Mediterranean Diet Adherence, Gut Microbiota and Parkinson’s Disease: A Systematic Review
Abstract
1. Introduction
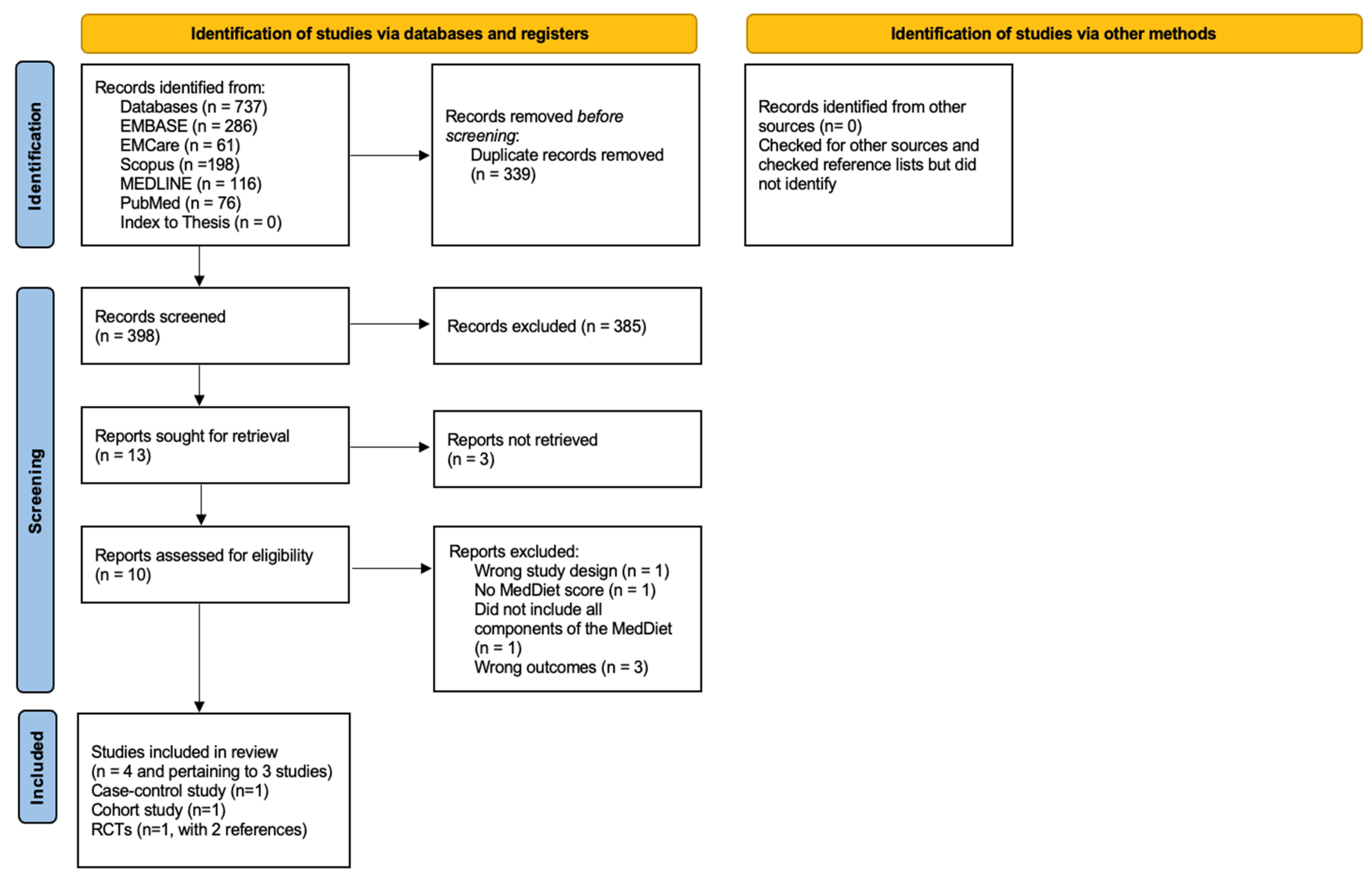
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Data Collection and Analysis
2.5. Assessment of Risk of Bias in Included Studies
2.6. Assessment for Heterogeneity and Synthesis of the Evidence
3. Results
3.1. Results of the Search
3.2. Excluded Studies
3.3. Included Studies
3.4. Characteristics of the Included Studies
3.4.1. Randomised Controlled Trials (RCT)
3.4.2. Case-Control Study
3.4.3. Cohort Study
3.5. Results of the Quality Assessment
3.6. Outcomes
3.6.1. Evidence from Randomised Controlled Trials (RCTs)
Effect of the Mediterranean Diet (MedDiet) on Global Cognitive Function and Specific Cognitive Domains
Effect of the Mediterranean Diet (MedDiet) on Serum Total Antioxidant Capacity, Motor and Non-Motor Symptoms beyond Cognitive Dysfunction
3.6.2. Evidence from the Case-Control Study
Mediterranean Diet (MedDiet) Adherence, Microbial Communities and Gastrointestinal Function
3.6.3. Evidence from the Cohort Study
Non-Motor Symptoms in Parkinson’s Disease (PD)
4. Discussion
4.1. Principal Findings
4.2. Effect of the Mediterranean Diet (MedDiet) on Motor Symptoms
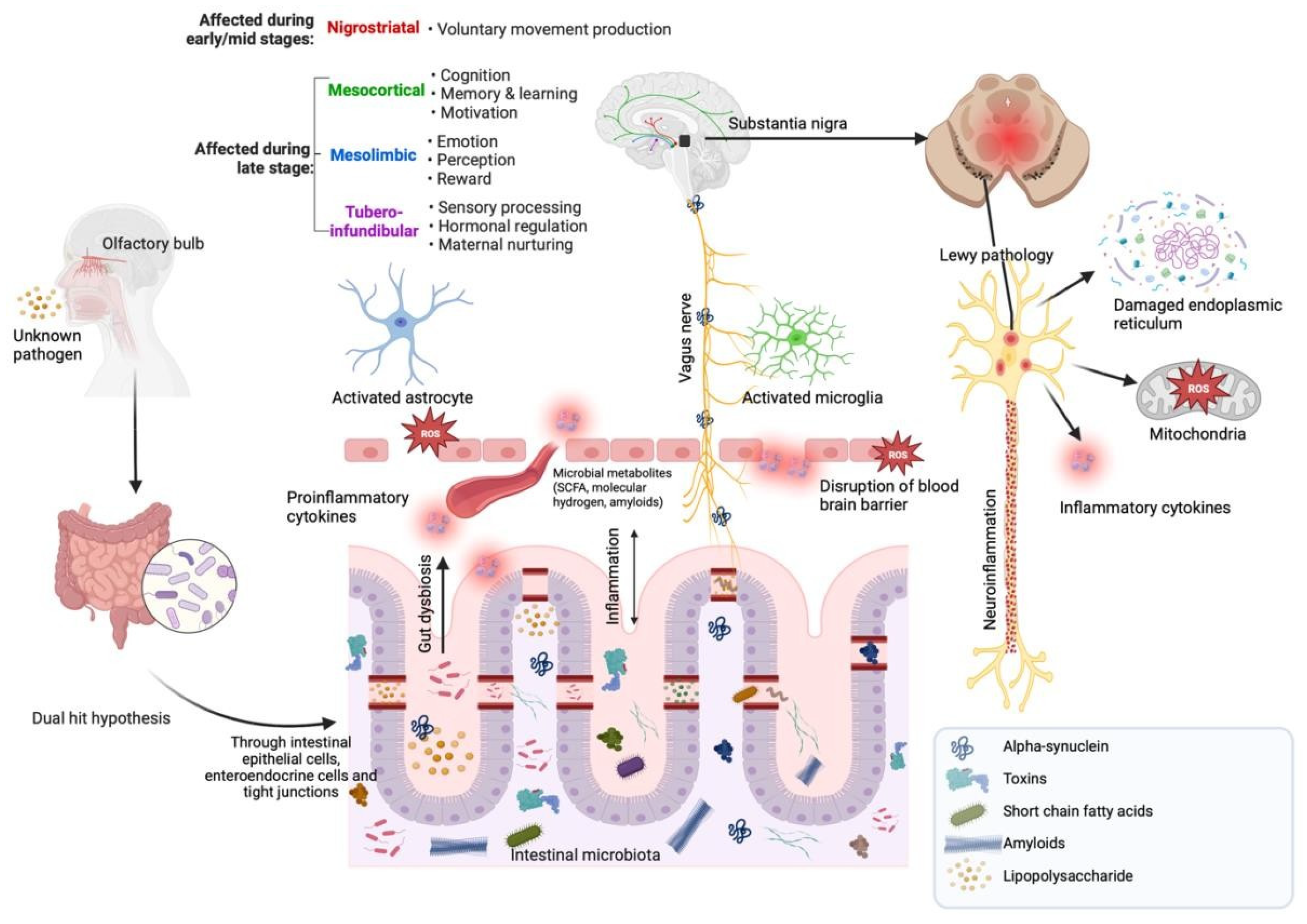
4.3. The Link between Short-Chain Fatty Acids (SCFAs), Mediterranean Diet (MedDiet) Adherence and Gastrointestinal Disturbances in Parkinson’s Disease (PD)
4.4. The Effect of Mediterranean Diet (MedDiet) on Cognitive Function in Parkinson’s Disease (PD)
4.5. Strengths and Limitations of this Systematic Review
4.6. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World-Health-Organization. Parkinson Disease: A Public Health Approach. Technical Brief; World-Health-Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240050983#:~:text=Download%20(1.2%20MB)-,Overview,%2Dincome%20countries%20(LMIC) (accessed on 19 April 2024).
- Rajput, A.H.; Rajput, A. Accuracy of Parkinson disease diagnosis unchanged in 2 decades. Neurology 2014, 83, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Parkinson’s-UK. The Incidence and Prevalence of Parkinson’s in the UK. Results from the Clinical Practice Research Datalink. 2022. Available online: https://www.parkinsons.org.uk/sites/default/files/2018-01/CS2960%20Incidence%20and%20prevalence%20report%20branding%20summary%20report.pdf (accessed on 19 April 2024).
- Deuschl, G.; Beghi, E.; Fazekas, F.; Varga, T.; Christoforidi, K.A.; Sipido, E.; Bassetti, C.L.; Vos, T.; Feigin, V.L. The burden of neurological diseases in Europe: An analysis for the Global Burden of Disease Study 2017. Lancet Public Health 2020, 5, e551–e567. [Google Scholar] [CrossRef]
- NHS-Digital. Health Survey for England, 2019. 2019. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2019 (accessed on 19 April 2024).
- Beard, J.R.; Officer, A.; de Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.P.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; Peeters, G.; Mahanani, W.R.; et al. The World report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef]
- Carter, J.; Mathers, J.; Fairweather-Tait, S.; Jebb, S.; Sattar, N.; Jennings, A.; Minihane, A.-M. Medical Research Council Hot Topic workshop report: Planning a UK Nutrition and Healthy Life Expectancy Trial. Nutr. Bull. 2021, 46, 395–408. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef]
- Parkinson’s-UK. The Cost of Parkinson’s: The Financial Impact of Living with the Condition. 2017. Available online: https://www.parkinsons.org.uk/sites/default/files/2017-07/CS2547%20Cost%20of%20Parkinson%27s%20report%202017%20-%20UK_1.pdf (accessed on 19 April 2024).
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.C.; Ulane, C.M.; Burke, R.E. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 2010, 67, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Mhyre, T.R.; Boyd, J.T.; Hamill, R.W.; Maguire-Zeiss, K.A. Parkinson’s disease. Subcell. Biochem. 2012, 65, 389–455. [Google Scholar] [CrossRef]
- Postuma, R.B.; Aarsland, D.; Barone, P.; Burn, D.J.; Hawkes, C.H.; Oertel, W.; Ziemssen, T. Identifying prodromal Parkinson’s disease: Pre-motor disorders in Parkinson’s disease. Mov. Disord. 2012, 27, 617–626. [Google Scholar] [CrossRef]
- Kalaitzakis, M.E.; Graeber, M.B.; Gentleman, S.M.; Pearce, R.K. The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease: A critical analysis of alpha-synuclein staging. Neuropathol. Appl. Neurobiol. 2008, 34, 284–295. [Google Scholar] [CrossRef]
- Zaccai, J.; Brayne, C.; McKeith, I.; Matthews, F.; Ince, P.G. Patterns and stages of alpha-synucleinopathy: Relevance in a population-based cohort. Neurology 2008, 70, 1042–1048. [Google Scholar] [CrossRef]
- Rietdijk, C.D.; Perez-Pardo, P.; Garssen, J.; van Wezel, R.J.; Kraneveld, A.D. Exploring Braak’s Hypothesis of Parkinson’s Disease. Front. Neurol. 2017, 8, 37. [Google Scholar] [CrossRef]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. Parkinson’s disease: A dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 2007, 33, 599–614. [Google Scholar] [CrossRef]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. Parkinson’s disease: The dual hit theory revisited. Ann. N. Y. Acad. Sci. 2009, 1170, 615–622. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Solch, R.J.; Aigbogun, J.O.; Voyiadjis, A.G.; Talkington, G.M.; Darensbourg, R.M.; O’Connell, S.; Pickett, K.M.; Perez, S.R.; Maraganore, D.M. Mediterranean diet adherence, gut microbiota, and Alzheimer’s or Parkinson’s disease risk: A systematic review. J. Neurol. Sci. 2022, 434, 120166. [Google Scholar] [CrossRef]
- Martucci, M.; Ostan, R.; Biondi, F.; Bellavista, E.; Fabbri, C.; Bertarelli, C.; Salvioli, S.; Capri, M.; Franceschi, C.; Santoro, A. Mediterranean diet and inflammaging within the hormesis paradigm. Nutr. Rev. 2017, 75, 442–455. [Google Scholar] [CrossRef]
- Korre, M.; Tsoukas, M.A.; Frantzeskou, E.; Yang, J.; Kales, S.N. Mediterranean Diet and Workplace Health Promotion. Curr. Cardiovasc. Risk Rep. 2014, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef]
- Seidl, S.; Santiago, J.; Bilyk, H.; Potashkin, J. The emerging role of nutrition in Parkinson’s disease. Front. Aging Neurosci. 2014, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villegas, A.; Galbete, C.; Martinez-González, M.A.; Martinez, J.A.; Razquin, C.; Salas-Salvadó, J.; Estruch, R.; Buil-Cosiales, P.; Martí, A. The effect of the Mediterranean diet on plasma brain-derived neurotrophic factor (BDNF) levels: The PREDIMED-NAVARRA randomized trial. Nutr. Neurosci. 2011, 14, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, H.; Fung, T.T.; Logroscino, G.; Schwarzschild, M.A.; Hu, F.B.; Ascherio, A. Prospective study of dietary pattern and risk of Parkinson disease. Am. J. Clin. Nutr. 2007, 86, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Alcalay, R.N.; Gu, Y.; Mejia-Santana, H.; Cote, L.; Marder, K.S.; Scarmeas, N. The association between Mediterranean diet adherence and Parkinson’s disease. Mov. Disord. 2012, 27, 771–774. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Löf, M.; Pedersen, N.L.; Sandin, S.; Fang, F. Mediterranean Dietary Pattern at Middle Age and Risk of Parkinson’s Disease: A Swedish Cohort Study. Mov. Disord. 2021, 36, 255–260. [Google Scholar] [CrossRef]
- Metcalfe-Roach, A.; Yu, A.C.; Golz, E.; Cirstea, M.; Sundvick, K.; Kliger, D.; Foulger, L.H.; Mackenzie, M.; Finlay, B.B.; Appel-Cresswell, S. MIND and Mediterranean Diets Associated with Later Onset of Parkinson’s Disease. Mov. Disord. 2021, 36, 977–984. [Google Scholar] [CrossRef]
- Alcalay, R.N.; Gu, Y.; Mehia-Santana, H.; Cote, L.; Marder, K.S.; Scarmeas, N. The association between mediterranean-type diet adherence and Parkinson’s disease. Ann. Neurol. 2011, 70 (Suppl. 15), S19–S20. [Google Scholar] [CrossRef]
- Agarwal, P.; Wang, Y.; Buchman, A.S.; Holland, T.M.; Bennett, D.A.; Morris, M.C. MIND Diet Associated with Reduced Incidence and Delayed Progression of Parkinsonism in Old Age. J. Nutr. Health Aging 2018, 22, 1211–1215. [Google Scholar] [CrossRef]
- Maraki, M.I.; Yannakoulia, M.; Stamelou, M.; Stefanis, L.; Xiromerisiou, G.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Anastasiou, C.A.; et al. Mediterranean diet adherence is related to reduced probability of prodromal Parkinson’s disease. Mov. Disord. 2019, 34, 48–57. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, X.; Ye, Y.; Yan, X.; Cheng, Y.; Zhao, L.; Chen, F.; Ling, Z. Gut Microbiota: A Novel Therapeutic Target for Parkinson’s Disease. Front. Immunol. 2022, 13, 937555. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.A.; Voigt, R.M.; Cantu-Jungles, T.M.; Hamaker, B.; Engen, P.A.; Shaikh, M.; Raeisi, S.; Green, S.J.; Naqib, A.; Forsyth, C.B.; et al. An open label, non-randomized study assessing a prebiotic fiber intervention in a small cohort of Parkinson’s disease participants. Nat. Commun. 2023, 14, 926. [Google Scholar] [CrossRef] [PubMed]
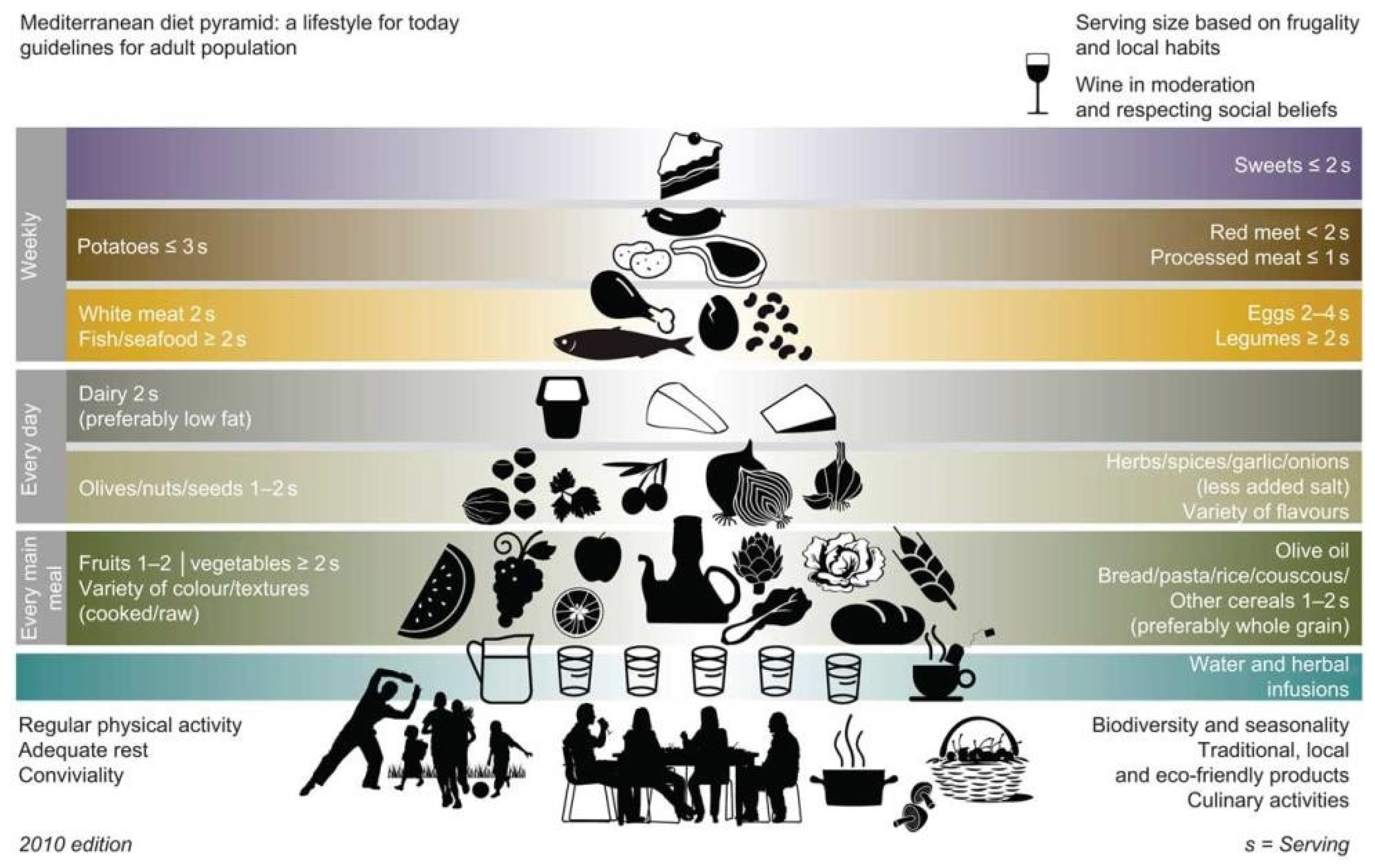
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Swarztrauber, K.; Anau, J.; Peters, D. Identifying and distinguishing cases of parkinsonism and Parkinson’s disease using ICD-9 CM codes and pharmacy data. Mov. Disord. 2005, 20, 964–970. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; a Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Academy-of-Nutrition-and-Dietetics. Evidence Analysis Manuel: Steps in the Academy Evidence Analysis Process. 2022. Available online: https://www.andeal.org/evidence-analysis-manual (accessed on 20 July 2023).
- Paknahad, Z.; Sheklabadi, E.; Derakhshan, Y.; Bagherniya, M.; Chitsaz, A. The effect of the Mediterranean diet on cognitive function in patients with Parkinson’s disease: A randomized clinical controlled trial. Complement. Ther. Med. 2020, 50, 102366. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, Z.; Sheklabadi, E.; Moravejolahkami, A.R.; Chitsaz, A.; Hassanzadeh, A. The effects of Mediterranean diet on severity of disease and serum Total Antioxidant Capacity (TAC) in patients with Parkinson’s disease: A single center, randomized controlled trial. Nutr. Neurosci. 2022, 25, 313–320. [Google Scholar] [CrossRef]
- Rusch, C.; Beke, M.; Tucciarone, L.; Nieves, C., Jr.; Ukhanova, M.; Tagliamonte, M.S.; Mai, V.; Suh, J.H.; Wang, Y.; Chiu, S.; et al. Mediterranean Diet Adherence in People With Parkinson’s Disease Reduces Constipation Symptoms and Changes Fecal Microbiota After a 5-Week Single-Arm Pilot Study. Front. Neurol. 2021, 12, 794640. [Google Scholar] [CrossRef]
- Fox, D.J.; Park, S.J.; Mischley, L.K. Comparison of Associations between MIND and Mediterranean Diet Scores with Patient-Reported Outcomes in Parkinson’s Disease. Nutrients 2022, 14, 5185. [Google Scholar] [CrossRef] [PubMed]
- Hutchins-Wiese, H.L.; Bales, C.W.; Porter Starr, K.N. Mediterranean diet scoring systems: Understanding the evolution and applications for Mediterranean and non-Mediterranean countries. Br. J. Nutr. 2022, 128, 1371–1392. [Google Scholar] [CrossRef] [PubMed]
- WHO Consultation on Obesity; World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000; Available online: https://iris.who.int/handle/10665/42330 (accessed on 19 April 2024).
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of Diet and Nutritional Supplements in Parkinson’s Disease Progression. Oxid. Med. Cell. Longev. 2017, 2017, 6405278. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, L.; Brayne, C. A systematic review of nutritional risk factors of Parkinson’s disease. Nutr. Res. Rev. 2005, 18, 259–282. [Google Scholar] [CrossRef] [PubMed]
- Keramati, M.; Musazadeh, V.; Kheirouri, S. Association between Mediterranean diet and Parkinson’s disease in adults: A systematic review and meta-analysis of cohort studies. Mediterr. J. Nutr. Metab. 2022, 15, 1–10. [Google Scholar] [CrossRef]
- Bianchi, V.E.; Rizzi, L.; Somaa, F. The role of nutrition on Parkinson’s disease: A systematic review. Nutr. Neurosci. 2023, 26, 605–628. [Google Scholar] [CrossRef] [PubMed]
- Bisaglia, M. Mediterranean Diet and Parkinson’s Disease. Int. J. Mol. Sci. 2022, 24, 42. [Google Scholar] [CrossRef]
- Rees, J.; Ryan, J.; Laws, M.; Devine, A. A comprehensive examination of the evidence for whole of diet patterns in Parkinson’s disease: A scoping review. Nutr. Neurosci. 2024, 27, 547–565. [Google Scholar] [CrossRef]
- Wu, L.; Chu, L.; Pang, Y.; Huo, J.; Cao, H.; Tian, Q.; Gao, Q. Effects of dietary supplements, foods, and dietary patterns in Parkinson’s disease: Meta-analysis and systematic review of randomized and crossover studies. Eur. J. Clin. Nutr. 2024, 78, 365–375. [Google Scholar] [CrossRef]
- Keramati, M.; Kheirouri, S.; Etemadifar, M. Dietary approach to stop hypertension (DASH), but not Mediterranean and MIND, dietary pattern protects against Parkinson’s disease. Food Sci. Nutr. 2024, 12, 943–951. [Google Scholar] [CrossRef] [PubMed]
- van Kessel, S.P.; El Aidy, S. Contributions of Gut Bacteria and Diet to Drug Pharmacokinetics in the Treatment of Parkinson’s Disease. Front. Neurol. 2019, 10, 1087. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Bonazzi, P.; Scarpellini, E.; Bendia, E.; Lauritano, E.C.; Fasano, A.; Ceravolo, M.G.; Capecci, M.; Rita Bentivoglio, A.; Provinciali, L.; et al. Prevalence of Small Intestinal Bacterial Overgrowth in Parkinson’s Disease. Mov. Disord. 2011, 26, 889–892. [Google Scholar] [CrossRef]
- Fasano, A.; Bove, F.; Gabrielli, M.; Petracca, M.; Zocco, M.A.; Ragazzoni, E.; Barbaro, F.; Piano, C.; Fortuna, S.; Tortora, A.; et al. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov. Disord. 2013, 28, 1241–1249. [Google Scholar] [CrossRef]
- Puris, E.; Gynther, M.; Auriola, S.; Huttunen, K.M. L-Type amino acid transporter 1 as a target for drug delivery. Pharm. Res. 2020, 37, 88. [Google Scholar] [CrossRef] [PubMed]
- Rusch, C.; Flanagan, R.; Suh, H.; Subramanian, I. To restrict or not to restrict? Practical considerations for optimizing dietary protein interactions on levodopa absorption in Parkinson’s disease. NPJ Park. Dis. 2023, 9, 98. [Google Scholar] [CrossRef]
- Kanai, Y.; Segawa, H.; Miyamoto, K.; Uchino, H.; Takeda, E.; Endou, H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem. 1998, 273, 23629–23632. [Google Scholar] [CrossRef]
- Pedrosa Carrasco, A.J.; Timmermann, L.; Pedrosa, D.J. Management of constipation in patients with Parkinson’s disease. NPJ Park. Dis. 2018, 4, 6. [Google Scholar] [CrossRef]
- Knudsen, K.; Fedorova, T.D.; Bekker, A.C.; Iversen, P.; Østergaard, K.; Krogh, K.; Borghammer, P. Objective Colonic Dysfunction is Far more Prevalent than Subjective Constipation in Parkinson’s Disease: A Colon Transit and Volume Study. J. Park. Dis. 2017, 7, 359–367. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Capuano, E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 2017, 57, 3543–3564. [Google Scholar] [CrossRef]
- Bellini, M.; Tonarelli, S.; Barracca, F.; Rettura, F.; Pancetti, A.; Ceccarelli, L.; Ricchiuti, A.; Costa, F.; de Bortoli, N.; Marchi, S.; et al. Chronic Constipation: Is a Nutritional Approach Reasonable? Nutrients 2021, 13, 3386. [Google Scholar] [CrossRef]
- Jouët, P.; Sabaté, J.-M.; Coffin, B.; Lémann, M.; Jian, R.; Flourié, B. Fermentation of starch stimulates propagated contractions in the human colon. Neurogastroenterol. Motil. 2011, 23, 450–456 e176. [Google Scholar] [CrossRef]
- Haro, C.; García-Carpintero, S.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Landa, B.B.; Clemente, J.C.; Pérez-Martínez, P.; López-Miranda, J.; Pérez-Jiménez, F.; Camargo, A. Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Mol. Nutr. Food Res. 2017, 61, 1700300. [Google Scholar] [CrossRef]
- van der Beek, C.M.; Dejong, C.H.C.; Troost, F.J.; Masclee, A.A.M.; Lenaerts, K. Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr. Rev. 2017, 75, 286–305. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Peng, L.; He, Z.; Chen, W.; Holzman, I.R.; Lin, J. Effects of Butyrate on Intestinal Barrier Function in a Caco-2 Cell Monolayer Model of Intestinal Barrier. Pediatr. Res. 2007, 61, 37–41. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshida, S.; Hara, H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br. J. Nutr. 2008, 100, 297–305. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef]
- Thangaraju, M.; Cresci, G.A.; Liu, K.; Ananth, S.; Gnanaprakasam, J.P.; Browning, D.D.; Mellinger, J.D.; Smith, S.B.; Digby, G.J.; Lambert, N.A.; et al. GPR109A Is a G-protein–Coupled Receptor for the Bacterial Fermentation Product Butyrate and Functions as a Tumor Suppressor in Colon. Cancer Res. 2009, 69, 2826–2832. [Google Scholar] [CrossRef]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef]
- McRorie, J.W.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef]
- Visekruna, A.; Luu, M. The Role of Short-Chain Fatty Acids and Bile Acids in Intestinal and Liver Function, Inflammation, and Carcinogenesis. Front. Cell Dev. Biol. 2021, 9, 703218. [Google Scholar] [CrossRef]
- Colamatteo, A.; Carbone, F.; Bruzzaniti, S.; Galgani, M.; Fusco, C.; Maniscalco, G.T.; Di Rella, F.; de Candia, P.; De Rosa, V. Molecular Mechanisms Controlling Foxp3 Expression in Health and Autoimmunity: From Epigenetic to Post-translational Regulation. Front. Immunol. 2020, 10, 3136. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Zimmerman, M.A.; Singh, N.; Martin, P.M.; Thangaraju, M.; Ganapathy, V.; Waller, J.L.; Shi, H.; Robertson, K.D.; Munn, D.H.; Liu, K. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 302, G1405–G1415. [Google Scholar] [CrossRef] [PubMed]
- Vareiro, D.; Bach-Faig, A.; Raidó Quintana, B.; Bertomeu, I.; Buckland, G.; Vaz de Almeida, M.D.; Serra-Majem, L. Availability of Mediterranean and non-Mediterranean foods during the last four decades: Comparison of several geographical areas. Public Health Nutr. 2009, 12, 1667–1675. [Google Scholar] [CrossRef]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef]
- Gotsis, E.; Anagnostis, P.; Mariolis, A.; Vlachou, A.; Katsiki, N.; Karagiannis, A. Health Benefits of the Mediterranean Diet:An Update of Research Over the Last 5 Years. Angiology 2015, 66, 304–318. [Google Scholar] [CrossRef]
- Serhan, C.N.; Petasis, N.A. Resolvins and Protectins in Inflammation Resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Walker, D.I.; Lill, C.M.; Bloem, B.R.; Darweesh, S.K.L.; Pinto-Pacheco, B.; McNeil, B.; Miller, G.W.; Heath, A.K.; Frissen, M.; et al. Lipopolysaccharide-binding protein and future Parkinson’s disease risk: A European prospective cohort. J. Neuroinflamm. 2023, 20, 170. [Google Scholar] [CrossRef] [PubMed]
- Taepavarapruk, P.; Song, C. Reductions of acetylcholine release and nerve growth factor expression are correlated with memory impairment induced by interleukin-1β administrations: Effects of omega-3 fatty acid EPA treatment. J. Neurochem. 2010, 112, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Luchtman, D.W.; Meng, Q.; Wang, X.; Shao, D.; Song, C. Omega-3 fatty acid eicospentaenoic acid attenuates MPP+-induced neurodegeneration in fully differentiated human SH-SY5Y and primary mesencephalic cells. J. Neurochem. 2013, 124, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.A.; Holscher, H.D. Microbiome-Mediated Effects of the Mediterranean Diet on Inflammation. Adv. Nutr. 2018, 9, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Chauhan, V. Beneficial Effects of Walnuts on Cognition and Brain Health. Nutrients 2020, 12, 550. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Farooqui, T. Effects of Extra-Virgin Olive Oil in Neurological Disorders. In Neuroprotective Effects of Phytochemicals in Neurological Disorders; Wiley: Hoboken, NJ, USA, 2017; pp. 133–148. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Agarwal, P.; Dhana, K.; Barnes, L.L.; Holland, T.M.; Zhang, Y.; Evans, D.A.; Morris, M.C. Unhealthy foods may attenuate the beneficial relation of a Mediterranean diet to cognitive decline. Alzheimers Dement. 2021, 17, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Gardener, S.L.; Rainey-Smith, S.R.; Barnes, M.B.; Sohrabi, H.R.; Weinborn, M.; Lim, Y.Y.; Harrington, K.; Taddei, K.; Gu, Y.; Rembach, A.; et al. Dietary patterns and cognitive decline in an Australian study of ageing. Mol. Psychiatry 2015, 20, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Kanoski, S.E.; Davidson, T.L. Western diet consumption and cognitive impairment: Links to hippocampal dysfunction and obesity. Physiol. Behav. 2011, 103, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Shannon, O.M.; Stephan, B.C.M.; Granic, A.; Lentjes, M.; Hayat, S.; Mulligan, A.; Brayne, C.; Khaw, K.T.; Bundy, R.; Aldred, S.; et al. Mediterranean diet adherence and cognitive function in older UK adults: The European Prospective Investigation into Cancer and Nutrition-Norfolk (EPIC-Norfolk) Study. Am. J. Clin. Nutr. 2019, 110, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.Y.N.; Wareham, N.J.; Khaw, K.-T.; Imamura, F.; Forouhi, N.G. Prospective association of the Mediterranean diet with cardiovascular disease incidence and mortality and its population impact in a non-Mediterranean population: The EPIC-Norfolk study. BMC Med. 2016, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Kalantar-Zadeh, K. Observational studies versus randomized controlled trials: Avenues to causal inference in nephrology. Adv. Chronic Kidney Dis. 2012, 19, 11–18. [Google Scholar] [CrossRef]
- Mark, S.D.; Robins, J.M. A method for the analysis of randomized trials with compliance information: An application to the Multiple Risk Factor Intervention Trial. Control Clin. Trials 1993, 14, 79–97. [Google Scholar] [CrossRef]
| Study (Country) | Study Design | Type of Intervention | Type of Control | Sample Size of Intervention Group | Sample Size of Control Group | Severity Rating Scale Used | Mean Age, y | Males, % | PD Duration, y | Duration of Intervention | Adjustment | Main Findings—MedDiet Associated with: | Additional Notes | Quality Assessment Rating |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paknahad et al., 2020 [43] (Iran) | RCT | MedDiet | Typical Iranian diet | 35 | 35 | The Hoehn and Yahr scale and the Unified Parkinson Disease Rating Scale (UPDRS) | MedDiet: 59.3 ± 8.3 Control: 58.6 ± 9.3 | MedDiet: 61.8 Control: 55.6 | MedDiet: 6.6 ± 6.0; Control: 5.8 ± 4.9 | 10 weeks | Univariate analyses only | Executive function (p = 0.001); Language (p = 0.02); Attention, concentration, working memory (p = 0.04); global cognitive function (p = 0.001) No significant differences in: visuospatial abilities (p = 0.99), short term memory recall (p = 0.3), orientation to time and place (p = 0.24) | Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) test Funding status not mentioned | Positive |
| Paknahad et al., 2022 [44] (Iran) | RCT | MedDiet | Typical Iranian diet | 36 | 34 | The Hoehn and Yahr scale and the Unified Parkinson Disease Rating Scale (UPDRS) | MedDiet: 59.3 ± 8.3 Control: 58.6 ± 9.3 | MedDiet: 61.8 Control: 55.6 | MedDiet: 6.6 ± 6.0; Control: 5.8 ± 4.9 | 10 weeks | Univariate analyses only | Increased intakes of selenium (p = 0.04) and beta-carotene (p = 0.002), serum total antioxdiant capacity (p < 0.001), mentation, behaviour and mood (p = 0.03), activity of daily living (p = 0.003), complications of therapy (p = 0.04) and total UPDRS (p = 0.01) No significant changes for intakes in vitamin E (p = 0.68) and vitamin C (p = 0.32) and in motor symptoms (p = 0.8) | Funding status not mentioned | Positive |
| Study (Country) | Study Design | Type of Intervention | Type of Control | Sample Size of Intervention Group | Sample Size of Control Group | Severity Rating Scale Used | Mean Age, y | Males, % | PD Duration, y | Duration of Intervention | Adjustment | Main Findings—MedDiet Associated with: | Additional Notes | Quality Assessment Rating |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rusch et al., 2021 [45] (USA) | Case control | MedDiet | Baseline diet/usual diet | 8 | 8 | The Hoehn and Yahr scale and the Unified Parkinson Disease Rating Scale (UPDRS) | 71.4 ± 2.6 | 63.8 | Not specified | 5 weeks | Univariate analyses only | Increase in Proteobacteria proportion; Decrease in Desulfovibrionaceae, Clostridium bolteae, Ruminococous, Blautia, Dorea, Lachnospiraceae (p < 0.01), improvement in constipation (p = 0.04) and indigestion syndrome (p = 0.02) No observed significant differences in the proportion of Roseburia and in abdominal pain (p = 0.13), reflux syndrome (p = 0.50) and diarrhoea (p > 0.05) | MedDiet adherence was assessed using the 14-item MEDAS questionnaire GI symptoms were assessed using the Gastrointestinal Symptom Rating Scale (GSRS) Medications for PD did not change during the study protocol Study funded as part of the University of Florida’s Creating the Healthiest Generation Moonshot initiative | Positive |
| Study (Country) | Study Design | Type of Intervention | Sample Size of Intervention Group | PD Definition | Mean Age, y | Males, % | PD Duration, y | Adjustment | Main Findings—MedDiet Associated with: | Additional Notes | Quality Assessment Rating |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fox et al., (2022) [46] (USA) | Cohort | Adherence to the MedDiet | 1205 | Not specified | 66.4 ± 8.76 | 39 | 7.19 ± 5.44 | Age, gender, income, and years since diagnosis | Decrease in PRO-PD score by 13.0 points (19.1–6.94) for non-motor symptoms and by 9.78 (14.3–5.23) for motor symptoms for each 1-point increase in the MEDAS score (p < 0.001) | MedDiet adherence was assessed using the MEDAS questionnaire. Study was independently funded. | Positive |
| Relevance Questions | Study 1 (Paknahad et al., 2020 [43]) (Paknahad et al., 2022 [44]) | Study 2 (Fox et al., 2022 [46]) | Study 3 (Rusch et al., 2021 [45]) |
|---|---|---|---|
| 1. Would implementing the studied intervention or procedure (if found successful) result in improved outcomes for the patients/ clients/ population group? | |||
| 2. Did the authors study an outcome (dependent variable) or topic that the patients/ clients/ population would care about? | |||
| 3. Is the focus of the intervention or procedure (independent variable) or topic of study a common issue of concern to dietetics practice? | |||
| 4. Is the intervention or procedure feasible? (NA for some epidemiological studies) | |||
| Validity Questions | |||
| 1. Was the research question clearly stated? | |||
| 2. Was the selecion of study subjects/ patients free from bias? | |||
| 3. Were study groups comparable? | |||
| 4. Was method of handling withdrawals described? | |||
| 5. Was blinding used to prevent introduction of bias? | |||
| 6. Were intervention/ therapeutic regimens/ exposure factor or procedure and any comparison(s) described in detail? Were intervening factors described? | |||
| 7. Were outcomes clearly defined and the measurements valid and reliable? | |||
| 8. Was the statistical analysis appropriate for the study design and type of outcome indicators? | |||
| 9. Are conclusions supported by results with biases and limitations taken into consideration? | |||
| 10. Is bias due to study’s funding or sponsorship unlikely? | |||
| Overall Rating |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seelarbokus, B.A.; Menozzi, E.; Schapira, A.H.V.; Kalea, A.Z.; Macnaughtan, J. Mediterranean Diet Adherence, Gut Microbiota and Parkinson’s Disease: A Systematic Review. Nutrients 2024, 16, 2181. https://doi.org/10.3390/nu16142181
Seelarbokus BA, Menozzi E, Schapira AHV, Kalea AZ, Macnaughtan J. Mediterranean Diet Adherence, Gut Microbiota and Parkinson’s Disease: A Systematic Review. Nutrients. 2024; 16(14):2181. https://doi.org/10.3390/nu16142181
Chicago/Turabian StyleSeelarbokus, Bibi Aliya, Elisa Menozzi, Anthony H. V. Schapira, Anastasia Z. Kalea, and Jane Macnaughtan. 2024. "Mediterranean Diet Adherence, Gut Microbiota and Parkinson’s Disease: A Systematic Review" Nutrients 16, no. 14: 2181. https://doi.org/10.3390/nu16142181
APA StyleSeelarbokus, B. A., Menozzi, E., Schapira, A. H. V., Kalea, A. Z., & Macnaughtan, J. (2024). Mediterranean Diet Adherence, Gut Microbiota and Parkinson’s Disease: A Systematic Review. Nutrients, 16(14), 2181. https://doi.org/10.3390/nu16142181








