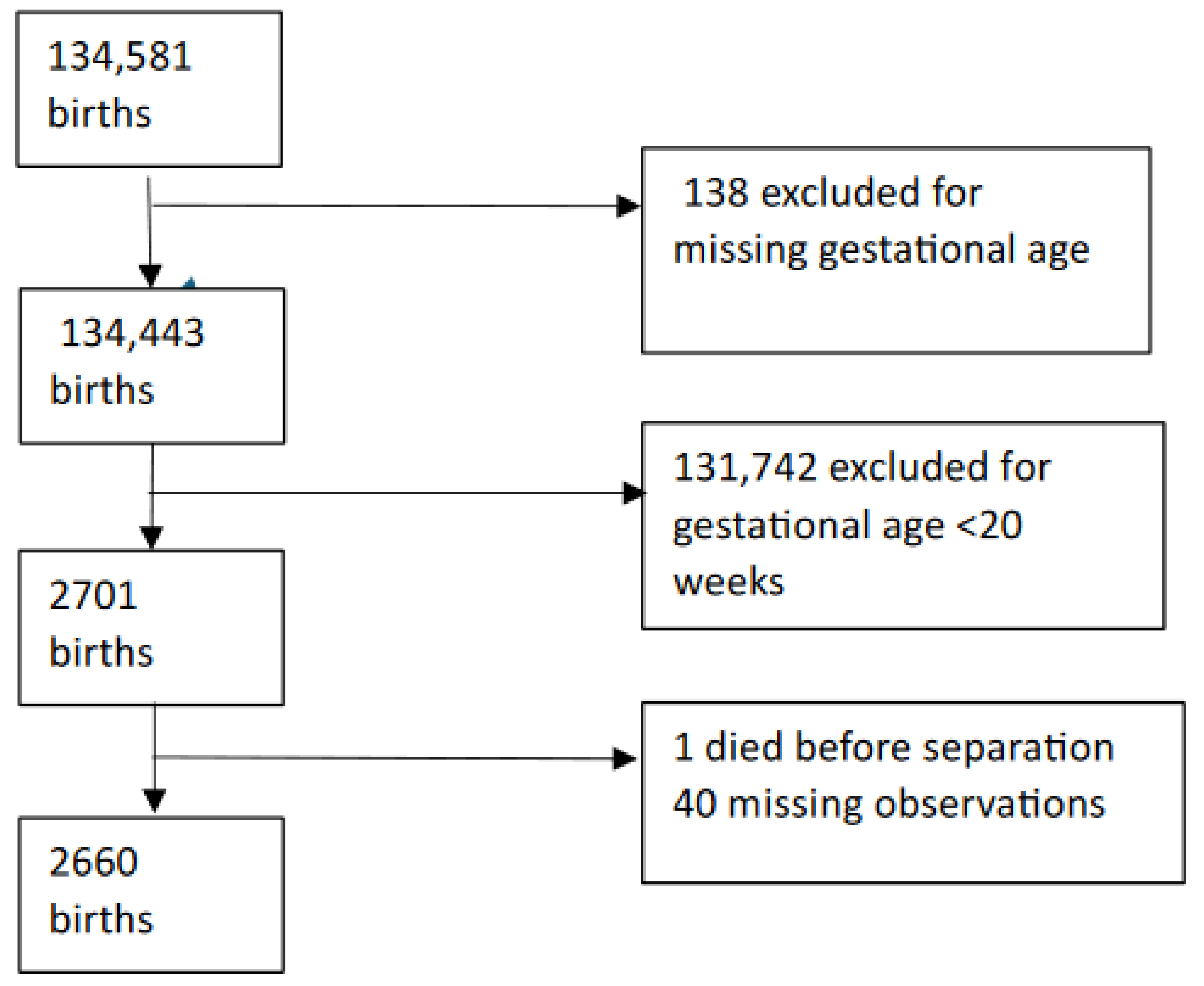
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Respiratory Infection Related Hospitalisations | ||||||
|---|---|---|---|---|---|---|
| J10–18 | J10 | J20–22 | J20 | J06.9 | J22 | J98.8 |
| J10.0 | J20.0 | |||||
| J10.1 | J20.1 | |||||
| J10.8 | J20.2 | |||||
| J11 | J20.3 | |||||
| J11.0 | J20.4 | |||||
| J11.1 | J20.5 | |||||
| J11.8 | J20.6 | |||||
| J12 | J20.7 | |||||
| J12.0 | J20.8 | |||||
| J12.1 | J20.9 | |||||
| J12.2 | J21 | |||||
| J12.3 | J21.0 | |||||
| J12.8 | J21.8 | |||||
| J12.9 | J21.9 | |||||
| J13 | J22 | |||||
| J14 | ||||||
| J15 | ||||||
| J15.0 | ||||||
| J15.1 | ||||||
| J15.2 | ||||||
| J15.3 | ||||||
| J15.4 | ||||||
| J15.5 | ||||||
| J15.6 | ||||||
| J15.7 | ||||||
| J15.8 | ||||||
| J15.9 | ||||||
| J16 | ||||||
| J16.0 | ||||||
| J16.8 | ||||||
| J17 | ||||||
| J17.0 | ||||||
| J17.1 | ||||||
| J17.2 | ||||||
| J17.3 | ||||||
| J17.8 | ||||||
| J18 |
| A08 |
| A08.0 |
| A08.1 |
| A08.11 |
| A08.19 |
| A08.2 |
| A08.3 |
| A08.31 |
| A08.32 |
| A08.39 |
| A08.4 |
| A08.8 |
| A09 |
Appendix B
| Variable | N | Excluded (n) | Epoch 1 (n) | Epoch 2 (n) |
|---|---|---|---|---|
| 134,581 | 62,552 | 72,029 | ||
| GA missing | 138 | 62,490 | 71,953 | |
| GA < 22 or > 32 weeks | 134,443 | 1267 | 1434 | |
| 2-year follow-up | 2701 | 131,742 | 1266 | 1434 |
| Records of death before separation | 2700 | 1 | 1 | 0 |
| Other exclusions | 2699 | 9 | 27 | 12 |
| Numbers included | 1238 | 1422 |
Appendix C
| Model 1 | Adjusted for GA | Adjusted for GA, Smoking, and Ethnicity | Adjusted for GA, Smoking, Ethnicity, Maternal Age, and SES | Restricted for GA ≤ 28 Weeks | Restricted to GA ≤ 28 Weeks, and Adjusted for GA | |
|---|---|---|---|---|---|---|
| Characteristic | IRR 1 (95% CI 2) | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) |
| Epoch 1 | ||||||
| Epoch 2 | 0.94 (0.88, 1.00) | 0.93 (0.87, 0.99) | 0.92 (0.87, 0.98) | 0.92 (0.87, 0.98) | 0.96 (0.87, 1.05) | 0.93 (0.85, 1.02) |
| GA (reversed) | 0.97 (0.96, 0.97) | 0.97 (0.96, 0.97) | 0.97 (0.96, 0.97) | 0.88 (0.87, 0.90) | ||
| Smoking | ||||||
| No | ||||||
| Yes | 1.09 (1.00, 1.18) | 1.07 (0.99, 1.17) | ||||
| <20 | - | |||||
| 20–39 | 0.90 (0.82, 1.00) | |||||
| 40–59 | 0.96 (0.87, 1.07) | |||||
| 60–79 | 0.87 (0.78, 0.96) | |||||
| >80 | 0.95 (0.86, 1.06) | |||||
| Maternal age (years) | ||||||
| 25–29 | ref | |||||
| <20 | 0.91 (0.80, 1.03) | |||||
| 20–24 | 0.96 (0.86, 1.06) | |||||
| 30–34 | 0.89 (0.82, 0.96) | |||||
| 35–39 | 0.87 (0.78, 0.96) | |||||
| 40+ | 0.83 (0.68, 1.02) | |||||
Appendix D
| GI Infection (Unadjusted) | GI Infection (Adjusted for Smoking, Ethnicity, Maternal Age, and SES) | Respiratory Infection (Unadjusted) | Respiratory Infection (Adjusted for Smoking, Ethnicity, Maternal Age, and SES) | |
|---|---|---|---|---|
| Epoch 1 | ||||
| Epoch 2 | 1.10 (0.73, 1.68) | 1.00 (0.66, 1.52) | 0.80 (0.67, 0.94) | 0.82 (0.69, 0.98) |
| GA reversed | 0.88 (0.82, 0.93) | 0.94 (0.91, 0.97) | ||
| Smoking | ||||
| No | ||||
| Yes | 1.51 (0.91, 2.49) | 1.21 (0.98, 1.50) | ||
| SES quintiles | ||||
| <20 | ||||
| 20–39 | 0.64 (0.32, 1.21) | 0.86 (0.66, 1.12) | ||
| 40–59 | 0.96 (0.51, 1.78) | 0.86 (0.66, 1.12) | ||
| 60–79 | 0.76 (0.40, 1.43) | 0.74 (0.56, 0.98) | ||
| >80 | 0.62 (0.29, 1.27) | 0.82 (0.62, 1.09) | ||
| Maternal age (yrs) | ||||
| 25–29 | ||||
| <20 | 0.47 (0.19, 1.01) | 0.91 (0.66, 1.23) | ||
| 20–24 | 0.65 (0.22, 1.21) | 1.15 (0.89, 1.49) | ||
| 30–34 | 0.66 (0.39, 1.10) | 0.78 (0.62, 0.98) | ||
| 35–39 | 0.39 (0.16, 0.84) | 0.69 (0.50, 0.92) | ||
| >40+ | 0.61 (0.10, 2.02) | 0.51 (0.23, 0.96) | ||
References
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Lopez, M.; Dinsmoor, A.M.; Ho, T.T.B.; Donovan, S.M. A systematic review of the factors influencing microbial colonization of the preterm infant gut. Gut Microbes 2021, 13, 1884514. [Google Scholar] [CrossRef]
- Baranowski, J.R.; Claud, E.C. Necrotizing Enterocolitis and the Preterm Infant Microbiome. Adv. Exp. Med. Biol. 2019, 1125, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Halloran, K.; Underwood, M.A. Probiotic mechanisms of action. Early Hum. Dev. 2019, 135, 58–65. [Google Scholar] [CrossRef]
- Campbell, C.; Kandalgaonkar, M.R.; Golonka, R.M.; Yeoh, B.S.; Vijay-Kumar, M.; Saha, P. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines 2023, 11, 294. [Google Scholar] [CrossRef]
- Morgan, R.L.; Preidis, G.A.; Kashyap, P.C.; Weizman, A.V.; Sadeghirad, B. Probiotics Reduce Mortality and Morbidity in Preterm, Low-Birth-Weight Infants: A Systematic Review and Network Meta-analysis of Randomized Trials. Gastroenterology 2020, 159, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Florez, I.D.; Morgan, R.L.; Foroutan, F.; Chang, Y.; Crandon, H.N.; Zeraatkar, D.; Bala, M.M.; Mao, R.Q.; Tao, B.; et al. Probiotics, Prebiotics, Lactoferrin, and Combination Products for Prevention of Mortality and Morbidity in Preterm Infants: A Systematic Review and Network Meta-Analysis. JAMA Pediatr. 2023, 177, 1158–1167. [Google Scholar] [CrossRef]
- Batta, V.K.; Rao, S.C.; Patole, S.K. Bifidobacterium infantis as a probiotic in preterm infants: A systematic review and meta-analysis. Pediatr. Res. 2023, 94, 1887–1905. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, M.; Patole, S. Prophylactic Probiotic Supplementation for Preterm Neonates—A Systematic Review and Meta-Analysis of Nonrandomized Studies. Adv. Nutr. 2021, 12, 1411–1423. [Google Scholar] [CrossRef]
- Rath, C.P.; Athalye-Jape, G.; Nathan, E.; Doherty, D.; Rao, S.; Patole, S. Benefits of routine probiotic supplementation in preterm infants. Acta Paediatr. 2023, 112, 2352–2358. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, G.; Rao, S.; Patole, S. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight: A systematic review of randomised controlled trials. Lancet 2007, 369, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Srinivasjois, R.; Gebremedhin, A.; Silva, D.; Rao, S.; Pereira, G. Probiotic supplementation in neonates and long-term gut colonisation: A systematic review of randomised controlled trials. J. Paediatr. Child Health 2023, 59, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, A.; Tounian, P.; Adel-Patient, K.; Thomas, M. Pre-, pro-, syn-, and Postbiotics in Infant Formulas: What Are the Immune Benefits for Infants? Nutrients 2023, 15, 1231. [Google Scholar] [CrossRef] [PubMed]
- Patole, S.K.; Rao, S.C.; Keil, A.D.; Nathan, E.A.; Doherty, D.A.; Simmer, K.N. Benefits of Bifidobacterium breve M-16V Supplementation in Preterm Neonates—A Retrospective Cohort Study. PLoS ONE 2016, 11, e0150775. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, G.; Rao, S.; Patole, S.; Bulsara, M. Updated Meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 2010, 125, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Patole, S.; Keil, A.D.; Chang, A.; Nathan, E.; Doherty, D.; Simmer, K.; Esvaran, M.; Conway, P. Effect of bifidobacterium breve M-16V supplementation on fecal bifidobacteria in preterm neonates—A randomised double blind placebo controlled trial. PLoS ONE 2014, 9, e89511. [Google Scholar] [CrossRef] [PubMed]
- Schneuer, F.J.; Demetriou, E.; Bond, D.; Lain, S.J.; Guastella, A.J.; Nassar, N. Child characteristics and health conditions associated with paediatric hospitalisations and length of stay: A population-based study. Lancet Reg. Health—West. Pac. 2023, 32, 100706. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, R.; Elsherbini, A.M.A.; Harms, M.; Alber, S.; Stemmler, R.; Peschel, A. Secretory IgA impacts the microbiota density in the human nose. Microbiome 2023, 11, 233. [Google Scholar] [CrossRef]
- Cangiano, L.R.; Villot, C.; Amorin-Hegedus, R.; Malmuthuge, N.; Gruninger, R.; Guan, L.L.; Steele, M. Saccharomyces cerevisiae boulardii accelerates intestinal microbiota maturation and is correlated with increased secretory IgA production in neonatal dairy calves. Front. Microbiol. 2023, 14, 1129250. [Google Scholar] [CrossRef]
- Eladham, M.W.; Selvakumar, B.; Sharif-Askari, N.S.; Sharif-Askari, F.S.; Ibrahim, S.M.; Halwani, R. Unraveling the gut-Lung axis: Exploring complex mechanisms in disease interplay. Heliyon 2024, 10, e24032. [Google Scholar] [CrossRef] [PubMed]
- Chunxi, L.; Haiyue, L.; Yanxia, L.; Jianbing, P.; Jin, S. The Gut Microbiota and Respiratory Diseases: New Evidence. J. Immunol. Res. 2020, 2020, 2340670. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, J.; Zhu, Z.; Liu, X.; Shen, T.; Wang, Y.; Ma, Q.; Wang, X.; Yang, G.; Guo, G.; et al. Gut Microbiota and Respiratory Infections: Insights from Mendelian Randomization. Microorganisms 2023, 11, 2108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dong, B.R.; Hao, Q. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2022, 8, CD006895. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.-J.; Sun, Q.-M.; Nie, D.-Y.; Wang, Q.; Zhang, H.; Qin, F.-F.; Wang, Q.-S.; Lu, S.-F.; Pang, G.-M.; Lu, Z.-G. Probiotics protect against RSV infection by modulating the microbiota-alveolar-macrophage axis. Acta Pharmacol. Sin. 2021, 42, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Guo, S.; Liu, Y.; Wang, G.; Wu, H. Association between probiotics and bronchopulmonary dysplasia in preterm infants. Sci. Rep. 2021, 11, 17060. [Google Scholar] [CrossRef] [PubMed]
- Srinivasjois, R.; Slimings, C.; Einarsdóttir, K.; Burgner, D.; Leonard, H. Association of Gestational Age at Birth with Reasons for Subsequent Hospitalisation: 18 Years of Follow-Up in a Western Australian Population Study. PLoS ONE 2015, 10, e0130535. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.; Burgner, D.; Carville, K.; Jacoby, P.; Richmond, P.; Lehmann, D. Diverging trends for lower respiratory infections in non-Aboriginal and Aboriginal children. J. Paediatr. Child Health 2007, 43, 451–457. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, K.F.; Torzillo, P.J.; Chang, A.B. Hospitalisation of Indigenous children in the Northern Territory for lower respiratory illness in the first year of life. Med. J. Aust. 2010, 192, 586–590. [Google Scholar] [CrossRef]
- Self, A.; Van Buskirk, J.; Clark, J.; Cochrane, J.E.; Knibbs, L.; Cass-Verco, J.; Gupta, L. Respiratory syncytial virus disease morbidity in Australian infants aged 0 to 6 months: A systematic review with narrative synthesis. BMC Public Health 2023, 23, 2560. [Google Scholar] [CrossRef]
- Ritchie, B.K.; Brewster, D.R.; Tran, C.D.; Davidson, G.P.; McNeil, Y.; Butler, R.N. Efficacy of Lactobacillus GG in aboriginal children with acute diarrhoeal disease: A randomised clinical trial. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B. The gut-brain axis in Parkinson’s disease. Rev. Neurol. 2023, 180, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, G.; Traina, G. Neuroinflammation in the Brain and Role of Intestinal Microbiota: An Overview of the Players. J. Integr. Neurosci. 2024, 22, 148. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, D.M.S.; Jayasena, V.; Rainey-Smith, S.R.; Martins, R.N.; Fernando, W. The Role of Diet and Gut Microbiota in Alzheimer’s Disease. Nutrients 2024, 16, 412. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.; Galiè, S. The Microbiota–Gut–Brain Axis in Metabolic Syndrome and Sleep Disorders: A Systematic Review. Nutrients 2024, 16, 390. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Mohajeri, M.H. Potential effects of the most prescribed drugs on the microbiota-gut-brain-axis: A review. Brain Res. Bull. 2024, 207, 110883. [Google Scholar] [CrossRef]
- Mudaliar, S.B.; Poojary, S.S.; Prasad, A.S.B.; Mazumder, N. Probiotics and Paraprobiotics: Effects on Microbiota-Gut-Brain Axis and Their Consequent Potential in Neuropsychiatric Therapy. Probiotics Antimicrob. Proteins 2024, 1–25. [Google Scholar] [CrossRef]
- Wolstenholme, J.T.; Duong, N.K.; Brocato, E.R.; Bajaj, J.S. Gut-Liver-Brain Axis and Alcohol Use Disorder: Treatment Potential of Fecal Microbiota Transplantation. Alcohol Res. Curr. Rev. 2024, 44, 1. [Google Scholar] [CrossRef]
- Panchal, H.; Athalye-Jape, G.; Rao, S.; Patole, S. Growth and neuro-developmental outcomes of probiotic supplemented preterm infants—A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2023, 77, 855–871. [Google Scholar] [CrossRef]
- Sharif, S.; Meader, N.; Oddie, S.J.; Rojas-Reyes, M.X.; McGuire, W. Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst. Rev. 2023, 7, CD005496. [Google Scholar] [CrossRef]
- Holman, C.D.J.; A Bass, J.; Rosman, D.L.; Smith, M.B.; Semmens, J.B.; Glasson, E.J.; Brook, E.L.; Trutwein, B.; Rouse, I.L.; Watson, C.R.; et al. A decade of data linkage in Western Australia: Strategic design, applications and benefits of the WA data linkage system. Aust. Health Rev. 2008, 32, 766–777. [Google Scholar] [CrossRef] [PubMed]
| Variable | N (%) | Epoch 1 1238 (%) | Epoch 2 1422 (%) | p Value 1 |
|---|---|---|---|---|
| Maternal age (years) | 0.003 | |||
| 25–29 | 689 (26) | 309 (25) | 380 (27) | |
| <20 | 208 (7.8) | 120 (9.7) | 88 (6.2) | |
| 20–24 | 350 (13) | 172 (14) | 178 (13) | |
| 30–34 | 919 (35) | 405 (33) | 514 (36) | |
| 35–39 | 418 (16) | 204 (16) | 214 (15) | |
| >40 | 76 (2.9) | 28 (2.3) | 48 (3.4) | |
| Ethnicity | ||||
| Caucasian | 1814 (68) | 895 (72) | 919 (65) | |
| Others | 846 (32) | 343 (28) | 503 (35) | |
| Smoking during pregnancy | ||||
| Yes No | 510 (19) | 235 (19) | 275 (19) | 0.800 |
| 2150 (81) | 1003 (81) | 1147 (81) | ||
| Gestational age (weeks) | 0.049 | |||
| <24 | 535 (20) | 261 (21) | 274 (19) | |
| 24–28 | 555 (21) | 277 (22) | 278 (20) | |
| 28–32 | 1570 (59) | 600 (57) | 870 (61) | |
| SES percentiles 2 | ||||
| <20 | 460 (17) | 212 (17) | 248 (17) | |
| 20–39 | 555 (21) | 255 (21) | 300 (21) | |
| 40–59 | 526 (20) | 250 (20) | 276 (19) | |
| 60–79 | 583 (22) | 260 (21) | 323 (23) | |
| >80 | 536 (20) | 261 (21) | 275 (19) | |
| Hospitalisation (Unadjusted) | Adjusted for GA, Smoking, Ethnicity, Maternal Age, and SES | GI Infection (Unadjusted) | GI Infection (Adjusted for Smoking, Ethnicity, Maternal Age and SES) | Respiratory Infection (Unadjusted) | Respiratory Infection (Adjusted for Smoking, Ethnicity, Maternal Age, and SES) | |
|---|---|---|---|---|---|---|
| IRR 1 (95% CI 2) | IRR (95% CI) | |||||
| Epoch 1 | ||||||
| Epoch 2 | 0.94 (0.88, 1.00) | 0.92 (0.87, 0.98) | 1.10 (0.73, 1.68) | 1.00 (0.66, 1.52) | 0.80 (0.67, 0.94) | 0.82 (0.69, 0.98) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinivasjois, R.; Gebremedhin, A.; Silva, D.; Rao, S.C.; Tessema, G.A.; Pereira, G. Probiotic Supplementation in the Neonatal Age Group and the Risk of Hospitalisation in the First Two Years: A Data Linkage Study from Western Australia. Nutrients 2024, 16, 2094. https://doi.org/10.3390/nu16132094
Srinivasjois R, Gebremedhin A, Silva D, Rao SC, Tessema GA, Pereira G. Probiotic Supplementation in the Neonatal Age Group and the Risk of Hospitalisation in the First Two Years: A Data Linkage Study from Western Australia. Nutrients. 2024; 16(13):2094. https://doi.org/10.3390/nu16132094
Chicago/Turabian StyleSrinivasjois, Ravisha, Amanuel Gebremedhin, Desiree Silva, Shripada C. Rao, Gizachew A. Tessema, and Gavin Pereira. 2024. "Probiotic Supplementation in the Neonatal Age Group and the Risk of Hospitalisation in the First Two Years: A Data Linkage Study from Western Australia" Nutrients 16, no. 13: 2094. https://doi.org/10.3390/nu16132094
APA StyleSrinivasjois, R., Gebremedhin, A., Silva, D., Rao, S. C., Tessema, G. A., & Pereira, G. (2024). Probiotic Supplementation in the Neonatal Age Group and the Risk of Hospitalisation in the First Two Years: A Data Linkage Study from Western Australia. Nutrients, 16(13), 2094. https://doi.org/10.3390/nu16132094








