A Mediation Analysis of Obesity and Adiponectin Association with Postmenopausal Breast Cancer Risk: A Nested Cohort Study in the International Breast Cancer Intervention Study II (IBIS-II) Prevention Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Selection of Cases and Controls
2.3. Biomarkers
2.4. Statistical Analysis
- -
- The natural direct effect (NDE), which describes the effect of BMI on the outcome (time to breast cancer event) independent of mediator M1 (adiponectin);
- -
- The natural indirect effect (NIE), which indicates the effect of BMI on the outcome, is mediated by M1.
- -
- NDE, independent of both mediators;
- -
- NIE, through the second mediator M2;
- -
- NIE, through the first mediator M1, and, possibly also through M2.
3. Results
3.1. Baseline Characteristics, Analysis of Biomarkers, and Breast Cancer Risk
3.2. Mediation Analysis
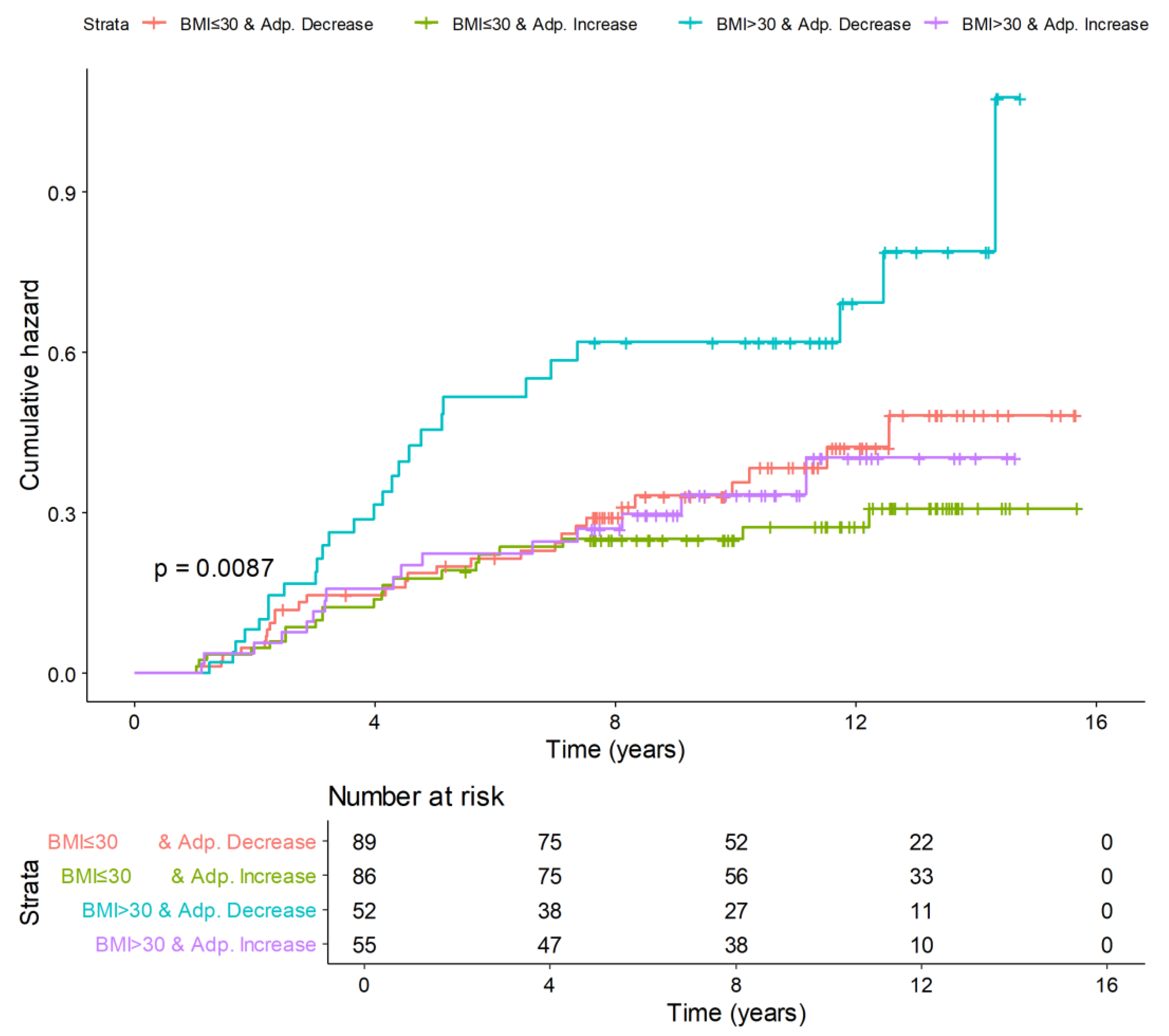
3.3. Replicated Analyses in Obese Subjects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Jemal, A.; Torre, L.A.; Forman, D.; Vineis, P. Long-Term Realism and Cost-Effectiveness: Primary Prevention in Combatting Cancer and Associated Inequalities Worldwide. J. Natl. Cancer Inst. 2015, 107, djv273. [Google Scholar] [CrossRef] [PubMed]
- Crew, K.D.; Silverman, T.B.; Vanegas, A.; Trivedi, M.S.; Dimond, J.; Mata, J.; Sin, M.; Jones, T.; Terry, M.B.; Tsai, W.Y.; et al. Study Protocol: Randomized Controlled Trial of Web-Based Decision Support Tools for High-Risk Women and Healthcare Providers to Increase Breast Cancer Chemoprevention. Contemp. Clin. Trials Commun. 2019, 16, 100433. [Google Scholar] [CrossRef] [PubMed]
- Freedman, A.N.; Graubard, B.I.; Rao, S.R.; Mccaskill-Stevens, W.; Ballard-Barbash, R.; Gail, M.H. Estimates of the Number of U.S. Women Who Could Benefit from Tamoxifen for Breast Cancer Chemoprevention. J. Natl. Cancer Inst. 2003, 95, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Ropka, M.E.; Keim, J.; Philbrick, J.T. Patient Decisions about Breast Cancer Chemoprevention: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2010, 28, 3090–3095. [Google Scholar] [CrossRef] [PubMed]
- Vilaprinyo, E.; Forné, C.; Carles, M.; Sala, M.; Pla, R.; Castells, X.; Domingo, L.; Rue, M.; Blanch, J.; Comas, M.; et al. Cost-Effectiveness and Harm-Benefit Analyses of Risk-Based Screening Strategies for Breast Cancer. PLoS ONE 2014, 9, e86858. [Google Scholar] [CrossRef] [PubMed]
- Thorat, M.A.; Balasubramanian, R. Breast Cancer Prevention in High-Risk Women. Best. Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Pashayan, N.; Antoniou, A.C.; Ivanus, U.; Esserman, L.J.; Easton, D.F.; French, D.; Sroczynski, G.; Hall, P.; Cuzick, J.; Evans, D.G.; et al. Personalized Early Detection and Prevention of Breast Cancer: ENVISION Consensus Statement. Nat. Rev. Clin. Oncol. 2020, 17, 687–705. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-Mass Index and Incidence of Cancer: A Systematic Review and Meta-Analysis of Prospective Observational Studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Renehan, A.G.; Zwahlen, M.; Egger, M. Adiposity and Cancer Risk: New Mechanistic Insights from Epidemiology. Nat. Rev. Cancer 2015, 15, 484–498. [Google Scholar] [CrossRef]
- Olson, T.P.; Dengel, D.R.; Leon, A.S.; Schmitz, K.H. Changes in Inflammatory Biomarkers Following One-Year of Moderate Resistance Training in Overweight Women. Int. J. Obes. 2007, 31, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and Cancer Risk: Emerging Biological Mechanisms and Perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Macis, D.; Guerrieri-Gonzaga, A.; Gandini, S. Circulating Adiponectin and Breast Cancer Risk: A Systematic Review and Meta-Analysis. Int. J. Epidemiol. 2014, 43, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.S.; Kwon, A.R.; Lee, Y.K.; Oh, S.W. Circulating Adipokines and Risk of Obesity Related Cancers: A Systematic Review and Meta-Analysis. Obes. Res. Clin. Pract. 2019, 13, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Frystyk, J.; Flyvbjerg, A. Obesity and Cancer Risk: The Role of the Insulin-IGF Axis. Trends Endocrinol. Metab. 2006, 17, 328–336. [Google Scholar] [CrossRef]
- Simó, R.; Sáez-López, C.; Barbosa-Desongles, A.; Hernández, C.; Selva, D.M. Novel Insights in SHBG Regulation and Clinical Implications. Trends Endocrinol. Metab. 2015, 26, 376–383. [Google Scholar] [CrossRef]
- VanderWeele, T.J. Mediation Analysis: A Practitioner’s Guide. Annu. Rev. Public Health 2016, 37, 17–32. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Forbes, J.F.; Dowsett, M.; Knox, J.; Cawthorn, S.; Saunders, C.; Roche, N.; Mansel, R.E.; Von Minckwitz, G.; et al. Anastrozole for Prevention of Breast Cancer in High-Risk Postmenopausal Women (IBIS-II): An International, Double-Blind, Randomised Placebo-Controlled Trial. Lancet 2014, 383, 1041–1048. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Forbes, J.F.; Dowsett, M.; Cawthorn, S.; Mansel, R.E.; Loibl, S.; Bonanni, B.; Evans, D.G.; Howell, A. Use of Anastrozole for Breast Cancer Prevention (IBIS-II): Long-Term Results of a Randomised Controlled Trial. Lancet 2020, 395, 117–122. [Google Scholar] [CrossRef]
- Tyrer, J.; Duffy, S.W.; Cuzick, J. A Breast Cancer Prediction Model Incorporating Familial and Personal Risk Factors. Stat. Med. 2004, 23, 1111–1130. [Google Scholar] [CrossRef]
- Macis, D.; Aristarco, V.; Johansson, H.; Guerrieri-gonzaga, A.; Raimondi, S.; Lazzeroni, M.; Sestak, I.; Cuzick, J.; De Censi, A.; Bonanni, B.; et al. A Novel Automated Immunoassay Platform to Evaluate the Association of Adiponectin and Leptin Levels with Breast Cancer Risk. Cancers 2021, 13, 3303. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Yang, H.I. Causal Mediation Analysis of Survival Outcome with Multiple Mediators. Epidemiology 2017, 28, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Delcoigne, B.; Colzani, E.; Prochazka, M.; Gagliardi, G.; Hall, P.; Abrahamowicz, M.; Czene, K.; Reilly, M. Breaking the Matching in Nested Case–Control Data Offered Several Advantages for Risk Estimation. J. Clin. Epidemiol. 2017, 82, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Borgan, Ø.; Langholz, B.; Samuelsen, S.O.; Goldstein, L.; Pogoda, J. Exposure Stratified Case-Cohort Designs. Lifetime Data Anal. 2000, 6, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, K.; Pischon, T. Obesity Biomarkers, Metabolism and Risk of Cancer: An Epidemiological Perspective. Recent Results Cancer Res. 2016, 208, 199–217. [Google Scholar] [PubMed]
- Bhardwaj, P.; Au, C.M.C.; Benito-Martin, A.; Ladumor, H.; Oshchepkova, S.; Moges, R.; Brown, K.A. Estrogens and Breast Cancer: Mechanisms Involved in Obesity-Related Development, Growth and Progression. J. Steroid Biochem. Mol. Biol. 2019, 189, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Devericks, E.N.; Carson, M.S.; McCullough, L.E.; Coleman, M.F.; Hursting, S.D. The Obesity-Breast Cancer Link: A Multidisciplinary Perspective. Cancer Metastasis Rev. 2022, 41, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity: Implications for Metabolic Syndrome, Diabetes, Hypertension, Dyslipidemia, Atherosclerosis, and Cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef] [PubMed]
- García-Estévez, L.; Cortés, J.; Pérez, S.; Calvo, I.; Gallegos, I.; Moreno-Bueno, G. Obesity and Breast Cancer: A Paradoxical and Controversial Relationship Influenced by Menopausal Status. Front. Oncol. 2021, 11, 705911. [Google Scholar] [CrossRef]
- Boyd, N.F.; Martin, L.J.; Yaffe, M.J.; Minkin, S. Mammographic Density and Breast Cancer Risk: Current Understanding and Future Prospects. Breast Cancer Res. 2011, 13, 223. [Google Scholar] [CrossRef]
- Vachon, C.M.; Sasano, H.; Ghosh, K.; Brandt, K.R.; Watson, D.A.; Reynolds, C.; Lingle, W.L.; Goss, P.E.; Li, R.; Aiyar, S.E.; et al. Aromatase Immunoreactivity Is Increased in Mammographically Dense Regions of the Breast. Breast Cancer Res. Treat. 2011, 125, 243–252. [Google Scholar] [CrossRef]
- Kaaks, R.; Rinaldi, S.; Key, T.J.; Berrinno, F.; Peeters, P.H.M.; Biessy, C.; Dossus, L.; Lukanova, A.; Bingham, S.; Khaw, K.T.; et al. Postmenopausal Serum Androgens, Oestrogens and Breast Cancer Risk: The European Prospective Investigation into Cancer and Nutrition. Endocr. Relat. Cancer 2005, 12, 1071–1082. [Google Scholar] [CrossRef]
- Fabian, C. Adiponectin: A Risk Biomarker and Attractive Target for Chemoprevention. J. Clin. Oncol. 2012, 30, 124–126. [Google Scholar] [CrossRef]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The Role of Adiponectin in Cancer: A Review of Current Evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef] [PubMed]
- Taliaferro-Smith, L.; Nagalingam, A.; Zhong, D.; Zhou, W.; Saxena, N.K.; Sharma, D. LKB1 Is Required for Adiponectin-Mediated Modulation of AMPK-S6K Axis and Inhibition of Migration and Invasion of Breast Cancer Cells. Oncogene 2009, 28, 2621–2633. [Google Scholar] [CrossRef]
- Kerr, J.; Anderson, C.; Lippman, S.M. Physical Activity, Sedentary Behaviour, Diet, and Cancer: An Update and Emerging New Evidence. Lancet Oncol. 2017, 18, e457–e471. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Barnett, J.B.; Spence, N.D. Fruit and Vegetable Consumption and Incident Breast Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. Br. J. Cancer 2021, 125, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Malandish, A.; Rosenkranz, S.K. The Impact of Exercise Training on Inflammatory Markers in Postmenopausal Women: A Systemic Review and Meta-Analysis. Exp. Gerontol. 2021, 150, 111398. [Google Scholar] [CrossRef]
- Janiszewska, J.; Ostrowska, J.; Szostak-Węgierek, D. The Influence of Nutrition on Adiponectin—A Narrative Review. Nutrients 2021, 13, 1394. [Google Scholar] [CrossRef]
- Fabian, C.J.; Befort, C.A.; Phillips, T.A.; Nydegger, J.L.; Kreutzjans, A.L.; Powers, K.R.; Metheny, T.; Klemp, J.R.; Carlson, S.E.; Sullivan, D.K.; et al. Change in Blood and Benign Breast Biomarkers in Women Undergoing a Weight-Loss Intervention Randomized to High-Dose v-3 Fatty Acids versus Placebo. Cancer Prev. Res. 2021, 14, 893–904. [Google Scholar] [CrossRef]
- Lim, S.; Quon, M.J.; Koh, K.K. Modulation of Adiponectin as a Potential Therapeutic Strategy. Atherosclerosis 2014, 233, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Manthravadi, S.; Shrestha, A.; Madhusudhana, S. Impact of Statin Use on Cancer Recurrence and Mortality in Breast Cancer: A Systematic Review and Meta-Analysis. Int. J. Cancer 2016, 139, 1281–1288. [Google Scholar] [CrossRef]
- Sebastián-Ochoa, A.; Fernández-García, D.; Reyes-García, R.; Mezquita-Raya, P.; Rozas-Moreno, P.; Alonso-Garcia, G.; Muñoz-Torres, M. Adiponectin and Leptin Serum Levels in Osteoporotic Postmenopausal Women Treated with Raloxifene or Alendronate. Menopause 2012, 19, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Macis, D.; Gandini, S.; Guerrieri-Gonzaga, A.; Johansson, H.; Magni, P.; Ruscica, M.; Lazzeroni, M.; Serrano, D.; Cazzaniga, M.; Mora, S.; et al. Prognostic Effect of Circulating Adiponectin in a Randomized 2 × 2 Trial of Low-Dose Tamoxifen and Fenretinide in Premenopausal Women at Risk for Breast Cancer. J. Clin. Oncol. 2012, 30, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Pfeiler, G.; Konigsberg, R.; Hadji, P.; Fitzal, F.; Tea, M.-K.M.; Vogl, S.; Berger, A.; Exner, R.; Seifert, M.; Singer, C.F.; et al. The Impact of Estrogen Depletion by Aromatase Inhibitors on Adiponectin Serum Levels in Postmenopausal Patients with Breast Cancer. J. Clin. Oncol. 2013, 31, e11601. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Pegington, M.; Zhen Tam, H.; Brentnall, A.; Sestak, I.; Adams, J.; Blake, G.M.; Gareth Evans, D.; Howell, A.; Cuzick, J.; Harvie, M. Body Composition Changes during Breast Cancer Preventive Treatment with Anastrozole: Findings from the IBIS-II Trial. Prev. Med. Rep. 2024, 38, 102620. [Google Scholar] [CrossRef] [PubMed]
- Secretan, B.L.; Scoccianti, C.; Loomis, D. Special Report Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2022, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Navarro Rosenblatt, D.; Thune, I.; Vieira, R.; Norat, T. Body Mass Index and Survival in Women with Breast Cancer—Systematic Literature Review and Meta-Analysis of 82 Follow-up Studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Pischon, T.; Nimptsch, K. Obesity and Risk of Cancer: An Introductory Overview. Recent Results Cancer Res. 2016, 208, 485. [Google Scholar]
- Tworoger, S.S.; Hankinson, S.E. Use of Biomarkers in Epidemiologic Studies: Minimizing the Influence of Measurement Error in the Study Design and Analysis. Cancer Causes Control 2006, 17, 889–899. [Google Scholar] [CrossRef] [PubMed]


| Controls (n = 195) | Cases (n = 92) | p-Value 1 | |
|---|---|---|---|
| Age (years), median [IQR] | 59.9 [56.3, 63.5] | 58.9 [55.8, 63.2] | 0.34 |
| BMI (kg/m2), median [IQR] | 27.7 [24.8, 31.2] | 28.9 [25.6, 34.2] | 0.05 |
| Tyrer–Cuzick score, median [IQR] | 0.08 [0.06, 0.10] | 0.09 [0.07, 0.13] | 0.01 |
| Smoking, n (%) | |||
| Never smoker | 118 (60.5%) | 49 (53.3%) | 0.33 |
| Current smoker | 21 (10.8%) | 15 (16.3%) | |
| Former smoker | 55 (28.2%) | 28 (30.4%) | |
| Oophorectomy, n (%) | |||
| Yes | 29 (14.9%) | 11 (12.0%) | 0.62 |
| No | 165 (84.6%) | 81 (88.0%) | |
| Concomitant medications | |||
| Beta-blockers, n (%) | |||
| Yes | 17 (8.7%) | 10 (10.9%) | 0.71 |
| No | 178 (91.3%) | 82 (89.1%) | |
| Insulin and hypoglycemic drugs, n (%) | |||
| Yes | 1 (0.5%) | 4 (4.3%) | 0.04 |
| No | 194 (99.5%) | 88 (95.7%) | |
| Lipid-lowering medications/supplements, n (%) | |||
| Yes | 55 (28.2%) | 16 (17.4%) | 0.07 |
| No | 140 (71.8%) | 76 (82.6%) | |
| Metformin, n (%) | |||
| Yes | 5 (2.6%) | 5 (5.4%) | 0.30 |
| No | 190 (97.4%) | 87 (94.6%) | |
| Psychotropic drugs, n (%) | |||
| Yes | 24 (12.3%) | 17 (18.5%) | 0.22 |
| No | 171 (87.7%) | 75 (81.5%) | |
| Thyroid drugs, n (%) | |||
| Yes | 18 (9.2%) | 9 (9.8%) | 1.00 |
| No | 177 (90.8%) | 83 (90.2%) | |
| Vitamin D, n (%) | |||
| Yes | 15 (7.7%) | 4 (4.3%) | 0.42 |
| No | 180 (92.3%) | 88 (95.7%) |
| Controls (n = 195) | Cases (n = 92) | p-Value 1 | |
|---|---|---|---|
| Adiponectin (μg/mL) | |||
| Baseline | 9.6 [7.2, 12.8] | 9.8 [7.0, 13.4] | 0.92 |
| 12 months | 9.9 [7.1, 13.1] | 9.4 [7.0, 13.3] | 0.62 |
| Change from baseline | 0.09 [−0.83, 1.13] | −0.21 [−1.05, 0.71] | 0.14 |
| Leptin (ng/mL) | |||
| Baseline | 29.7 [18.0, 48.3] | 34.8 [21.1, 54.5] | 0.17 |
| 12 months | 27.0 [17.1, 44.3] | 34.2 [19.0, 48.7] | 0.14 |
| Change from baseline | −0.55 [−7.26, 5.37] | −0.71 [−8.14, 5.23] | 0.48 |
| L/A ratio | |||
| Baseline | 3.0 [1.6, 6.3] | 3.7 [1.9, 6.3] | 0.39 |
| 12 months | 2.7 [1.5, 5.7] | 3.8 [1.6, 6.9] | 0.21 |
| Change from baseline | −0.10 [−0.80, 0.37] | 0 [−0.73, 0.63] | 0.24 |
| IGF-I (ng/mL) | |||
| Baseline | 121.0 [102.0, 145.0] | 127 [92.2, 147.0] | 0.87 |
| 12 months | 120.0 [101.0, 143.0] | 122.0 [98.0, 149.0] | 0.94 |
| Change from baseline | −1.86 [−10.9, 8.77] | −1.86 [−13.2, 11.5] | 0.96 |
| IGFBP-1 (ng/mL) | |||
| Baseline | 5.6 [2.6, 11.2] | 5.4 [2.2, 12.0] | 0.57 |
| 12 months | 5.1 [2.5, 10.3] | 5.0 [2.2, 9.9] | 0.73 |
| Change from baseline | −0.15 [−2.91, 2.64] | −0.11 [−3.09, 2.34] | 0.90 |
| Glycemia (mg/dL) | |||
| Baseline | 88.0 [80.0, 97.0] | 90.0 [81.5, 105.0] | 0.11 |
| 12 months | 87.0 [78.0, 98.0] | 90.5 [83.0, 103.0] | 0.06 |
| Change from baseline | −1.00 [−12.0, 13.0] | 0.50 [−10.8, 9.00] | 0.89 |
| Insulin (uU/mL) | |||
| Baseline | 7.90 [5.50, 15.8] | 10.7 [6.4, 20.1] | 0.06 |
| 12 months | 9.6 [5.5, 18.0] | 10.3 [6.5, 21.4] | 0.36 |
| Change from baseline | 0.50 [−2.50, 6.85] | −0.45 [−8.80, 3.78] | 0.07 |
| HOMA-IR index | |||
| Baseline | 1.8 [1.1, 4.1] | 2.5 [1.3, 5.1] | 0.06 |
| 12 months | 2.1 [1.1, 4.2] | 2.2 [1.4, 5.2] | 0.29 |
| Change from baseline | 0.11 [−0.74, 1.67] | −0.05 [−2.32, 0.97] | 0.07 |
| hs-CRP (mg/dL) | |||
| Baseline | 0.2 [0.1, 0.4] | 0.2 [0.1, 0.6] | 0.07 |
| 12 months | 0.2 [0.1, 0.39] | 0.2 [0.1, 0.5] | 0.10 |
| Change from baseline | 0 [−0.06, 0.06] | −0.01 [−0.09, 0.07] | 0.54 |
| SHBG (nmol/L) | |||
| Baseline | 46.8 [34.8, 61.5] | 43.8 [28.1, 58.1] | 0.06 |
| 12 months | 48.4 [36.5, 63.9] | 43.9 [30.4, 58.8] | 0.07 |
| Change from baseline | 0.40 [−4.10, 7.10] | 1.85 [−2.88, 5.58] | 0.49 |
| Hazard Ratio | 95% Confidence Interval | |
|---|---|---|
| Natural Direct Effect of BMI | 1.05 | [1.00, 1.09] |
| Natural Indirect Effect of BMI via adiponectin increase (M1) | 1.00 | [0.98, 1.02] |
| Total Effect of BMI | 1.05 | [1.00, 1.10] |
| Hazard Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Baseline BMI (continuous) | 1.05 | [1.00, 1.09] | 0.03 |
| Adiponectin increase (Yes vs. No) | 0.60 | [0.36, 1.00] | 0.05 |
| Tyrer–Cuzick score difference (High vs. Low) 1 | 1.74 | [1.05, 2.89] | 0.03 |
| Lipid-lowering medications and supplements (Yes vs. No) | 0.53 | [0.28, 1.02] | 0.06 |
| Hazard Ratio | 95% Confidence Interval | |
|---|---|---|
| Natural Direct Effect of BMI > 30 | 1.71 | [1.03, 2.85] |
| Natural Indirect Effect of BMI > 30 via adiponectin increase (M1) | 0.95 | [0.68, 1.25] |
| Total Effect of BMI > 30 | 1.62 | [0.90, 2.90] |
| Hazard Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| BMI > 30 (Yes vs. No) | 1.71 | [1.03, 2.86] | 0.04 |
| Adiponectin increase (Yes vs. No) | 0.60 | [0.36, 0.99] | 0.04 |
| Tyrer–Cuzick score difference (High vs. Low) 1 | 1.82 | [1.10, 2.99] | 0.02 |
| Lipid-lowering medications and supplements (Yes vs. No) | 0.57 | [0.30, 1.06] | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macis, D.; Bellerba, F.; Aristarco, V.; Johansson, H.; Guerrieri-Gonzaga, A.; Lazzeroni, M.; Sestak, I.; Cuzick, J.; DeCensi, A.; Bonanni, B.; et al. A Mediation Analysis of Obesity and Adiponectin Association with Postmenopausal Breast Cancer Risk: A Nested Cohort Study in the International Breast Cancer Intervention Study II (IBIS-II) Prevention Trial. Nutrients 2024, 16, 2098. https://doi.org/10.3390/nu16132098
Macis D, Bellerba F, Aristarco V, Johansson H, Guerrieri-Gonzaga A, Lazzeroni M, Sestak I, Cuzick J, DeCensi A, Bonanni B, et al. A Mediation Analysis of Obesity and Adiponectin Association with Postmenopausal Breast Cancer Risk: A Nested Cohort Study in the International Breast Cancer Intervention Study II (IBIS-II) Prevention Trial. Nutrients. 2024; 16(13):2098. https://doi.org/10.3390/nu16132098
Chicago/Turabian StyleMacis, Debora, Federica Bellerba, Valentina Aristarco, Harriet Johansson, Aliana Guerrieri-Gonzaga, Matteo Lazzeroni, Ivana Sestak, Jack Cuzick, Andrea DeCensi, Bernardo Bonanni, and et al. 2024. "A Mediation Analysis of Obesity and Adiponectin Association with Postmenopausal Breast Cancer Risk: A Nested Cohort Study in the International Breast Cancer Intervention Study II (IBIS-II) Prevention Trial" Nutrients 16, no. 13: 2098. https://doi.org/10.3390/nu16132098
APA StyleMacis, D., Bellerba, F., Aristarco, V., Johansson, H., Guerrieri-Gonzaga, A., Lazzeroni, M., Sestak, I., Cuzick, J., DeCensi, A., Bonanni, B., & Gandini, S. (2024). A Mediation Analysis of Obesity and Adiponectin Association with Postmenopausal Breast Cancer Risk: A Nested Cohort Study in the International Breast Cancer Intervention Study II (IBIS-II) Prevention Trial. Nutrients, 16(13), 2098. https://doi.org/10.3390/nu16132098








