The Role of Diet and the Gut Microbiota in Reactive Aggression and Adult ADHD—An Exploratory Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Experimental Procedure
2.2. Statistical Analysis of Diet
2.2.1. Dietary Patterns
2.2.2. Diet–Behavior Associations
2.3. Statistical Analysis of the Gut Microbiota
2.3.1. Microbiota–Behavior Associations
2.3.2. Mediation Analysis of the Gut Microbiota on Diet and Behavior
3. Results
3.1. Demographic Description of the Sample
3.2. Diet
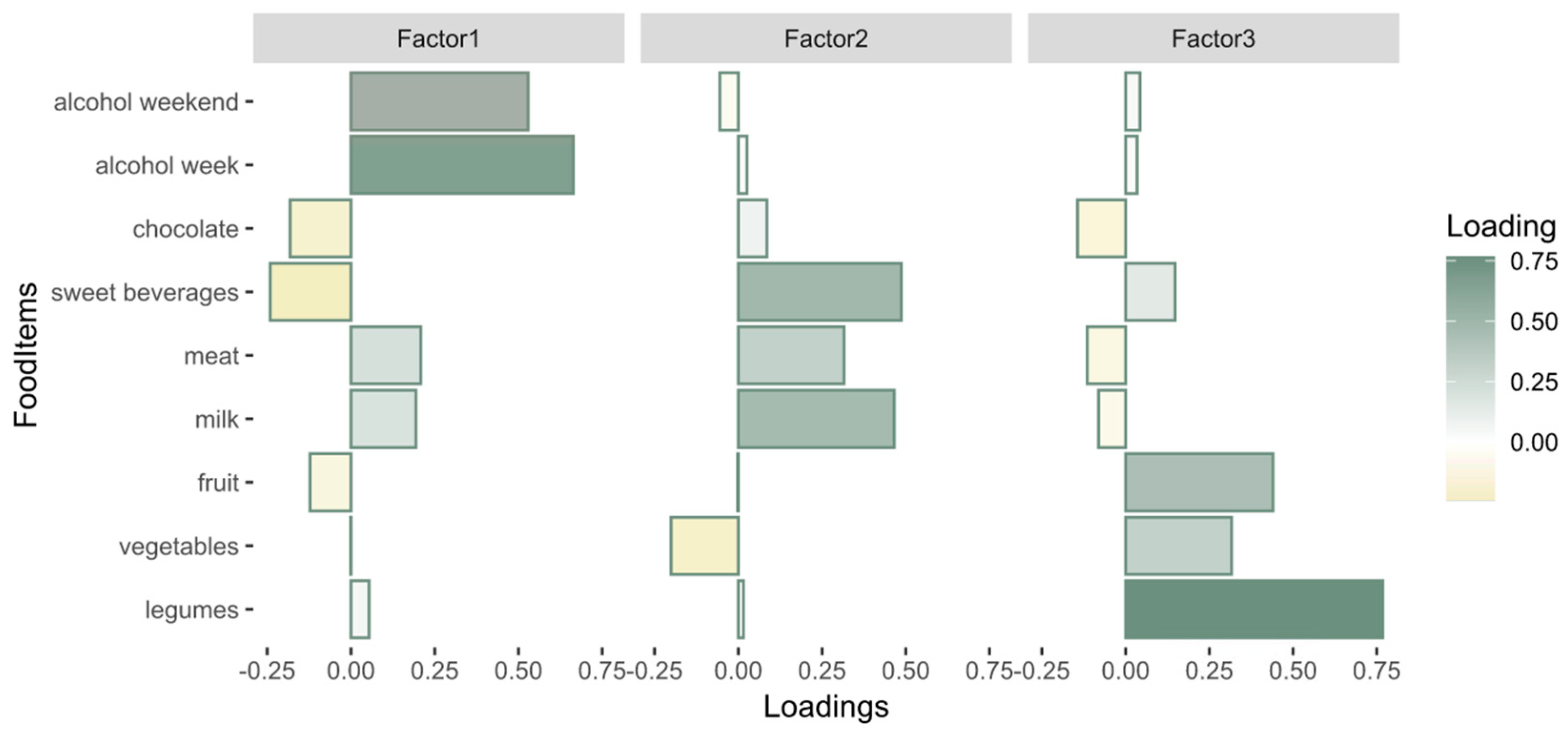
3.2.1. Dietary Patterns
3.2.2. Diet–Behavior Associations
3.3. Microbiota
3.3.1. Microbiota–Behavior Associations
3.3.2. The Gut Microbiota as a Mediator of Diet and Behavior
4. Discussion
4.1. Diet
4.2. Gut Microbiota
4.3. Mediation
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faraone, S.; Asherson, P.; Banaschewski, T.; Biederman, J.; Buitelaar, J.; Ramos-Quiroga, J. 482 ADHD. Nat. Rev. Dis. Primers 2015, 1, 15027. [Google Scholar]
- Shaw, P.; Stringaris, A.; Nigg, J.; Leibenluft, E. Emotion dysregulation in attention deficit hyperactivity disorder. Focus 2016, 14, 127–144. [Google Scholar] [CrossRef] [PubMed]
- King, S.; Waschbusch, D.A. Aggression in children with attention-deficit/hyperactivity disorder. Expert Rev. Neurother. 2010, 10, 1581–1594. [Google Scholar] [CrossRef] [PubMed]
- Abel, M.R.; Poquiz, J.L.; Fite, P.J.; Doyle, R.L. Reactive aggression and suicidal behaviors in children receiving outpatient psychological services: The moderating role of hyperactivity and inattention. Child Psychiatry Hum. Dev. 2020, 51, 2–12. [Google Scholar] [CrossRef]
- Slaughter, K.E.; Leaberry, K.D.; Fogleman, N.D.; Rosen, P.J. Reactive and proactive aggression in children with and without ADHD and negative emotional lability. Soc. Dev. 2020, 29, 320–338. [Google Scholar] [CrossRef]
- Evans, S.C.; Fite, P.J.; Hendrickson, M.L.; Rubens, S.L.; Mages, A.K. The role of reactive aggression in the link between hyperactive–impulsive behaviors and peer rejection in adolescents. Child Psychiatry Hum. Dev. 2015, 46, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Martel, M.M. Research review: A new perspective on attention-deficit/hyperactivity disorder: Emotion dysregulation and trait models. J. Child Psychol. Psychiatry 2009, 50, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; Nelson, L.H. Microglia and beyond: Innate immune cells as regulators of brain development and behavioral function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Hoogman, M.; Bralten, J.; Hibar, D.P.; Mennes, M.; Zwiers, M.P.; Schweren, L.S.; van Hulzen, K.J.; Medland, S.E.; Shumskaya, E.; Jahanshad, N. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: A cross-sectional mega-analysis. Lancet Psychiatry 2017, 4, 310–319. [Google Scholar] [CrossRef]
- Hoogman, M.; Muetzel, R.; Guimaraes, J.P.; Shumskaya, E.; Mennes, M.; Zwiers, M.P.; Jahanshad, N.; Sudre, G.; Wolfers, T.; Earl, E.A. Brain imaging of the cortex in ADHD: A coordinated analysis of large-scale clinical and population-based samples. Am. J. Psychiatry 2019, 176, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Jakobi, B.; Arias-Vasquez, A.; Hermans, E.; Vlaming, P.; Buitelaar, J.K.; Franke, B.; Hoogman, M.; Van Rooij, D. Neural Correlates of Reactive Aggression in Adult ADHD. Front. Psychiatry 2022, 13, 840095. [Google Scholar] [CrossRef]
- Tylee, D.S.; Sun, J.; Hess, J.L.; Tahir, M.A.; Sharma, E.; Malik, R.; Worrall, B.B.; Levine, A.J.; Martinson, J.J.; Nejentsev, S. Genetic correlations among psychiatric and immune-related phenotypes based on genome-wide association data. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2018, 177, 641–657. [Google Scholar] [CrossRef]
- Seroczynski, A.D.; Bergeman, C.; Coccaro, E.F. Etiology of the impulsivity/aggression relationship: Genes or environment? Psychiatry Res. 1999, 86, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, J.Y.; Lee, J.; Jeong, G.H.; Lee, E.; Lee, S.; Lee, K.H.; Kronbichler, A.; Stubbs, B.; Solmi, M. Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: An umbrella review. Lancet Psychiatry 2020, 7, 955–970. [Google Scholar] [CrossRef] [PubMed]
- Tcherni-Buzzeo, M. Dietary interventions, the gut microbiome, and aggressive behavior: Review of research evidence and potential next steps. Aggress. Behav. 2023, 49, 15–32. [Google Scholar] [CrossRef]
- Pinto, S.; Correia-de-Sá, T.; Sampaio-Maia, B.; Vasconcelos, C.; Moreira, P.; Ferreira-Gomes, J. Eating Patterns and Dietary Interventions in ADHD: A Narrative Review. Nutrients 2022, 14, 4332. [Google Scholar] [CrossRef] [PubMed]
- Gkougka, D.; Mitropoulos, K.; Tzanakaki, G.; Panagouli, E.; Psaltopoulou, T.; Thomaidis, L.; Tsolia, M.; Sergentanis, T.N.; Tsitsika, A. Gut microbiome and attention deficit/hyperactivity disorder: A systematic review. Pediatr. Res. 2022, 92, 1507–1519. [Google Scholar] [CrossRef]
- Langmajerová, M.; Roubalová, R.; Šebela, A.; Vevera, J. The effect of microbiome composition on impulsive and violent behavior: A systematic review. Behav. Brain Res. 2022, 440, 114266. [Google Scholar] [CrossRef]
- Del-Ponte, B.; Quinte, G.C.; Cruz, S.; Grellert, M.; Santos, I.S. Dietary patterns and attention deficit/hyperactivity disorder (ADHD): A systematic review and meta-analysis. J. Affect. Disord. 2019, 252, 160–173. [Google Scholar] [CrossRef]
- Ly, V.; Bottelier, M.; Hoekstra, P.J.; Arias Vasquez, A.; Buitelaar, J.K.; Rommelse, N.N. Elimination diets’ efficacy and mechanisms in attention deficit hyperactivity disorder and autism spectrum disorder. Eur. Child Adolesc. Psychiatry 2017, 26, 1067–1079. [Google Scholar] [CrossRef]
- Uldall Torp, N.M.; Thomsen, P.H. The use of diet interventions to treat symptoms of ADHD in children and adolescents–a systematic review of randomized controlled trials. Nord. J. Psychiatry 2020, 74, 558–568. [Google Scholar] [CrossRef]
- Lipsanen, J.; Elovainio, M.; Hakulinen, C.; Tremblay, M.S.; Rovio, S.; Lagström, H.; Jaakkola, J.M.; Jula, A.; Rönnemaa, T.; Viikari, J. Temperament profiles are associated with dietary behavior from childhood to adulthood. Appetite 2020, 151, 104681. [Google Scholar] [CrossRef]
- Riggs, N.R.; Spruijt-Metz, D.; Sakuma, K.-L.; Chou, C.-P.; Pentz, M.A. Executive cognitive function and food intake in children. J. Nutr. Educ. Behav. 2010, 42, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, M.E.; Stene-Larsen, K.; Tonstad, S.; Rothbart, M.K.; Hampson, S.E. Associations between temperament at age 1.5 years and obesogenic diet at ages 3 and 7 years. J. Dev. Behav. Pediatr. JDBP 2012, 33, 721–727. [Google Scholar] [CrossRef]
- Holt, M. Association of Dietary Intake Patterns with Emotion Regulation. Ph.D. Thesis, Loma Linda University, Loma Linda, CA, USA, 2013. [Google Scholar]
- Abiri, B.; Amini, S.; Ehsani, H.; Ehsani, M.; Adineh, P.; Mohammadzadeh, H.; Hashemi, S. Evaluation of dietary food intakes and anthropometric measures in middle-aged men with aggressive symptoms. BMC Nutr. 2023, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Gow, R.V.; Sumich, A.; Vallee-Tourangeau, F.; Crawford, M.A.; Ghebremeskel, K.; Bueno, A.A.; Hibbeln, J.R.; Taylor, E.; Wilson, D.A.; Rubia, K. Omega-3 fatty acids are related to abnormal emotion processing in adolescent boys with attention deficit hyperactivity disorder. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Harty, S.; Johnson, K.V.A.; Moeller, A.H.; Carmody, R.N.; Lehto, S.M.; Erdman, S.E.; Dunbar, R.I.; Burnet, P.W. The role of the microbiome in the neurobiology of social behaviour. Biol. Rev. 2020, 95, 1131–1166. [Google Scholar] [CrossRef]
- Mulder, D.; Aarts, E.; Arias Vasquez, A.; Bloemendaal, M. A systematic review exploring the association between the human gut microbiota and brain connectivity in health and disease. Mol. Psychiatry 2023, 28, 5037–5061. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Dam, S.A.; Mostert, J.C.; Szopinska-Tokov, J.W.; Bloemendaal, M.; Amato, M.; Arias-Vasquez, A. The role of the gut-brain axis in attention-deficit/hyperactivity disorder. Gastroenterol. Clin. 2019, 48, 407–431. [Google Scholar] [CrossRef]
- Fox, M.; Lee, S.M.; Wiley, K.S.; Lagishetty, V.; Sandman, C.A.; Jacobs, J.P.; Glynn, L.M. Development of the infant gut microbiome predicts temperament across the first year of life. Dev. Psychopathol. 2022, 34, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J.K.; Kassem, H.; Ngo, A.L.; Abd-Elsayed, A.; Simopoulos, T.; et al. Current perspectives on gut microbiome dysbiosis and depression. Adv. Ther. 2020, 37, 1328–1346. [Google Scholar] [CrossRef]
- Kirchoff, N.S.; Udell, M.A.; Sharpton, T.J. The gut microbiome correlates with conspecific aggression in a small population of rescued dogs (Canis familiaris). PeerJ 2019, 7, e6103. [Google Scholar] [CrossRef]
- Jia, Y.; Jin, S.; Hu, K.; Geng, L.; Han, C.; Kang, R.; Pang, Y.; Ling, E.; Tan, E.K.; Pan, Y. Gut microbiome modulates Drosophila aggression through octopamine signaling. Nat. Commun. 2021, 12, 2698. [Google Scholar] [CrossRef]
- Ren, C.C.; Sylvia, K.E.; Munley, K.M.; Deyoe, J.E.; Henderson, S.G.; Vu, M.P.; Demas, G.E. Photoperiod modulates the gut microbiome and aggressive behavior in Siberian hamsters. J. Exp. Biol. 2020, 223, jeb212548. [Google Scholar] [CrossRef] [PubMed]
- Carbia, C.; Lannoy, S.; Maurage, P.; López-Caneda, E.; O’Riordan, K.J.; Dinan, T.G.; Cryan, J.F. A biological framework for emotional dysregulation in alcohol misuse: From gut to brain. Mol. Psychiatry 2021, 26, 1098–1118. [Google Scholar] [CrossRef] [PubMed]
- Kooij, J.; Francken, M. DIVA 2.0. Diagnostic Interview Voor ADHD in Adults Bij Volwassenen [DIVA 2 0 Diagnostic Interview ADHD in Adults]. DIVA Foundation. 2010. Available online: http://www.divacenter.eu/DIVA.aspx (accessed on 5 July 2024).
- Kooij, J.; Bijlenga, D.; Salerno, L.; Jaeschke, R.; Bitter, I.; Balazs, J.; Thome, J.; Dom, G.; Kasper, S.; Filipe, C.N. Updated European Consensus Statement on diagnosis and treatment of adult ADHD. Eur. Psychiatry 2019, 56, 14–34. [Google Scholar] [CrossRef]
- Raine, A.; Dodge, K.; Loeber, R.; Gatzke-Kopp, L.; Lynam, D.; Reynolds, C.; Stouthamer-Loeber, M.; Liu, J. The reactive–proactive aggression questionnaire: Differential correlates of reactive and proactive aggression in adolescent boys. Aggress. Behav. Off. J. Int. Soc. Res. Aggress. 2006, 32, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Bloemendaal, M.; Vlaming, P.; de Boer, A.; Vermeulen-Kalk, K.; Bouman, A.; Kleefstra, T.; Arias Vasquez, A. The role of the gut microbiota in patients with Kleefstra syndrome. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2023, 192, 124–138. [Google Scholar] [CrossRef]
- Mirzayi, C.; Renson, A.; Zohra, F.; Elsafoury, S.; Geistlinger, L.; Kasselman, L.; Eckenrode, K.; van de Wijgert, J.; Loughman, A.; Marques, F.Z. Strengthening the Organization and Reporting of Microbiome Studies (STORMS): A reporting checklist for human microbiome research. BioRxiv 2020. [Google Scholar] [CrossRef]
- Revelle, W.R. psych: Procedures for Personality and Psychological Research. 2017. Available online: https://www.scholars.northwestern.edu/en/publications/psych-procedures-for-personality-and-psychological-research/ (accessed on 5 July 2024).
- Kloke, J.D.; McKean, J.W. Rfit: Rank-based estimation for linear models. R J. 2012, 4, 57–64. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The vegan package. Community Ecol. Package 2007, 10, 719. [Google Scholar]
- Meinshausen, N.; Bühlmann, P. Stability selection. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2010, 72, 417–473. [Google Scholar] [CrossRef]
- Gloor, G. ALDEx2: ANOVA-Like Differential Expression tool for compositional data. ALDEX Man. Modul. 2015, 20, 1–11. [Google Scholar]
- Yu, Q.; Li, B. mma: An R package for mediation analysis with multiple mediators. J. Open Res. Softw. 2017, 5, 11. [Google Scholar] [CrossRef]
- Tingley, D.; Yamamoto, T.; Hirose, K.; Keele, L.; Imai, K. Mediation: R package for causal mediation analysis. J. Stat. Softw. 2014, 59, 1–38. [Google Scholar] [CrossRef]
- Shi, H.; Ter Horst, R.; Nielen, S.; Bloemendaal, M.; Jaeger, M.; Joosten, I.; Koenen, H.; Joosten, L.A.; Schweren, L.J.; Vasquez, A.A. The gut microbiome as mediator between diet and its impact on immune function. Sci. Rep. 2022, 12, 5149. [Google Scholar] [CrossRef]
- Shareghfarid, E.; Sangsefidi, Z.S.; Salehi-Abargouei, A.; Hosseinzadeh, M. Empirically derived dietary patterns and food groups intake in relation with Attention Deficit/Hyperactivity Disorder (ADHD): A systematic review and meta-analysis. Clin. Nutr. ESPEN 2020, 36, 28–35. [Google Scholar] [CrossRef]
- Holton, K.F.; Johnstone, J.M.; Brandley, E.T.; Nigg, J.T. Evaluation of dietary intake in children and college students with and without attention-deficit/hyperactivity disorder. Nutr. Neurosci. 2019, 22, 664–677. [Google Scholar] [CrossRef]
- Li, L.; Taylor, M.J.; Bälter, K.; Kuja-Halkola, R.; Chen, Q.; Hegvik, T.A.; Tate, A.E.; Chang, Z.; Arias-Vásquez, A.; Hartman, C.A. Attention-deficit/hyperactivity disorder symptoms and dietary habits in adulthood: A large population-based twin study in Sweden. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2020, 183, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Weissenberger, S.; Ptacek, R.; Vnukova, M.; Raboch, J.; Klicperova-Baker, M.; Domkarova, L.; Goetz, M. ADHD and lifestyle habits in Czech adults, a national sample. Neuropsychiatr. Dis. Treat. 2018, 14, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Molag, M. Towards Transparent Development of Food Frequency Questionnaires: Scientific Basis of the Dutch FFQ-TOOL tm: A Computer System to Generate, Apply and Process FFQs; Wageningen University and Research: Wageningen, The Netherlands, 2010. [Google Scholar]
- Della Corte, K.W.; Perrar, I.; Penczynski, K.J.; Schwingshackl, L.; Herder, C.; Buyken, A.E. Effect of dietary sugar intake on biomarkers of subclinical inflammation: A systematic review and meta-analysis of intervention studies. Nutrients 2018, 10, 606. [Google Scholar] [CrossRef] [PubMed]
- Wärnberg, J.; Gomez-Martinez, S.; Romeo, J.; Díaz, L.E.; Marcos, A. Nutrition, inflammation, and cognitive function. Ann. N. Y. Acad. Sci. 2009, 1153, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Jakobi, B.; Vlaming, P.; Mulder, D.; Ribases, M.; Richarte, V.; Ramos-Quiroga, J.A.; Tendolkar, I.; van Eijndhoven, P.; Vrijsen, J.; Buitelaar, J.; et al. The gut-microbiome in adult Attention-deficit/hyperactivity disorder-A Meta-analysis. medRxiv 2023, 12. [Google Scholar] [CrossRef]
- Bao, J.; Zheng, H.; Wang, Y.; Zheng, X.; He, L.; Qi, W.; Wang, T.; Guo, B.; Guo, G.; Zhang, Z. Echinococcus granulosus infection results in an increase in Eisenbergiella and Parabacteroides genera in the gut of mice. Front. Microbiol. 2018, 9, 2890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Li, J.; Xing, T.; Jiang, Y.; Zhang, L.; Gao, F. Dietary resistant starch modifies the composition and function of caecal microbiota of broilers. J. Sci. Food Agric. 2020, 100, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, D.; Law, H.K.-W.; Wu, Y.; Zhu, G.-h.; Huang, W.-y.; Kang, Y. Integrative Analysis of Gut Microbiota and Fecal Metabolites in Rats after Prednisone Treatment. Microbiol. Spectr. 2021, 9, e00650-21. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Gao, X.; Wang, Z.; Cao, S.; Liang, G.; He, D.; Lv, Z.; Wang, L.; Xu, P.; Zhang, Q. Comparison of gut microbiota in autism spectrum disorders and neurotypical boys in China: A case-control study. Synth. Syst. Biotechnol. 2021, 6, 120–126. [Google Scholar] [CrossRef]
- Wan, X.; Eguchi, A.; Qu, Y.; Yang, Y.; Chang, L.; Shan, J.; Mori, C.; Hashimoto, K. Gut–microbiota–brain axis in the vulnerability to psychosis in adulthood after repeated cannabis exposure during adolescence. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 1297–1309. [Google Scholar] [CrossRef]
- Ma, S.; You, Y.; Huang, L.; Long, S.; Zhang, J.; Guo, C.; Zhang, N.; Wu, X.; Xiao, Y.; Tan, H. Alterations in gut microbiota of gestational diabetes patients during the first trimester of pregnancy. Front. Cell. Infect. Microbiol. 2020, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Ventura, M.; Buttó, L.F.; Duranti, S.; O’Toole, P.W.; Motherway, M.O.C.; van Sinderen, D. Molecular dialogue between the human gut microbiota and the host: A Lactobacillus and Bifidobacterium perspective. Cell. Mol. Life Sci. 2014, 71, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Rode, J.; Edebol Carlman, H.M.; König, J.; Repsilber, D.; Hutchinson, A.N.; Thunberg, P.; Andersson, P.; Persson, J.; Kiselev, A.; Lathrop Stern, L. Probiotic mixture containing lactobacillus helveticus, Bifidobacterium longum and Lactiplantibacillus plantarum affects brain responses toward an emotional task in healthy subjects: A randomized clinical trial. Front. Nutr. 2022, 9, 865. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Park, Y.-H.; Sim, M.; Kim, S.-A.; Joung, H.; Shin, D.-M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res. Microbiol. 2019, 170, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Wade, W.G. The genus Eubacterium and related genera. Prokaryotes 2006, 4, 823–835. [Google Scholar]
- Hu, R.; Wu, S.; Li, B.; Tan, J.; Yan, J.; Wang, Y.; Tang, Z.; Liu, M.; Fu, C.; Zhang, H. Dietary ferulic acid and vanillic acid on inflammation, gut barrier function and growth performance in lipopolysaccharide-challenged piglets. Anim. Nutr. 2022, 8, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Diotallevi, C.; Fava, F.; Gobbetti, M.; Tuohy, K. Healthy dietary patterns to reduce obesity-related metabolic disease: Polyphenol-microbiome interactions unifying health effects across geography. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 437–444. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, Z.; Zhang, X.; Fan, M.; Hong, Y.; Feng, Y.; Dong, Q.; Diao, H.; Wang, G. Sodium butyrate modulates gut microbiota and immune response in colorectal cancer liver metastatic mice. Cell Biol. Toxicol. 2020, 36, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Pla-Pagà, L.; Pedret, A.; Rubió, L.; Gosalbes, M.J.; Yuste, S.; Solà, R.; Valls, R.M. Interplay between dietary phenolic compound intake and the human gut microbiome in hypertension: A cross-sectional study. Food Chem. 2021, 344, 128567. [Google Scholar] [CrossRef]
- Calvete-Torre, I.; Sabater, C.; Antón, M.J.; Moreno, F.J.; Riestra, S.; Margolles, A.; Ruiz, L. Prebiotic potential of apple pomace and pectins from different apple varieties: Modulatory effects on key target commensal microbial populations. Food Hydrocoll. 2022, 133, 107958. [Google Scholar] [CrossRef]
- Holdeman, L.V.; Cato, E.P.; Burmeister, J.; Moore, W. Descriptions of Eubacterium timidum sp. nov., Eubacterium brachy sp. nov., and Eubacterium nodatum sp. nov. isolated from human periodontitis. Int. J. Syst. Evol. Microbiol. 1980, 30, 163–169. [Google Scholar] [CrossRef]
- Yang, J.; Kurnia, P.; Henning, S.M.; Lee, R.; Huang, J.; Garcia, M.C.; Surampudi, V.; Heber, D.; Li, Z. Effect of standardized grape powder consumption on the gut microbiome of healthy subjects: A pilot study. Nutrients 2021, 13, 3965. [Google Scholar] [CrossRef] [PubMed]
- Cade, J.E. Measuring diet in the 21st century: Use of new technologies. Proc. Nutr. Soc. 2017, 76, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Breda, V.; Cerqueira, R.O.; Ceolin, G.; Koning, E.; Fabe, J.; McDonald, A.; Gomes, F.A.; Brietzke, E. Is there a place for dietetic interventions in adult ADHD? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 119, 110613. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson AJ, A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Gloor, G.B.; Macklaim, J.M.; Pawlowsky-Glahn, V.; Egozcue, J.J. Microbiome datasets are compositional: And this is not optional. Front. Microbiol. 2017, 8, 2224. [Google Scholar] [CrossRef]
- Nearing, J.T.; Douglas, G.M.; Hayes, M.G.; MacDonald, J.; Desai, D.K.; Allward, N.; Jones, C.M.; Wright, R.J.; Dhanani, A.S.; Comeau, A.M. Microbiome differential abundance methods produce different results across 38 datasets. Nat. Commun. 2022, 13, 342. [Google Scholar] [CrossRef]
- Lahti, L.; Shetty, S. Microbiome R Package. 2017. Available online: https://microbiome.github.io/tutorials (accessed on 5 July 2024).

| ADHD | Controls | |
|---|---|---|
| N | 77 | 76 |
| Age in years, Mean (SD) | 34.09 (10.37) | 34.43 (12.9) |
| Sex, % male | 42.86% | 47.82% |
| BMI as kg/m2, Mean (SD) | 24.90 (4.51) | 24.82 (4.1) |
| Smoking,% current non-smokers | 70.13% | 90.91% |
| Stimulant medication, % current users | 57.14% | 0% |
| Reactive aggression 1, Mean (SD) | 8.23 (4.04) | 5.64 (3.2) |
| Number of inattentive symptoms 2, Mean (SD) | 7.34 (1.90) | 0.83 (1.2) |
| Number of hyperactive/impulsive symptoms 2, Mean (SD) | 5.62 (2.22) | 0.81 (1.1) |
| Standard Estimate | Estimate | Std. Error | z-Value/ t-Value | p-Value | p-Value FDR | |
|---|---|---|---|---|---|---|
| Reactive Aggression ~ Factor1 + Factor2 + Factor3 + age + sex + BMI + smoking + ADHD diagnosis | ||||||
| Factor1 | 0.37 | 0.16 | 0.22 | 0.74 | 4.6 × 10−01 | 1 |
| Factor2 | 0.82 | 0.51 | 0.19 | 2.73 | 7.0 × 10−03 | 4.22 × 10−02 |
| Factor3 | 0.30 | 0.12 | 0.21 | 0.60 | 5.5 × 10−01 | 1 |
| Age | 0.06 | 0.00 | 0.02 | 0.11 | 9.1 × 10−01 | n.a. |
| Sex | −0.76 | −2.03 | 0.55 | −3.72 | 2.8 × 10−04 | n.a. |
| BMI | 0.33 | 0.04 | 0.06 | 0.67 | 5.0 × 10−01 | n.a. |
| Smoke | 0.07 | 0.05 | 0.40 | 0.13 | 8.9 × 10−01 | n.a. |
| ADHD diagnosis | 0.75 | 2.22 | 0.55 | 4.04 | 8.4 × 10−05 | 5.85 × 10−04 |
| ADHD Diagnosis ~ Factor1 + Factor2 + Factor3 + age + sex + BMI + smoking | ||||||
| Factor1 | −0.26 | −0.11 | 0.15 | −0.73 | 4.7 × 10−01 | 1 |
| Factor2 | −0.01 | 0.00 | 0.12 | −0.03 | 9.7 × 10−01 | 1 |
| Factor3 | 0.02 | 0.01 | 0.13 | 0.06 | 9.5 × 10−01 | 1 |
| Age | 0.02 | 0.00 | 0.01 | 0.04 | 9.7 × 10−01 | n.a. |
| Sex | 0.36 | 0.36 | 0.36 | 1.01 | 3.2 × 10−01 | n.a. |
| BMI | 0.02 | 0.00 | 0.04 | 0.05 | 9.6 × 10−01 | n.a. |
| Smoke | 1.13 | 0.87 | 0.27 | 3.21 | 1.6 × 10−03 | n.a. |
| Feature Selection | Logistic Regression/Rank-Based Regression | ALDEx2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Genus | Sel. Prob. | Std. Error | Estimate | z | p | pFDR | Estimate | Std. Error | t | p | pFDR |
| Reactive Aggression | |||||||||||
| Lactobacillus | 0.27 | 0.11 | 0.28 | 2.54 | 1.2 × 10−02 | 1.7 × 10−02 | −0.23 | 0.1 | −2.42 | 1.9 × 10−02 | 5.2 × 10−02 |
| Slackia | 0.35 | 0.08 | 0.24 | 2.88 | 4.5 × 10−03 | 1.4 × 10−02 | −0.32 | 0.13 | −2.52 | 1.7 × 10−02 | 5.2 × 10−02 |
| Eubacterium xylanophilum group * | 0.11 | 0.12 | −0.26 | −2.23 | 2.7 × 10−02 | 2.9 × 10−02 | 0.21 | 0.1 | 2.23 | 3.3 × 10−02 | 5.3 × 10−02 |
| Dialister | 0.11 | 0.07 | −0.16 | −2.28 | 2.4 × 10−02 | 2.9 × 10−02 | 0.28 | 0.15 | 1.95 | 5.8 × 10−02 | 9.1 × 10−02 |
| Succiniclasticum | 0.19 | 0.15 | 0.33 | 2.25 | 2.6 × 10−02 | 2.9 × 10−02 | −0.24 | 0.1 | −2.46 | 4.4 × 10−02 | 9.9 × 10−02 |
| Allhorhizobium Neorhizobium Pararhizobium Rhizobium | 0.26 | 0.38 | 1.14 | 3.03 | 2.9 × 10−03 | 1.4 × 10−02 | −0.12 | 0.08 | −1.53 | 2.3 × 10−01 | 2.5 × 10−01 |
| Murdochiella | 0.21 | 0.35 | 0.91 | 2.6 | 1.0 × 10−02 | 1.6 × 10−02 | −0.11 | 0.08 | −1.51 | 2.3 × 10−01 | 2.5 × 10−01 |
| Lachnospiraceae | 0.13 | 0.22 | 0.51 | 2.31 | 2.2 × 10−02 | 2.9 × 10−02 | −0.11 | 0.09 | −1.29 | 2.7 × 10−01 | 2.8 × 10−01 |
| Atopobium | 0.11 | 0.26 | −0.18 | −0.69 | 4.9 × 10−01 | 4.9 × 10−01 | 0.09 | 0.08 | 1.16 | 3.3 × 10−01 | 3.4 × 10−01 |
| ADHD Diagnosis | |||||||||||
| Tyzzerella | 0.59 | 0.06 | 0.22 | 3.82 | 1.3 × 10−04 | 2.4 × 10−03 | −3.6 | 0.99 | −3.65 | 9.7 × 10−04 | 9.4 × 10−03 |
| RF39 | 0.25 | 0.06 | −0.19 | −3.39 | 7.1 × 10−04 | 6.4 × 10−03 | 3.13 | 0.9 | 3.49 | 1.2 × 10−03 | 9.4 × 10−03 |
| Eubacterium fissicatena group | 0.11 | 0.11 | 0.28 | 2.55 | 1.1 × 10−02 | 1.6 × 10−02 | −1.48 | 0.59 | −2.53 | 2.4 × 10−02 | 5.0 × 10−02 |
| Sutterella | 0.19 | 0.08 | −0.24 | −2.85 | 4.4 × 10−03 | 1.4 × 10−02 | 1.75 | 0.66 | 2.66 | 9.3 × 10−03 | 5.2 × 10−02 |
| uncultured.6 | 0.15 | 0.06 | −0.18 | −2.78 | 5.4 × 10−03 | 1.4 × 10−02 | 2.3 | 0.9 | 2.57 | 2.1 × 10−02 | 5.2 × 10−02 |
| Eisenbergiella | 0.12 | 0.09 | 0.25 | 2.77 | 5.6 × 10−03 | 1.4 × 10−02 | −1.79 | 0.67 | −2.67 | 1.6 × 10−02 | 5.2 × 10−02 |
| Ruminiclostridium | 0.15 | 0.11 | 0.3 | 2.63 | 8.7 × 10−03 | 1.6 × 10−02 | −1.47 | 0.71 | −2.07 | 9.3 × 10−02 | 1.1 × 10−01 |
| Caulobacter | 0.11 | 0.16 | 0.43 | 2.64 | 8.3 × 10−03 | 1.6 × 10−02 | −0.97 | 0.56 | −1.74 | 1.4 × 10−01 | 1.5 × 10−01 |
| Sanguibacteroides | 0.16 | 0.15 | −0.4 | −2.58 | 9.8 × 10−03 | 1.6 × 10−02 | 1.25 | 0.65 | 1.93 | 1.3 × 10−01 | 2.1 × 10−01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakobi, B.; Cimetti, C.; Mulder, D.; Vlaming, P.; Franke, B.; Hoogman, M.; Arias-Vasquez, A. The Role of Diet and the Gut Microbiota in Reactive Aggression and Adult ADHD—An Exploratory Analysis. Nutrients 2024, 16, 2174. https://doi.org/10.3390/nu16142174
Jakobi B, Cimetti C, Mulder D, Vlaming P, Franke B, Hoogman M, Arias-Vasquez A. The Role of Diet and the Gut Microbiota in Reactive Aggression and Adult ADHD—An Exploratory Analysis. Nutrients. 2024; 16(14):2174. https://doi.org/10.3390/nu16142174
Chicago/Turabian StyleJakobi, Babette, Chiara Cimetti, Danique Mulder, Priscilla Vlaming, Barbara Franke, Martine Hoogman, and Alejandro Arias-Vasquez. 2024. "The Role of Diet and the Gut Microbiota in Reactive Aggression and Adult ADHD—An Exploratory Analysis" Nutrients 16, no. 14: 2174. https://doi.org/10.3390/nu16142174
APA StyleJakobi, B., Cimetti, C., Mulder, D., Vlaming, P., Franke, B., Hoogman, M., & Arias-Vasquez, A. (2024). The Role of Diet and the Gut Microbiota in Reactive Aggression and Adult ADHD—An Exploratory Analysis. Nutrients, 16(14), 2174. https://doi.org/10.3390/nu16142174






