IL-17A Drives Oxidative Stress and Cell Growth in A549 Lung Epithelial Cells: Potential Protective Action of Oleuropein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Biochemicals
2.2. Cell Cultures and Stimuli
2.3. Detection of Intracellular Reactive Oxygen Species (ROS) and Mitochondrial Membrane Potential (JC1) Measurement
2.4. Cell Apoptosis
2.5. γH2AX by Flow Cytometry
2.6. Immunofluorescence of γH2AX Foci Formation
2.7. DNA Damage Analysis
2.8. MTS AssayA549 Cells
2.9. Clonogenic Assay
2.10. Long Term Exposure of A549 Cells
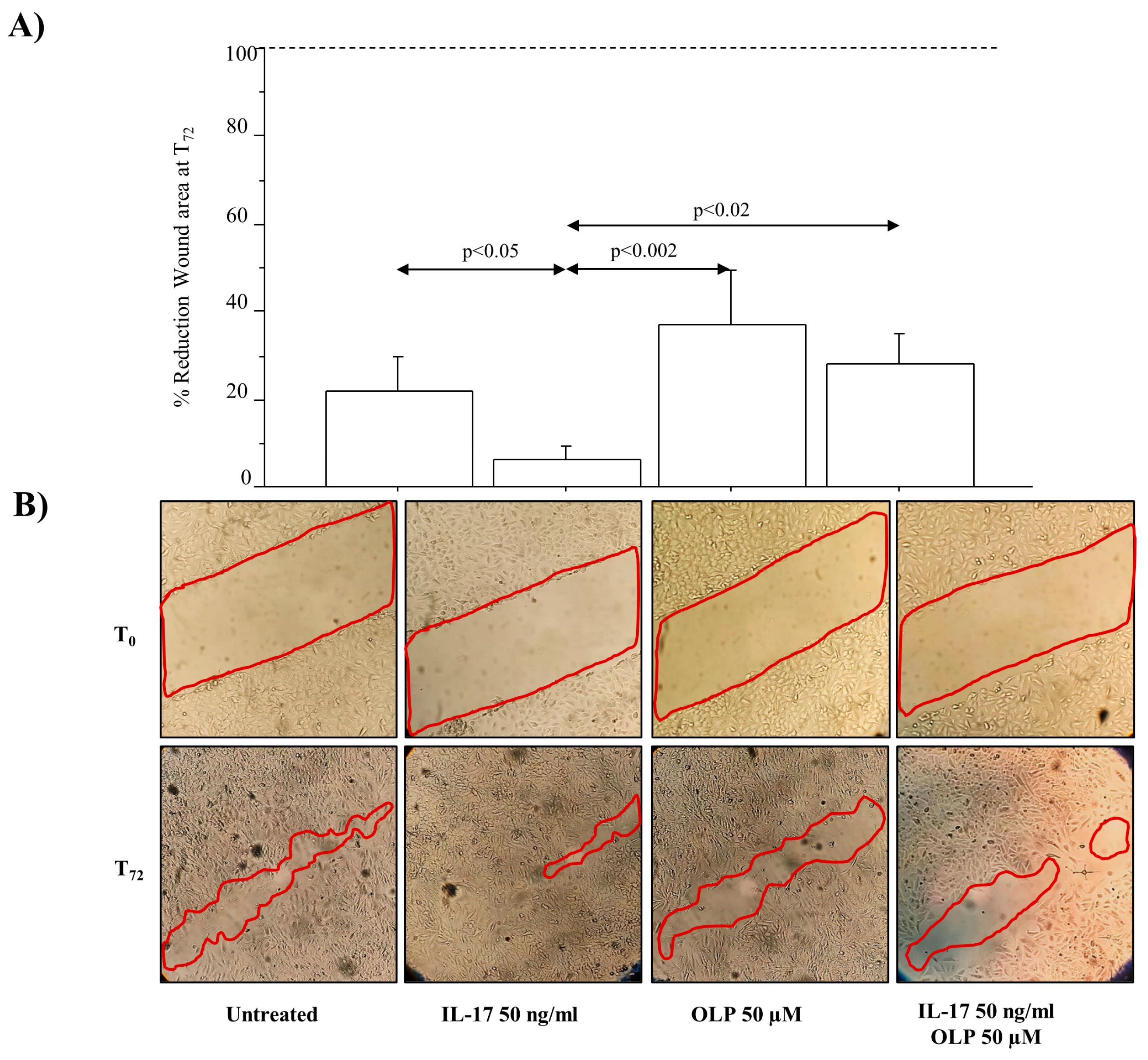
2.11. Cell Migration Assay (Scratch/Wound Healing Assay)
2.12. Statistical Analysis
3. Results
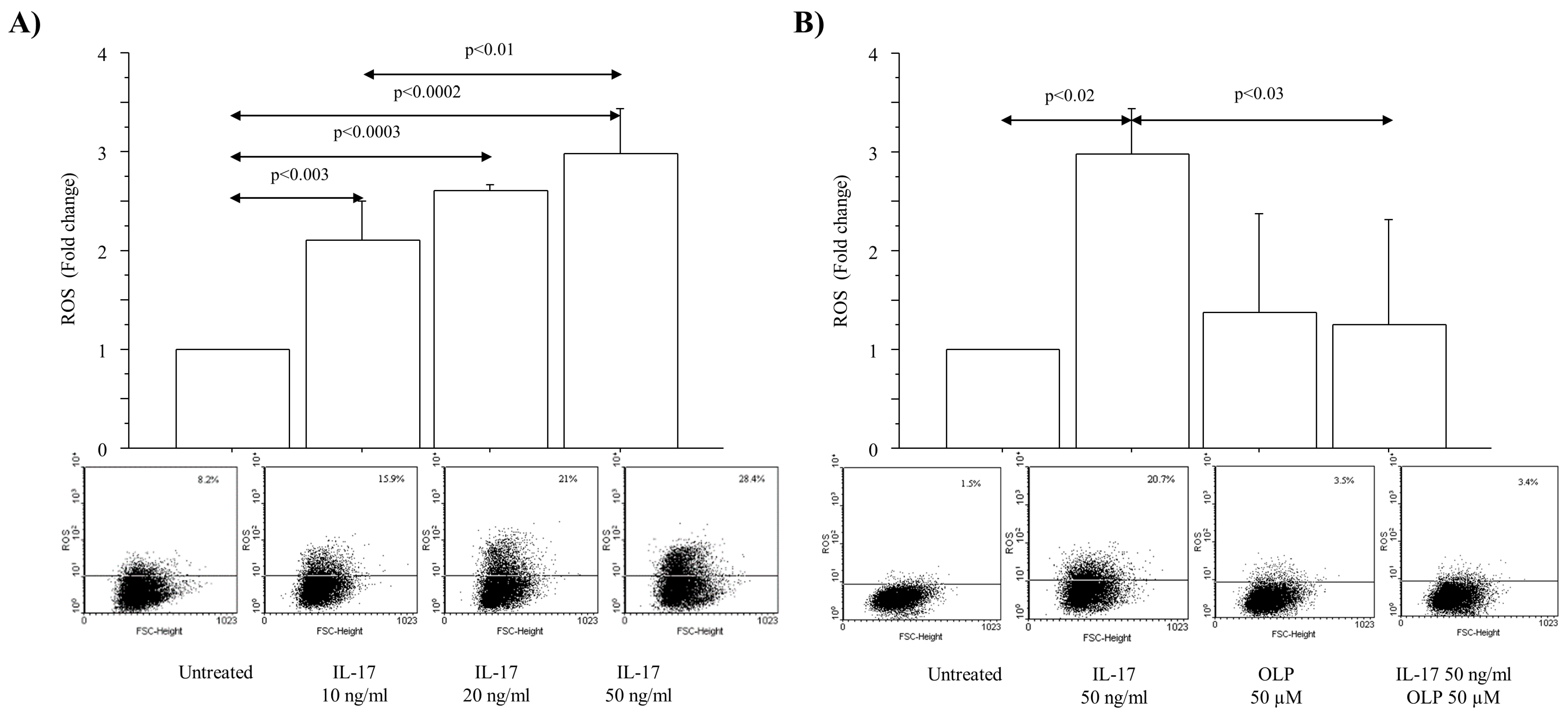
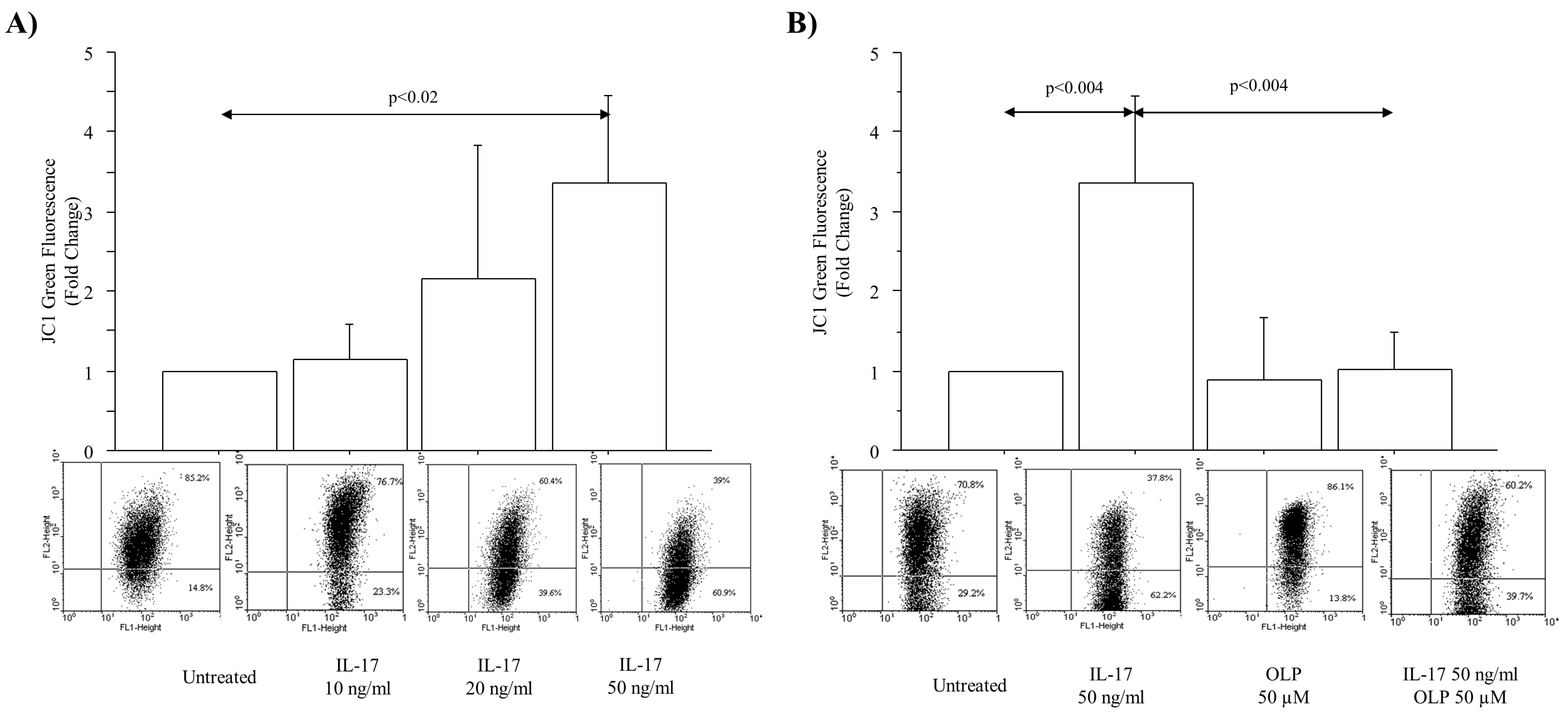
3.1. IL-17A Drives ROS Production and Mitochondrial Injury in A549 Cells: Effect of OLP
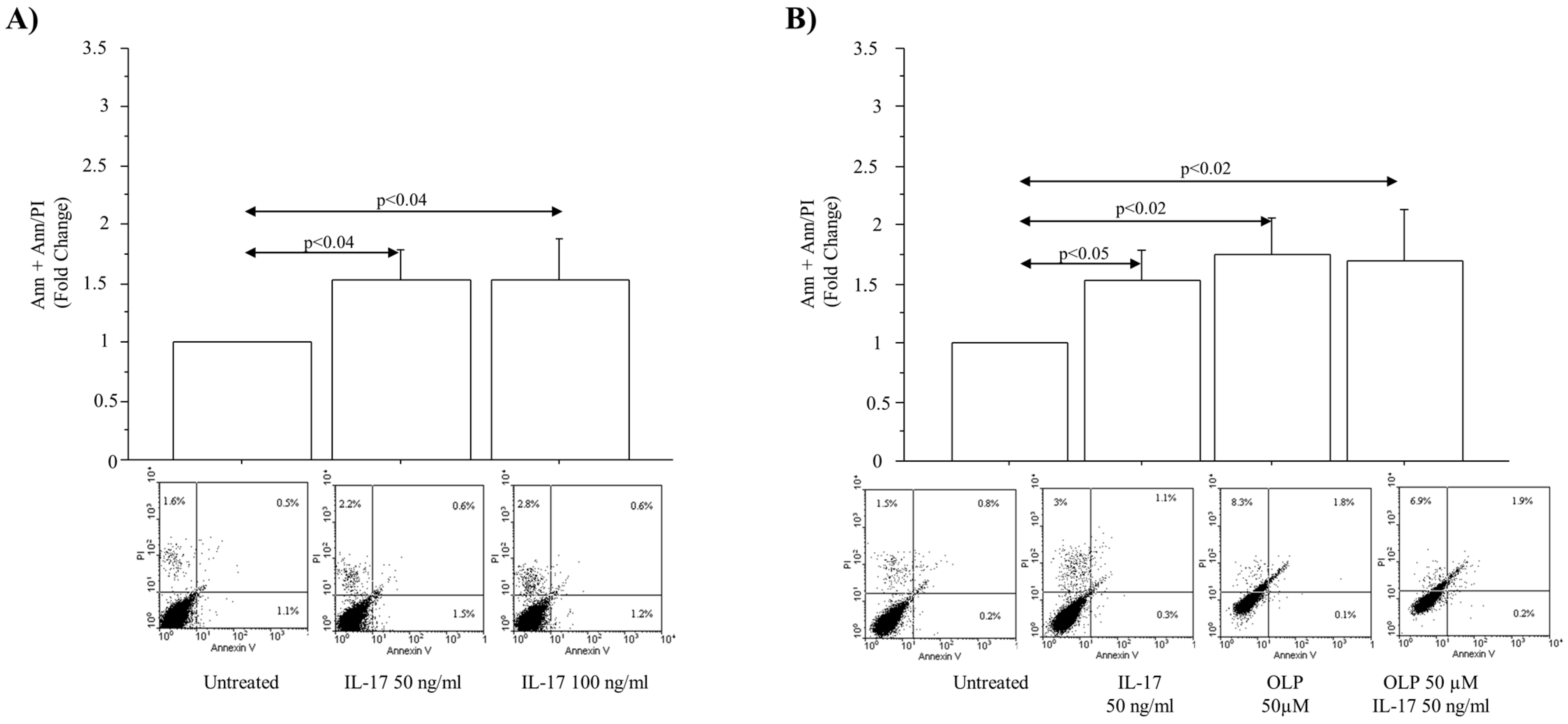
3.2. IL-17A Drives Cell Apoptosis in A549 Cells: Effect of OLP
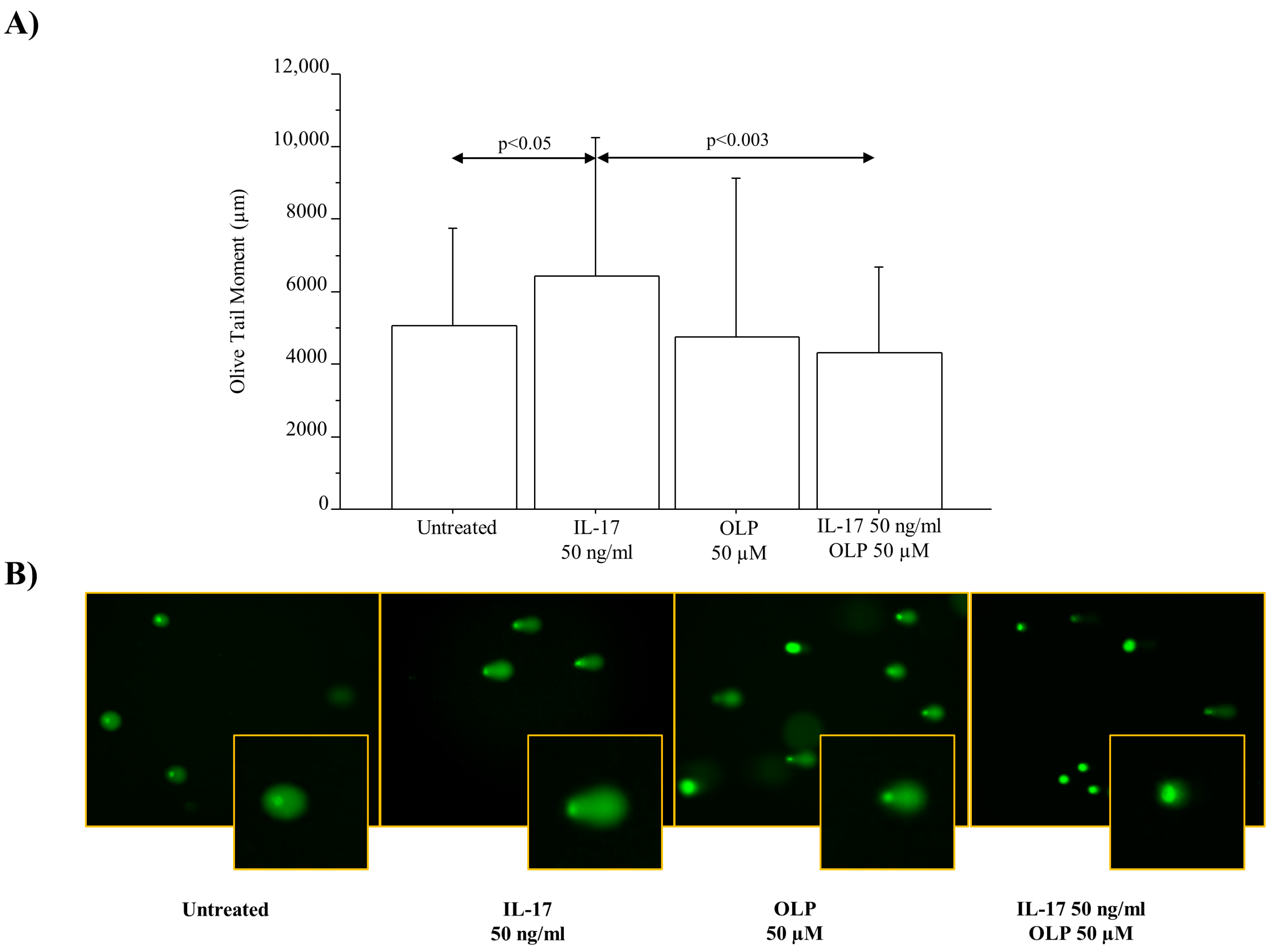
3.3. IL-17A Drives DNA Damage/Repair Mechanisms in A549 Cells: Effect of OLP
3.4. IL-17A Drives Cell Viability during Proliferation: Effect of OLP
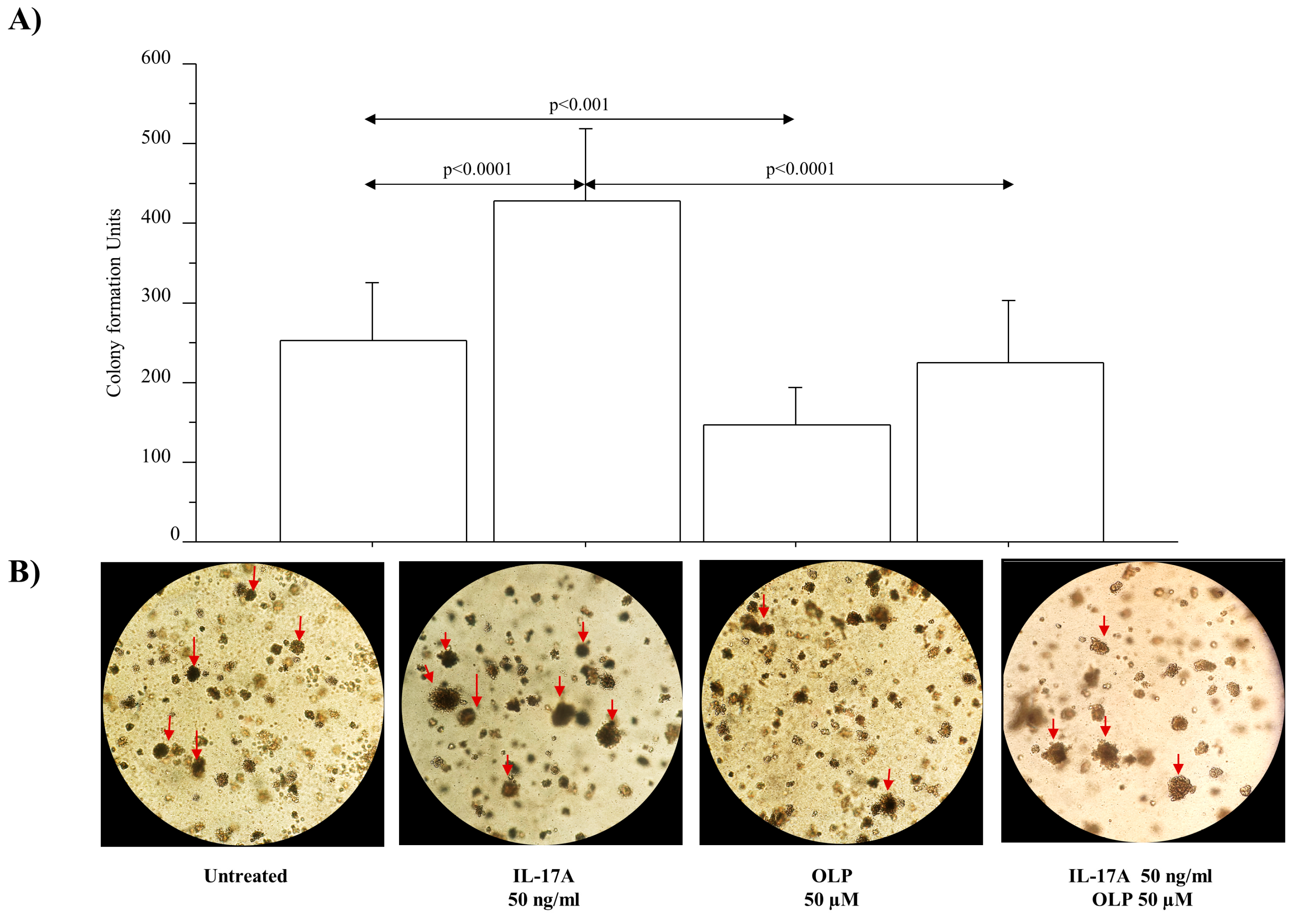
3.5. IL-17A Drives Colony Formation and Invasiveness in A549 Cells and OLP Downregulates These Phenomena
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Song, M.; Liang, J.; Wang, L.; Li, W.; Jiang, S.; Xu, S.; Tang, L.; Du, Q.; Liu, G.; Meng, H.; et al. IL-17A Functions and the Therapeutic Use of IL-17A and IL-17RA Targeted Antibodies for Cancer Treatment. Int. Immunopharmacol. 2023, 123, 110757. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, A.M.; Riccobono, L.; Siena, L.; Chiappara, G.; Di Sano, C.; Anzalone, G.; Gagliardo, R.; Ricciardolo, F.L.M.; Sorbello, V.; Pipitone, L.; et al. Cigarette Smoke Affects IL-17A, IL-17F and IL-17 Receptor Expression in the Lung Tissue: Ex Vivo and in Vitro Studies. Cytokine 2015, 76, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Dharshini, L.C.P.; Rasmi, R.R.; Kathirvelan, C.; Kumar, K.M.; Saradhadevi, K.M.; Sakthivel, K.M. Regulatory Components of Oxidative Stress and Inflammation and Their Complex Interplay in Carcinogenesis. Appl. Biochem. Biotechnol. 2023, 195, 2893–2916. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Tian, B.; Geng, X.; Zhou, H.; Xu, Z.; Lai, T.; Wu, Y.; Bao, Z.; Chen, Z.; Li, W.; et al. IL-17-Mediated Inflammation Promotes Cigarette Smoke-Induced Genomic Instability. Cells 2021, 10, 1173. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Long, H.; He, L.; Han, X.; Lin, K.; Liang, Z.; Zhuo, W.; Xie, R.; Zhu, B. Interleukin-17 Produced by Tumor Microenvironment Promotes Self-Renewal of CD133+ Cancer Stem-like Cells in Ovarian Cancer. Oncogene 2015, 34, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Q.; Chen, C.; Ge, D.; Qu, Y.; Chen, R.; Fan, Y.-M.; Li, N.; Tang, W.W.; Zhang, W.; et al. Hyperinsulinemia Enhances Interleukin-17-Induced Inflammation to Promote Prostate Cancer Development in Obese Mice through Inhibiting Glycogen Synthase Kinase 3-Mediated Phosphorylation and Degradation of Interleukin-17 Receptor. Oncotarget 2016, 7, 13651–13666. [Google Scholar] [CrossRef] [PubMed]
- Bouras, E.; Karhunen, V.; Gill, D.; Huang, J.; Haycock, P.C.; Gunter, M.J.; Johansson, M.; Brennan, P.; Key, T.; Lewis, S.J.; et al. Circulating Inflammatory Cytokines and Risk of Five Cancers: A Mendelian Randomization Analysis. BMC Med. 2022, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, Y.; Zhang, W.; Zhao, D.; Ma, L.; Ma, P.; Yang, F.; Wang, Y.; Shu, Y.; Qiu, W. IL-17 Induces NSCLC A549 Cell Proliferation via the Upregulation of HMGA1, Resulting in an Increased Cyclin D1 Expression. Int. J. Oncol. 2018, 52, 1579–1592. [Google Scholar] [CrossRef]
- Li, J.; Panganiban, R.; Kho, A.T.; McGeachie, M.J.; Farnam, L.; Chase, R.P.; Weiss, S.T.; Lu, Q.; Tantisira, K.G. Circulating MicroRNAs and Treatment Response in Childhood Asthma. Am. J. Respir. Crit. Care Med. 2020, 202, 65–72. [Google Scholar] [CrossRef]
- Xu, R.; Ke, X.; Shang, W.; Liu, S.; Fu, X.; Wang, T.; Jin, S. Distribution and Clinical Significance of IL-17A in Tumor-Infiltrating Lymphocytes of Non-Small Cell Lung Cancer Patients. Pathol. Oncol. Res. 2022, 28, 1610384. [Google Scholar] [CrossRef]
- Hays, L.E.; Zodrow, D.M.; Yates, J.E.; Deffebach, M.E.; Jacoby, D.B.; Olson, S.B.; Pankow, J.F.; Bagby, G.C. Cigarette Smoke Induces Genetic Instability in Airway Epithelial Cells by Suppressing FANCD2 Expression. Br. J. Cancer 2008, 98, 1653–1661. [Google Scholar] [CrossRef]
- Wu, H.-H.; Hwang-Verslues, W.W.; Lee, W.-H.; Huang, C.-K.; Wei, P.-C.; Chen, C.-L.; Shew, J.-Y.; Lee, E.Y.-H.P.; Jeng, Y.-M.; Tien, Y.-W.; et al. Targeting IL-17B-IL-17RB Signaling with an Anti-IL-17RB Antibody Blocks Pancreatic Cancer Metastasis by Silencing Multiple Chemokines. J. Exp. Med. 2015, 212, 333–349. [Google Scholar] [CrossRef]
- Kucukgul, A.; Isgor, M.M.; Duzguner, V.; Atabay, M.N.; Kucukgul, A. Antioxidant Effects of Oleuropein on Hydrogen Peroxide-Induced Neuronal Stress—An In Vitro Study. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2020, 19, 74–84. [Google Scholar] [CrossRef]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Würtele, G.; Spiegelhalder, B.; Bartsch, H. Olive-Oil Consumption and Health: The Possible Role of Antioxidants. Lancet Oncol. 2000, 1, 107–112. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.-H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea Europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Rosillo, M.A.; Alarcón de la Lastra, C. Oleuropein, a Secoiridoid Derived from Olive Tree, Inhibits the Proliferation of Human Colorectal Cancer Cell through Downregulation of HIF-1α. Nutr. Cancer 2013, 65, 147–156. [Google Scholar] [CrossRef]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea Europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef]
- Nardi, M.; Baldelli, S.; Ciriolo, M.R.; Costanzo, P.; Procopio, A.; Colica, C. Oleuropein Aglycone Peracetylated (3,4-DHPEA-EA(P)) Attenuates H2O2-Mediated Cytotoxicity in C2C12 Myocytes via Inactivation of p-JNK/p-c-Jun Signaling Pathway. Molecules 2020, 25, 5472. [Google Scholar] [CrossRef]
- Piroddi, M.; Albini, A.; Fabiani, R.; Giovannelli, L.; Luceri, C.; Natella, F.; Rosignoli, P.; Rossi, T.; Taticchi, A.; Servili, M.; et al. Nutrigenomics of Extra-Virgin Olive Oil: A Review. BioFactors 2017, 43, 17–41. [Google Scholar] [CrossRef]
- D’Anna, C.; Di Sano, C.; Di Vincenzo, S.; Taverna, S.; Cammarata, G.; Scurria, A.; Pagliaro, M.; Ciriminna, R.; Pace, E. Mesoporous Silica Particles Functionalized with Newly Extracted Fish Oil (Omeg@Silica) Reducing IL-8 Counteract Cell Migration in NSCLC Cell Lines. Pharmaceutics 2022, 14, 2079. [Google Scholar] [CrossRef]
- Pojero, F.; Aiello, A.; Gervasi, F.; Caruso, C.; Ligotti, M.E.; Calabrò, A.; Procopio, A.; Candore, G.; Accardi, G.; Allegra, M. Effects of Oleuropein and Hydroxytyrosol on Inflammatory Mediators: Consequences on Inflammaging. Int. J. Mol. Sci. 2022, 24, 380. [Google Scholar] [CrossRef] [PubMed]
- Strøbech, J.E.; Giuriatti, P.; Erler, J.T. Neutrophil Granulocytes Influence on Extracellular Matrix in Cancer Progression. Am. J. Physiol. Cell Physiol. 2022, 323, C486–C493. [Google Scholar] [CrossRef] [PubMed]
- Geyikoglu, F.; Isikgoz, H.; Onalan, H.; Colak, S.; Cerig, S.; Bakir, M.; Hosseinigouzdagani, M.; Koc, K.; Erol, H.S.; Saglam, Y.S.; et al. Impact of High-Dose Oleuropein on Cisplatin-Induced Oxidative Stress, Genotoxicity and Pathological Changes in Rat Stomach and Lung. J. Asian Nat. Prod. Res. 2017, 19, 1214–1231. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Choi, Y.-J.; Kang, M.-K.; Lee, E.-J.; Kim, D.Y.; Oh, H.; Kang, Y.-H. Oleuropein Curtails Pulmonary Inflammation and Tissue Destruction in Models of Experimental Asthma and Emphysema. J. Agric. Food Chem. 2018, 66, 7643–7654. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhu, X.; Du, L. P38 MAP Kinase Is Involved in Oleuropein-Induced Apoptosis in A549 Cells by a Mitochondrial Apoptotic Cascade. Biomed. Pharmacother. 2017, 95, 1425–1435. [Google Scholar] [CrossRef]
- Antognelli, C.; Frosini, R.; Santolla, M.F.; Peirce, M.J.; Talesa, V.N. Oleuropein-Induced Apoptosis Is Mediated by Mitochondrial Glyoxalase 2 in NSCLC A549 Cells: A Mechanistic Inside and a Possible Novel Nonenzymatic Role for an Ancient Enzyme. Oxidative Med. Cell. Longev. 2019, 2019, 8576961. [Google Scholar] [CrossRef]
- Montalbano, A.M.; Albano, G.D.; Anzalone, G.; Moscato, M.; Gagliardo, R.; Di Sano, C.; Bonanno, A.; Ruggieri, S.; Cibella, F.; Profita, M. Cytotoxic and Genotoxic Effects of the Flame Retardants (PBDE-47, PBDE-99 and PBDE-209) in Human Bronchial Epithelial Cells. Chemosphere 2020, 245, 125600. [Google Scholar] [CrossRef] [PubMed]
- Albano, G.D.; Moscato, M.; Montalbano, A.M.; Anzalone, G.; Gagliardo, R.; Bonanno, A.; Giacomazza, D.; Barone, R.; Drago, G.; Cibella, F.; et al. Can PBDEs Affect the Pathophysiologic Complex of Epithelium in Lung Diseases? Chemosphere 2020, 241, 125087. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, A.M.; Di Sano, C.; Chiappara, G.; Riccobono, L.; Bonanno, A.; Anzalone, G.; Vitulo, P.; Pipitone, L.; Gjomarkaj, M.; Pieper, M.P.; et al. Cigarette Smoke and Non-Neuronal Cholinergic System in the Airway Epithelium of COPD Patients. J. Cell. Physiol. 2018, 233, 5856–5868. [Google Scholar] [CrossRef]
- Przychodzen, P.; Wyszkowska, R.; Gorzynik-Debicka, M.; Kostrzewa, T.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Anticancer Potential of Oleuropein, the Polyphenol of Olive Oil, With 2-Methoxyestradiol, Separately or in Combination, in Human Osteosarcoma Cells. Anticancer. Res. 2019, 39, 1243–1251. [Google Scholar] [CrossRef]
- Ma, W.; Wang, Z.; Zhao, Y.; Wang, Q.; Zhang, Y.; Lei, P.; Lu, W.; Yan, S.; Zhou, J.; Li, X.; et al. Salidroside Suppresses the Proliferation and Migration of Human Lung Cancer Cells through AMPK-Dependent NLRP3 Inflammasome Regulation. Oxidative Med. Cell. Longev. 2021, 2021, 6614574. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xin, M.; Li, Q.; Sun, L.; Han, X.; Wang, J. IL-17A Promotes the Migration, Invasion and the EMT Process of Lung Cancer Accompanied by NLRP3 Activation. BioMed Res. Int. 2022, 2022, 7841279. [Google Scholar] [CrossRef]
- Ritzmann, F.; Lunding, L.P.; Bals, R.; Wegmann, M.; Beisswenger, C. IL-17 Cytokines and Chronic Lung Diseases. Cells 2022, 11, 2132. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Danielsen, P.H.; Karottki, D.G.; Jantzen, K.; Roursgaard, M.; Klingberg, H.; Jensen, D.M.; Christophersen, D.V.; Hemmingsen, J.G.; Cao, Y.; et al. Oxidative Stress and Inflammation Generated DNA Damage by Exposure to Air Pollution Particles. Mutat. Res. Rev. Mutat. Res. 2014, 762, 133–166. [Google Scholar] [CrossRef] [PubMed]
- Kopp, B.; Khoury, L.; Audebert, M. Validation of the ΓH2AX Biomarker for Genotoxicity Assessment: A Review. Arch. Toxicol. 2019, 93, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Canton, C.; Anadón, A.; Meredith, C. ΓH2AX as a Novel Endpoint to Detect DNA Damage: Applications for the Assessment of the in Vitro Genotoxicity of Cigarette Smoke. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2012, 26, 1075–1086. [Google Scholar] [CrossRef]
- Riches, L.C.; Lynch, A.M.; Gooderham, N.J. Early Events in the Mammalian Response to DNA Double-Strand Breaks. Mutagenesis 2008, 23, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Cann, K.L.; Dellaire, G. Heterochromatin and the DNA Damage Response: The Need to Relax. Biochem. Cell Biol. 2011, 89, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Lynch, A.M.; Sasaki, J.C.; Elespuru, R.; Jacobson-Kram, D.; Thybaud, V.; De Boeck, M.; Aardema, M.J.; Aubrecht, J.; Benz, R.D.; Dertinger, S.D.; et al. New and Emerging Technologies for Genetic Toxicity Testing. Environ. Mol. Mutagen. 2011, 52, 205–223. [Google Scholar] [CrossRef]
- Martin-Orozco, N.; Muranski, P.; Chung, Y.; Yang, X.O.; Yamazaki, T.; Lu, S.; Hwu, P.; Restifo, N.P.; Overwijk, W.W.; Dong, C. T Helper 17 Cells Promote Cytotoxic T Cell Activation in Tumor Immunity. Immunity 2009, 31, 787–798. [Google Scholar] [CrossRef]
- Antoniou, C.; Hull, J. The Anti-Cancer Effect of Olea Europaea L. Products: A Review. Curr. Nutr. Rep. 2021, 10, 99–124. [Google Scholar] [CrossRef] [PubMed]
- ArulJothi, K.N.; Kumaran, K.; Senthil, S.; Nidhu, A.B.; Munaff, N.; Janitri, V.B.; Kirubakaran, R.; Singh, S.K.; Gupt, G.; Dua, K.; et al. Implications of Reactive Oxygen Species in Lung Cancer and Exploiting It for Therapeutic Interventions. Med. Oncol. 2022, 40, 43. [Google Scholar] [CrossRef]
- Dayi, T.; Oniz, A. Effects of the Mediterranean Diet Polyphenols on Cancer Development. J. Prev. Med. Hyg. 2022, 63, E74–E80. [Google Scholar] [CrossRef] [PubMed]
- Yadegar, N.; Dadashi, Z.; Shams, K.; Mohammadi, M.; Abyar, M.; Rafat, M. The Prominent Role of MiR-942 in Carcinogenesis of Tumors. Adv. Biomed. Res. 2022, 11, 63. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in Olive and Its Pharmacological Effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, L.; Zhao, J.; Jiao, Y.; Zhang, M.; Xu, G.; Jiang, Y. PI3K/AKT1 Signaling Pathway Mediates Sinomenine-Induced Hepatocellular Carcinoma Cells Apoptosis: An in Vitro and in Vivo Study. Biol. Pharm. Bull. 2022, 45, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, Z.; Yang, X.; Liu, L.; Ahn, K.S. An Updated Review on the Potential Antineoplastic Actions of Oleuropein. Phytother. Res. 2022, 36, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, D.W.; Lee, Y.H.; Oh, J.H.; Yoon, S.; Choi, M.S.; Lee, S.K.; Kim, J.W.; Lee, K.; Song, C.-W. Silver Nanoparticles Induce Apoptosis and G2/M Arrest via PKCζ-Dependent Signaling in A549 Lung Cells. Arch. Toxicol. 2011, 85, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Miro-Casas, E.; Covas, M.-I.; Farre, M.; Fito, M.; Ortuño, J.; Weinbrenner, T.; Roset, P.; de la Torre, R. Hydroxytyrosol Disposition in Humans. Clin. Chem. 2003, 49, 945–952. [Google Scholar] [CrossRef]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally Relevant Concentrations of Resveratrol and Hydroxytyrosol Mitigate Oxidative Burst of Human Granulocytes and Monocytes and the Production of Pro-Inflammatory Mediators in LPS-Stimulated RAW 264.7 Macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Su, H.; Jiao, K.; Liu, J. Association Between IL-17 and Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J. Food Polyphenols as Preventive Medicine. Antioxidants 2023, 12, 2103. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalbano, A.M.; Di Sano, C.; Albano, G.D.; Gjomarkaj, M.; Ricciardolo, F.L.M.; Profita, M. IL-17A Drives Oxidative Stress and Cell Growth in A549 Lung Epithelial Cells: Potential Protective Action of Oleuropein. Nutrients 2024, 16, 2123. https://doi.org/10.3390/nu16132123
Montalbano AM, Di Sano C, Albano GD, Gjomarkaj M, Ricciardolo FLM, Profita M. IL-17A Drives Oxidative Stress and Cell Growth in A549 Lung Epithelial Cells: Potential Protective Action of Oleuropein. Nutrients. 2024; 16(13):2123. https://doi.org/10.3390/nu16132123
Chicago/Turabian StyleMontalbano, Angela Marina, Caterina Di Sano, Giusy Daniela Albano, Mark Gjomarkaj, Fabio Luigi Massimo Ricciardolo, and Mirella Profita. 2024. "IL-17A Drives Oxidative Stress and Cell Growth in A549 Lung Epithelial Cells: Potential Protective Action of Oleuropein" Nutrients 16, no. 13: 2123. https://doi.org/10.3390/nu16132123




